Abstract
Although gemcitabine has been accepted as the first-line chemotherapeutic reagent for advanced pancreatic cancer, improvement of response rate and survival is not sufficient and patients often develop resistance. We hypothesized that the inhibition of phosphorylation of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) on tumor cells and tumor-associated endothelial cells, combined with gemcitabine, would overcome the resistance to gemcitabine in orthotopic pancreatic tumor animal model. L3.6pl, human pancreatic cancer cells growing in the pancreas, and tumor-associated endothelial cells in microorgan environment highly expressed phosphorylated EGFR, VEGFR, and Akt, which regulates antiapoptotic mechanism. Oral administration of AEE788 (dual tyrosine kinase inhibitor against EGFR and VEGFR) inhibited the phosphorylation of EGFR, VEGFR, and Akt on tumor-associated endothelial cells as well as tumor cells. Although intraperitoneal (i.p.) injection of gemcitabine showed limited inhibitory effect on tumor growth, combination with AEE788 and gemcitabine produced nearly 95% inhibition of tumor growth in parallel with a high level of apoptosis on tumor cells and tumor-associated endothelial cells, and decreased microvascular density and proliferation rate. Collectively, these data indicate that dual inhibition of phosphorylation of EGFR and VEGFR, in combination with gemcitabine, produces apoptosis of tumor-associated endothelial cells and significantly suppresses human pancreatic cancer in nude mice.
Keywords: AEE788, EGFR, VEGFR, gemcitabine, pancreatic cancer
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States with 28,000 new cases diagnosed annually [1]. The difficulty in detecting pancreatic cancer at an early stage, the aggressive nature of the disease, and the lack of effective therapy are all responsible for the high mortality from this disease [2], a 1-year survival rate of 18%, and a 5-year survival rate of less than 3% [1,3]. Systemic therapy with gemcitabine (2′,2′-difluorodeoxycytidine), a deoxycytidine analogue most recently used against pancreatic cancer, has not increased the median survival of patients beyond 6 months and often leads to resistance [3,4]. Clearly, an effective treatment for this devastating disease is urgently needed.
The molecular biology of pancreatic cancer may present therapeutic opportunities. Recent advances have identified a number of biologic and genetic abnormalities, including overexpression of such growth factors as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and basic fibroblast growth factor (bFGF) and their receptors [5–8]. The binding of ligands to their receptors has been shown to enhance the malignant phenotype of cancer by activating downstream protein kinase cascades, such as the one involving PI3K/Akt, MAPK, and nuclear factor-kappa B (NF-κB) that controls cell proliferation, angiogenesis, chemoresistance, and apoptosis [9–14].
The increase in VEGF production seen in many cancers leads to greater microvascular density (MVD) and is associated with a poor prognosis [15]. This growth factor acts not only as a mitogenic and permeability factor but also as an antiapoptotic survival factor for vascular endothelial cells [16,17]. A vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor and an antibody against VEGF have been shown to inhibit angiogenesis and the progressive growth of tumors in animals. For example, bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA) is a recombinant humanized monoclonal antibody against VEGF that inhibits its binding to VEGFR [18]. In stage IV pancreatic cancer patients, bevacizumab, combined with gemcitabine, produced a 21% partial response rate and 45% of the patients had stable disease. The increase in median survival to 9 months was also encouraging [19]. The overexpression of EGF and epidermal growth factor receptor (EGFR) correlates with development of cancer metastasis and resistance to chemotherapy [14,20–22]. In a phase II clinical trial, the administration of cetuximab (IMC C225, Erbitux; ImClone, New York, NY), a monoclonal antibody targeting EGFR [23], in combination with gemcitabine, produced 12% partial response and stable disease in 63% of the advanced pancreatic cancer patients [24].
Collectively, these data imply that dual inhibition of EGFR and VEGFR signaling pathways could be useful in the treatment of pancreatic cancer [25,26]. One possible inhibitor is AEE788, a recently described specific tyrosine kinase inhibitor of EGFR and VEGFR tyrosine kinases [27]. To establish this inhibitor's potential, we determined whether AEE788, administered alone or in combination with gemcitabine, can inhibit the progressive growth and spread of human pancreatic cancer cells implanted into the pancreas of nude mice.
Materials and Methods
Pancreatic Cancer Cell Line and Culture Conditions
The human pancreatic cancer cell line, L3.6pl [26], was maintained in minimal essential medium supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, non-essential amino acids, l-glutamine, a two-fold vitamin solution (Life Technologies, Grand Island, NY), and a penicillin-streptomycin mixture (Flow Laboratories, Rockville, MD). The cultures were free of mycoplasma and the following pathogenic murine viruses: reovirus type 3, pneumonia virus, K virus, Theiler's encephalitis virus, Sendai virus, minute virus, mouse adenovirus, mouse hepatitis virus, lymphocytic choriomeningitis virus, ectromelia virus, and lactate dehydrogenase virus (assayed by Science Applications International Corp., Frederick, MD).
Nucleotide Sequence Analysis of EGFR in Pancreatic Cancer L3.6pl Cell Line
We analyzed for DNA sequence variations in exons 18, 19, and 21 of the kinase domain of EGFR shown to have mutations in patients who responded to therapy [28]. DNA was extracted from log phase cultures of L3.6pl cells using the DNeasy Tissue Kit No. 69504 (Qiagen, Inc., Valencia, CA). Mutational analysis was performed by the Molecular Diagnostic Laboratory of the M. D. Anderson Cancer Center (Houston, TX). Nested PCR products of exons 18, 19, and 21, obtained using primers previously described [28], were directly sequenced in sense and antisense directions. All sequences were screened for the presence of mutations both manually and using the SeqScape software and confirmed by two independent PCR amplifications. The results clearly indicate that the L3.6pl cells contain a wild-type EGFR.
Reagents
AEE788 (Novartis Pharma, Basel, Switzerland), 7H-pyrrolo[2,3-d]pyrimidine lead scaffold, is an ATP-competitive dual EGFR and VEGFR tyrosine kinase family inhibitor [27]. For in vitro use, AEE788 was dissolved in dimethyl sulfoxide (Sigma-Aldrich Corp., St. Louis, MO) to a concentration of 20 mM and further diluted to an appropriate final concentration in RPMI 1640 medium with 10% FBS. Dimethyl sulfoxide in the final solution did not exceed 0.1% vol/vol. For in vivo administration (oral), AEE788 was dissolved in N-methylpyrrolidone and polyethylene glycol 300 1:9 (vol/vol). The AEE788 solution was prepared just before it was administered to the mice. Paclitaxel (Mead Johnson, Princeton, NJ) was diluted 1:6 in phosphate-buffered saline (PBS) for intraperitoneal (i.p.) injections.
The following primary antibodies were purchased from their respective manufacturers: rabbit anti-p-VEGFR 2/3 (Flk-1) (Oncogene, Boston, MA); rabbit anti-human VEGF (SC152); rabbit anti-human mouse, rat VEGFR (Flk-1) (C1158) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-human p-EGFR (Tyr 1173) (Biosource, Camarillo, CA); rabbit anti-human EGF (SC275) and rabbit anti-human EGFR (SC03) for paraffin samples (Santa Cruz Biotechnology); rabbit anti-human EGFR for frozen samples (Zymed, San Francisco, CA); rat anti-mouse CD31 (BD PharMingen, San Diego, CA); mouse anti-proliferating cell nuclear antigen (PCNA) clone PC 10 (Dako A/S, Copenhagen, Denmark); rabbit anti-mouse, human, rat phospho-Akt (Ser 473) and rabbit anti-human phosphorylated MAPK/ERK1/2 (phospho-p44/42 MAP [ERK1/2] kinase antibody) (Cell Signaling, Beverly, MA); and donkey anti-rabbit IGG-HRP (Santa Cruz Biotechnology). The following secondary antibodies were used for colorimetric immunohistochemistry: peroxidase-conjugated goat antirabbit IgG; F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA); biotinylated goat antirabbit (Biocare Medical, Walnut Creek, CA); streptavidin horseradish peroxidase (Dako A/S); rat anti-mouse IgG2a horseradish peroxidase (Serotec; Harlan Bioproducts for Science, Indianapolis, IN); and goat anti-rat horseradish peroxidase (Jackson ImmunoResearch Laboratories). We obtained the fluorescent secondary antibodies goat anti-rabbit Alexa 488 and goat anti-rat Alexa 594 from Molecular Probes (Eugene, OR). Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining was performed using a commercial apoptosis detection kit (Promega, Madison, WI).
Animals and Orthotopic Implantation of Tumor Cells
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and the National Institutes of Health. The mice were used in accordance with institutional guidelines when they were 8 to 12 weeks old.
To produce pancreatic tumors, we harvested L3.6pl cells from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped with medium containing 10% FBS, and the cells were washed once in serum-free medium and resuspended in Hanks' balanced salt solution (HBSS). Only suspensions consisting of single cells with greater than 90% viability were used for the injections. One million cells suspended in 50 µl of HBSS were injected into the pancreas of nude mice as described previously [26,29].
Treatment of Nude Mice with Established Orthotopic Human Pancreatic Cancer
Fourteen days after the injection of L3.6pl tumor cells into the pancreas, all mice were randomized to one of the following four treatment groups (n = 10): (a) oral gavage three times per week with water diluted 1:20 with DMSO-0.5% Tween 80 (diluent), and two i.p. injections per week of PBS (control group); (b) oral gavage of diluent three times per week, and two i.p. injections per week of gemcitabine (50 mg/kg); (c) oral gavage of AEE788 (50 mg/kg) three times per week, and two i.p. injections per week of PBS; and (d) oral gavage of AEE788 (50 mg/kg) three times per week, and two i.p. injections per week of gemcitabine (50 mg/kg). The treatment continued for 5 weeks, at which time the mice were killed and necropsied.
Necropsy Procedure and Histologic Studies
Before necropsy, mice were weighed, and tumors were then excised and weighed. For histology and immunohistochemical (IHC) analyses, one part of the tumor tissue was fixed in formalin and embedded in paraffin, and another was embedded in OCT compound (Miles, Inc., Elkhart, IN), rapidly frozen in liquid nitrogen, and stored at -70°C.
IHC Analysis to Detect EGF, VEGF, EGFR, VEGFR, p-EGFR, and p-VEGFR
Paraffin-embedded pancreas from tumor-free normal mice and pancreatic tumors from mice in all treatment groups were immunostained to evaluate the expression of EGF, VEGF, EGFR, and VEGFR. Sections were also stained for p-EGFR and p-VEGFR.
Determination of PCNA, CD31/PECAM-1 (Endothelial Cells), and TUNEL
Paraffin-embedded tissues were used to determine PCNA. Sections were deparaffinized and rehydrated in PBS as described previously, microwaved for 5 minutes for antigen retrieval, incubated at 4°C with the primary antibody overnight (mouse IgG2a anti-PCNA), and incubated for 1 hour at room temperature with a secondary antibody (peroxidase-conjugated rat anti-mouse IgG2a). Frozen tissues used to identify CD31/PECAM-1 were sectioned (8–10 µm), mounted on positive-charged slides, and air-dried for 30 minutes. Frozen sections were fixed in cold acetone (5 minutes), in acetone/chloroform (vol/vol; 5 minutes), and again in acetone (5 minutes), and washed with PBS. Positive reaction was visualized by incubating the slides for 10 to 20 minutes with stable DAB. The positive sections were rinsed with distilled water, counterstained with Gill's hematoxylin for 30 seconds, and mounted with Universal Mount (Research Genetics, Huntsville AL). Control samples exposed to a secondary had no specific staining. For the quantification of MVD in sections stained for CD31, 10 random 0.159-mm2 fields at x100 magnification were captured for each tumor, and microvessels were quantified according to the method described previously [29]. For quantification of PCNA expression, the number of positive cells was determined in ten 0.159-mm2 fields at x100 magnification.
Analysis of apoptotic cells was performed by using a commercially available TUNEL kit with the following modifications: samples were fixed with 4% paraformaldehyde (methanol free) for 10 minutes at room temperature, washed twice with PBS for 5 minutes, and then incubated with 0.2% Triton X-100 for 15 minutes at room temperature. After being washed with PBS, the samples were incubated with equilibration buffer for 10 minutes at room temperature, and a reaction buffer was added (containing equilibration buffer, nucleotide mix, and terminal deoxynucleotidyl transferase enzyme). Samples were incubated in a humidified chamber at 37°C for 1 hour in the dark and immersed in 2x SSC for 15 minutes to terminate the reaction. Immunofluorescence microscopy was performed on an epifluorescence microscope equipped with narrow bandpass excitation filters mounted in a filter wheel (Ludl Electronic Products, Hawthorne, NY). Images were captured by using a chilled, cooled charge-coupled device camera (Photometrics, Tucson, AZ) and Smart Capture software (Digital Scientific, Cambridge, UK) on a Macintosh computer. Images were further processed with Adobe Photoshop software (Adobe Systems, Mountain View, CA). The total number of apoptotic cells was quantified by counting 10 randomly selected microscope fields.
Double Immunofluorescence Staining for CD31/PECAM-1 and EGFR, p-EGFR, VEGFR, p-VEGFR, p-Akt, and TUNEL (Apoptotic Cells)
Frozen sections of pancreatic tissue from normal mice and L3.6pl tumors in the pancreas of nude mice were mounted on slides and fixed. IHC procedures for CD31 assay were performed as described above. Samples were again blocked briefly in blocking solution (5% normal horse serum and 1% normal goat serum in PBS) and incubated overnight at 4°C with antibody against human EGFR, p-EGFR, VEGFR, p-VEGFR, or p-Akt. After a wash in PBS, samples were blocked briefly with blocking solution and incubated for 1 hour with the streptavidin-Alexa 488 antibody. To visualize nuclei, we briefly incubated the samples with Hoechst stain. Endothelial cells were identified by red fluorescence, and EGFR, p-EGFR, VEGFR, p-VEGFR, or p-Akt was identified by a green fluorescence. The presence of these growth factor receptors, phosphorylated receptors, or p-Akt in endothelial cells was detected by colocalization of red and green fluorescence, which yields a yellow color. TUNEL-positive apoptotic cells were detected by localized green fluorescence within cell nuclei, and endothelial cells were identified by red fluorescence. Apoptotic endothelial cells were identified by yellow fluorescence within the nuclei. Quantification of apoptotic endothelial cells was expressed as the ratio of apoptotic endothelial cells to the total number of endothelial cells in ten 0.159-mm2 fields at x100 magnification.
Statistical Analysis
Body weight, the weight of the tumors, PCNA-positive cells, MVD (CD31/PECAM-1), TUNEL-positive cells, and ratio of CD31/PECAM-1/TUNEL colocalized cells were compared using the Mann-Whitney U test.
Results
Therapy of Human Pancreatic Cancer in an Orthotopic Nude Mouse Model by AEE788 or AEE788 Combined with Gemcitabine
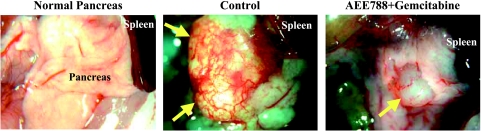
First, we determined the therapeutic efficacy of AEE788, gemcitabine, or the combination of AEE788 and gemcitabine on well-established (visible tumors) human pancreatic cancer in the pancreas of nude mice. At the end of the study, treatment with AEE788 alone, gemcitabine alone, or the combination of AEE788 and gemcitabine did not affect body weight (Table 1). Mice in the control group had the largest tumors. Mice treated with gemcitabine had a similar size (weight) of tumors as the control mice. The tumors in mice receiving oral AEE788 were nearly 70% smaller than controls. The combination treatment reduced the size of the tumors by nearly 95% (Table 1). The gross appearance of a normal pancreas, pancreas from tumor-bearing control-treated mice, and pancreas from tumor-bearing mice treated with AEE788 and gemcitabine are shown in Figure 1. The extensively vascularized tumors in control mice nearly replaced the entire organ. Tumors from mice treated with AEE788 and gemcitabine were small and had few blood vessels.
Table 1.
Therapy of L3.6pl Human Pancreatic Cancer Cells Growing in the Pancreas of Nude Mice.
| Treatment | Body Weight (g) | Tumor Incidence | Tumor Weight (g) | ||
| Median | Range | Median | Range | ||
| Control | 22.1 | 18.7–26.6 | 10/10 | 1.97 | 0.8–3.78 |
| Gemcitabine | 22.6 | 19.9–25.6 | 9/9 | 1.70 | 0.27–3.13 |
| AEE788 | 23.8 | 22.7–26.5 | 10/10 | 0.59* | 0.19–1.1 |
| AEE788 + gemcitabine | 23.1 | 20.6–25.8 | 10/10 | 0.11† | 0.02–0.25 |
L3.6p.l cells (1 x 106) were injected into the pancreas of nude mice. Two weeks later, treatment began and continued for 5 weeks.
The mice were killed on day 36 of treatment (day 50 of the study) and subjected to necropsy.
Body weight, tumor incidence, and weight were determined.
This is one representative experiment of two.
P < .05.
P < .01 vs control.
Figure 1.
Gross appearance of a nude mouse normal pancreas and pancreas of mice implanted with L3.6pl human pancreas cancer cells after 5 weeks of treatment with vehicle (control) or the combination of AEE788 (oral gavage of 50 mg/kg; thrice per week) and gemcitabine (50 mg/kg, i.p.; twice per week). Arrowheads indicate pancreatic tumor. Note the extensive vascularization of pancreatic tumor in control mice.
IHC Analysis
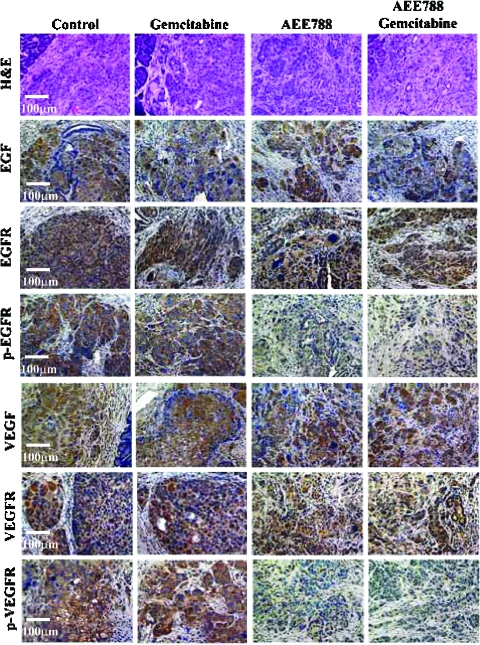
Expression of EGF, VEGF, EGFR, and VEGFR was detected in L3.6pl tumors from all treatment groups. Expression of p-EGFR and p-VEGFR was found only in tumors from control and gemcitabine-treated groups and not in tumors from mice treated with AEE788 or AEE788 plus gemcitabine (Figure 2). Parenchyma cells or endothelial cells in the normal pancreas expressed minimal levels of EGF, VEGF, EGFR, VEGFR, p-EGFR, or p-VEGFR (data not shown).
Figure 2.
IHC staining for EGF, EGFR, p-EGFR, VEGF, VEGFR, and p-VEGFR. The large L3.6pl tumors were harvested, fixed, and sectioned for histology and IHC analysis from mice treated with vehicle (control), AEE788, gemcitabine, or the combination of AEE788 and gemcitabine. Sections of tumors from all treatment groups were stained for expression of EGF, EGFR, p-EGFR, VEGF, VEGFR, and p-VEGFR as described in Materials and Methods section. Tumors from the mice treated with vehicle or gemcitabine positively stained positive for p-EGFR and p-VEGFR. Treatment with AEE788 alone, or AEE788 and gemcitabine significantly decreased the phosphorylation of EGFR and VEGFR.
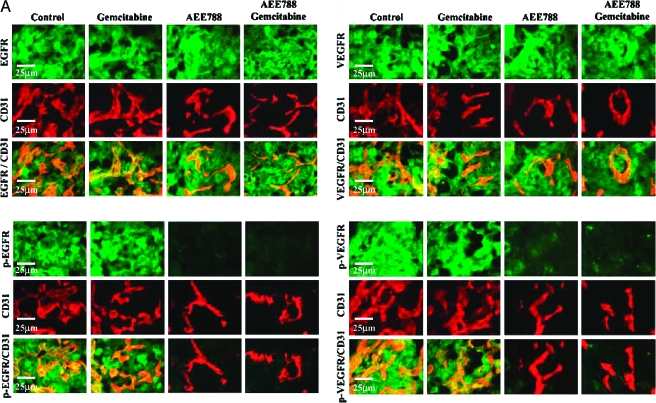
The double immunofluorescence staining showed that tumor-associated endothelial cells in the pancreas of all treatment groups expressed EGFR and VEGFR. Endothelial cells in tumors from the control treatment group or in the gemcitabine-treated group, but not from the AEE788 or AEE788 and gemcitabine-treated tumors, expressed phosphorylated EGFR and phosphorylated VEGFR (Figure 3A).
Figure 3.
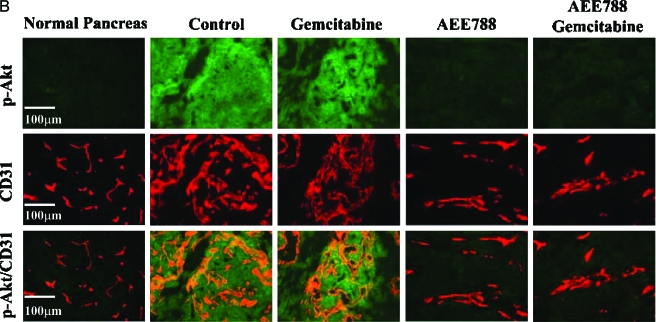
(A) Double immunofluorescence staining for CD31/PECAM-1 and EGFR, p-EGFR, VEGFR, and p-VEGFR in pancreatic tumors. Samples were stained with anti-CD31/PECAM1 antibody (red fluorescence) and anti-EGFR, p-EGFR, VEGFR, or p-VEGFR antibody in green fluorescence as described in Materials and Methods section. Colocalization of CD31 and EGFR, p-EGFR, VEGFR, and p-VEGFR yields a yellow fluorescence. Expression of EGFR and VEGFR by tumor-associated endothelial cells was found in tumors from all treatment groups. Phosphorylated EGFR and VEGFR on endothelial cells were decreased by treatment with AEE788 or AEE788 and gemcitabine. (B) Double immunofluorescence staining for CD31/PECAM-1 and p-Akt in normal mouse pancreas and pancreatic tumor of nude mice treated with control, gemcitabine, AEE788, or AEE788 plus gemcitabine. CD31/PECAM-1 was stained with red fluorescence and p-Akt was stained with green fluorescence. Yellow fluorescence indicates expression of phosphorylated Akt on tumor-associated endothelial cells.
Akt Phosphorylation on Tumor Cells and Tumor-Associated Endothelial Cells in Normal Nude Mouse Pancreas and Pancreatic Tumors
Parenchymal cells and vascular endothelial cells in the normal pancreas expressed minimal levels of phosphorylated Akt. In contrast, elevated phosphorylation of Akt was found in tumor-associated endothelial cells, as well as tumor cells from the control and gemcitabine-treated mice. Treatment with AEE788 alone or AEE788 combined with gemcitabine reduced the expression of p-Akt in both endothelial cells and tumor cells (Figure 3B).
Cell Proliferation, Apoptosis, and MVD
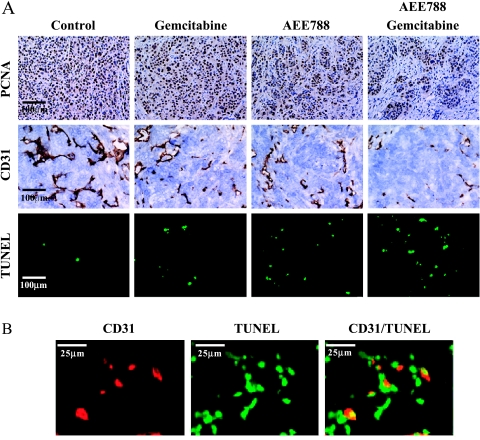
Cell proliferation in the pancreatic tumors was evaluated by staining for PCNA (Figure 4A). Treatment with AEE788 or gemcitabine significantly decreased PCNA staining compared to the control group. Treatment with both AEE788 and gemcitabine produced the largest reduction in PCNA staining (Table 2). ERK1/2 and MAPK signaling level, as measured by Western blot analysis, was reduced in pancreatic cancers from mice treated with AEE788 and AEE788 plus gemcitabine (data not shown).
Figure 4.
(A) Analysis of cell proliferation (PCNA), MVD (endothelial cells), and apoptosis (TUNEL) in L3.6pl cells growing in the pancreas of nude mice. Mice were treated with vehicle (control), AEE788, gemcitabine, or the combination of AEE788 and gemcitabine. (B) Double immunofluorescence staining of CD31/PECAM-1 and TUNEL in pancreatic tumors from mice treated with the combination of AEE788 and gemcitabine. Endothelial cells (CD31+) stained red fluorescence and apoptotic cells (TUNEL+) stained green fluorescence. Colocalization of endothelial cells undergoing apoptosis yields yellow fluorescence.
Table 2.
IHC Analysis of L3.6pl Human Pancreatic Cancer Cells Growing in the Pancreas of Nude Mice.
| Treatment Group | Tumor Cells | Endothelial Cells | |||
| PCNA* | TUNEL* | CD31* | TUNEL+ (%)† | ||
| Mean | Range | ||||
| Control | 488 ± 59 | 1 ± 1 | 55 ± 12 | 0 | 0–0 |
| Gemcitabine | 414 ± 49‡ | 9 ± 3‡ | 31 ± 12‡ | 1 | 0–3 |
| AEE788 | 272 ± 52§ | 15 ± 5§ | 26 ± 5§ | 3 | 0–7 |
| AEE788 + gemcitabine | 212 ± 51§ | 23 ± 6§ | 16 ± 6§ | 9 | 0–16§ |
Mean ± S.D. positive cells per field determined from measurement of 10 random 0.159-mm2 fields at x100 magnification.
Percentage of CD31/TUNEL+ cells in 10 random 0.011-mm2 fields at x400 magnification.
P < .01 vs control.
P < .001 vs control.
The induction of apoptosis was evaluated by the TUNEL method. In tumors from control mice, the mean number of apoptotic tumor cells was minimal. Treatment with gemcitabine or AEE788 increased the number of TUNEL-positive apoptotic cells. The largest number of apoptotic cells was found in tumors from mice treated with AEE788 and gemcitabine (Table 2).
MVD in the tumor from all treatment groups was determined subsequent to IHC staining with antibodies against CD31. Tumors treated with AEE788, gemcitabine, or AEE788 and gemcitabine had a significantly lower MVD than controls. The largest decrease in MVD occurred in tumors from mice treated with the combination of AEE788 and gemcitabine (Table 2).
Immunofluorescence Double Staining for CD31/PECAM-1 and TUNEL
Finally, we examined whether treatment with AEE788 and/or gemcitabine would produce significant apoptosis of endothelial cells. In control tumors, the CD31/TUNEL fluorescent double-labeling technique (colocalization) revealed a median of 0% apoptotic endothelial cells, whereas treatment with AEE788 and gemcitabine produced a median of 9% (range, 0–16%) apoptotic endothelial cells (Table 2). Apoptotic tumor-associated endothelial cells as well as tumor cells in mice treated with both AEE788 and gemcitabine are shown in Figure 4B.
Discussion
The level of expression of EGF, EGFR, VEGF, and VEGFR has been reported to correlate with the progressive growth and metastasis of cancer, its resistance to chemotherapy, and hence the poor survival of patients [5,8,20–22]. The rapid growth and spread of the human L3.6pl pancreatic cancer cells implanted into the pancreas of nude mice also correlated with the expression of EGF, VEGF, their receptors, and the phosphorylated receptors. Parenchymal cells and endothelial cells in a normal pancreas expressed minimal levels of ligands and receptors. In contrast, tumor-associated endothelial cells expressed elevated levels of EGFR and VEGFR, which were phosphorylated, suggesting that ligands released by tumor cells upregulate the expression of their receptors on tumor-associated endothelial cells in a paracrine manner.
EGF and VEGF are also known to be survival factors rendering cells more resistant to apoptosis and chemotherapy by activation of Akt, which regulates antiapoptotic mechanisms by activating NF-κB and inactivating the proapoptotic protein, BAD, and others [10,30–32]. We could find minimal phosphorylation of Akt in vascular endothelial cells or pancreatic parenchyma cells in the normal pancreas. In contrast, we found an intense phosphorylation of Akt on tumor-associated endothelial cells and pancreatic tumor cells in untreated mice or mice treated with gemcitabine. This phosphorylation of Akt was inhibited by treatment with AEE788 alone or AEE788 plus gemcitabine. These data indicate that EGF and VEGF released by tumor cells can phosphorylate Akt on tumor-associated endothelial cells in a paracrine manner and on tumor cells in an autocrine manner.
We found that L3.6pl cells were relatively resistant to gemcitabine treatment (Table 1); however, when combined with AEE788, gemcitabine reduced tumor growth by nearly 95%. The result that combination treatment was most effective on the relative gemcitabine-resistant tumor cells suggests that the primary target of treatment could be the tumor-associated endothelial cells rather than the tumor cells. The apoptosis of tumor-associated endothelial cells leads to hypoxia, resulting in decreased tumor cell proliferation and increased apoptosis of tumor cells.
The abrogation of Akt phosphorylation by AEE788 in tumor-associated endothelial cells and tumor cells would render these cells highly sensitive to chemotherapy because of loss of antiapoptotic mechanism. Endothelial cells in tumors undergo cell proliferation more frequently than those in normal tissues [33] and, therefore, the tumor-associated endothelial cells should be more attractive targets for chemotherapeutic reagents. Indeed, treatment with AEE788 and gemcitabine produced the highest incidence of apoptosis in tumor cells and tumor-associated endothelial cells, resulting in the lowest MVD and proliferating cells compared to either agent alone.
Another reason that combination treatment overcomes a low efficacy of gemcitabine is that combination treatment would improve the drug delivery of the gemcitabine into the tumor cells by inhibiting VEGFR signaling. Increased vascular permeability is a major cause of interstitial hypertension responsible for reduction in the delivery of molecules to tumor cells [34,35]. Indeed, the use of an anti-VEGFR monoclonal antibody has been shown to lower vascular permeability and reduce vascular resistance by normalization of vascular architecture and function [34]. Whether AEE788 can also lower interstitial hypertension and increase uptake of gemcitabine by cancer cells is under current investigation.
Published work from our laboratory demonstrated that either EGFR inhibition or VEGFR phosphorylation, combined with gemcitabine, produced therapy of human pancreatic carcinoma in nude mice [26,36]. The current data using a dual tyrosine kinase inhibitor of EGFR and VEGFR cannot distinguish between these signaling pathways. Using a chemotherapy-resistant human ovarian cancer implanted in nude mice, we found that AEE788 had longer-lasting inhibition of EGFR phosphorylation compared to VEGFR phosphorylation (manuscript submitted for publication), suggesting that inhibition of the EGFR signaling may be the dominant mechanism leading to induction of apoptosis in tumor-associated cells and, hence, tumor cells.
In conclusion, because both EGFR and VEGFR signalings in tumor cells and tumor-associated endothelial cells regulate the progressive growth of pancreatic cancer, the ability to abrogate their activation, and hence their signaling pathways, markedly decreased tumor cell proliferation and induced apoptosis in tumor cells and tumor-associated endothelial cells. The success in using a dual tyrosine kinase inhibitor with the conventional chemotherapeutic gemcitabine holds promise for the therapy of human pancreatic cancer.
Acknowledgements
We thank Novartis Pharma for providing AEE788 and for critical discussions and support. We also thank Rajyalashmi Luthra (Molecular Diagnostic Laboratory, M. D. Anderson Cancer Center) for sequencing the EGFR, Walter Pagel for critical editorial review, and Lola López for expert assistance with the preparation of this manuscript.
Abbreviations
- EGFR
epidermal growth factor receptor
- HBSS
Hanks' balanced salt solution
- VEGFR
vascular endothelial growth factor receptor
- MVD
microvascular density
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
Footnotes
This work was supported, in part, by Cancer Center Support grant CA16672, SPORE in Prostate Cancer grant CA90270, and SPORE in Pancreatic Cancer grant CA10193-06 from the National Cancer Institute, National Institutes of Health, and by a sponsored research agreement from Novartis Pharma (Basel, Switzerland). S. R. Hamilton is the recipient of the Frederick F. Becker Distinguished University Chair in Cancer Research from The University of Texas.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenbert ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Abbruzzese JL. New application of gemcitabine and future directions in the management of pancreatic cancer. Cancer. 2002;95:941–945. doi: 10.1002/cncr.10753. [DOI] [PubMed] [Google Scholar]
- 5.Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP. Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancr Surg. 2002;9:1–11. doi: 10.1007/s005340200000. [DOI] [PubMed] [Google Scholar]
- 6.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 7.Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control. 2000;7:421–427. doi: 10.1177/107327480000700504. [DOI] [PubMed] [Google Scholar]
- 8.Buchler P, Reber HA, Buchler MW, Friess H, Hines OJ. VEGF-RII influences the prognosis of pancreatic cancer. Ann Surg. 2002;236:738–749. doi: 10.1097/00000658-200212000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perugini RA, McDade TP, Vittimberga FJ, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:29–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson KM, Anderson NG. The protein kinase B/Akt signaling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 12.Douziech N, Calvo E, Laine J, Morisset J. Activation of MAP kinases in growth responsive pancreatic cancer cells. Cell Signal. 1999;11:591–602. doi: 10.1016/s0898-6568(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 13.Parikh AA, Liu WB, Fan F, Stoeltzing O, Reinmuth N, Bruns CJ, Bucana DB, Evans DB, Ellis LM. Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer. 2003;98:720–729. doi: 10.1002/cncr.11560. [DOI] [PubMed] [Google Scholar]
- 14.Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- 15.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 16.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 17.Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, Kerbel RS. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 19.Kindler HL, Friberg G, Stadler WM, Singh DA, Locker G, Nattam S, Kozloff M, Kasza K, Vokes EE. Bevacizumab (B) plus gemcitabine (G) in patients (pts) with advanced pancreatic cancer (PC): updated results of a multicenter phase II trial (abstract) Proc ASCO Meet. 2004;22:315. [Google Scholar]
- 20.Schmidt M, Lichtner RB. EGF receptor targeting in therapy-resistant human tumors. Drug Resist Updat. 2002;5:11–18. doi: 10.1016/s1368-7646(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- 22.Ozawa F, Friess H, Tempia-Caliera A, Kleeff J, Buchler MW. Growth factors and their receptors in pancreatic cancer. Teratog Carcinog Mutagen. 2001;21:27–44. doi: 10.1002/1520-6866(2001)21:1<27::aid-tcm4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr-Relat Cancer. 2001;8:3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 24.Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Ciardiello F, Bianco R, Caputo R, Damiano V, Troiani T, Melisi D, DeVita F, DePlacido S, Bianco AR, Tortora G. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to anti-epidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 26.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 27.Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 28.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 29.Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB, Fidler IJ. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2003;9:6534–6544. [PubMed] [Google Scholar]
- 30.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and Akt pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 31.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 32.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:736–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 33.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labeling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor-2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 35.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solorzano CC, Baker CH, Tsan R, Traxler P, Cohen P, Buchdunger E, Killion JJ, Fidler IJ. Optimization for blockade of the epidermal growth factor receptor signaling for therapy of human pancreatic carcinoma. Clin Cancer Res. 2001;8:2563–2572. [PubMed] [Google Scholar]