Abstract
The protein kinase, PAK1, is overexpressed in human breast cancer and may contribute to malignancy through induction of proliferation and invasiveness. In this study, we examined the role of PAK1 in the survival of detached MCF10A breast epithelial cells to test whether it may also regulate the early stages of neoplasia. MCF10A cells undergo anoikis, as measured by the cleavage of caspase 3 and poly(ADP-ribose) polymerase (PARP), after more than 8 hours of detachment. Endogenous Akt, PAK1, and BAD are phosphorylated in attached MCF10A cells, but these phosphorylation events are all lost during the first 8 hours of detachment. Expression of constitutively active PAK1 or Akt suppresses the cleavage of caspase 3 and PARP in detached MCF10A cells. Co-overexpression of active PAK1 with dominant-negative Akt, or of active Akt with dominant-negative PAK1, still suppresses anoikis. Thus, Akt and PAK1 enhance survival through pathways that are at least partially independent. PAK1-dependent regulation of anoikis is likely to occur early in the apoptotic cascade as expression of dominant-negative PAK1 increased the cleavage of the upstream caspase 9, while constitutively active PAK1 inhibited caspase 9 activation. These results support a role for activated PAK1 in the suppression of anoikis in MCF10A epithelial cells.
Keywords: Breast carcinoma, apoptosis, cell survival, caspase activation, protein kinases
Introduction
Epithelial cells undergo apoptosis when they lose contact with the extracellular matrix (ECM), or bind to an inappropriate integrin [1]. This phenomenon has been called anoikis, and it prevents shed epithelial cells from colonizing elsewhere and thus protects against neoplasia [2]. Anoikis is also an important mechanism in the initial cavitation step of embryonic development [3] and in mammary gland involution [4], and has been studied extensively in angiogenesis research [5]. Failure of anoikis possibly contributes to the malignancy of mammary glands and other carcinomas [6–8].
The p21-activated kinase (PAK) family is homologous to the yeast sterile 20 (Ste 20), and regulates a wide variety of cellular responses, including cell morphology, proliferation, and survival. PAKs were first identified in screens for binding targets of Rac and Cdc42 [9]. Multiple pathways lead to activation of PAK1 [10], some of which [e.g., Gβγ subunits that stimulate PAK1 through activation of phosphatidylinositol 3-kinase (PI3-kinase) and Akt] are independent of Rac1/Cdc42 [11]. Activation of PAK1 by diverse signals leads to autophosphorylation at several sites, including threonine-423 (T423) within the activation loop of the kinase. PAK1 phosphorylation at T423 has been linked to its activation, as substitution of the acidic residue glutamic acid (E) at this site yields a constitutively active T423E PAK1 enzyme [12].
PAKs are involved in the regulation of the apoptotic death pathway. In Jurkat cells, for example, expression of dominant-negative PAK1 inhibited fragmentation into apoptotic bodies, and PAK function is also required for the stimulation of JNK by Fas [13]. However, in HeLa and NIH3T3 cells, overexpression of wild-type or constitutively active PAK4 protects these cells from apoptosis in response to serum withdrawal, UV irradiation, and TNFα treatment. Expression of PAK4 inhibits the activation of caspase 3-like enzymes, and specifically promotes the phosphorylation of BAD on serine-112 (Ser-112) [14]. PAK1 is activated by IL-3 (a cytokine) in FL5.12 lymphoid cells, and active PAK1 protects these cells from apoptosis by phosphorylating BAD [15]. Inhibition of PAK1 has also been reported during detachment-induced death of NIH-3T3 fibroblasts [16].
PAK1 has been strongly implicated in breast cancer. It is overexpressed in human breast cancer [17,18], probably, at least in some cases, due to gene amplification [19]. PAK1 has been shown to mediate cellular effects of polypeptide growth factors on the motility and invasiveness of human breast cancer cells, and to promote their anchorage-independent growth [20,21]. In murine models, it was shown that inhibition of PAK1 kinase activity by a dominant-negative fragment or by short-interference RNA drastically reduced transactivation functions of estrogen receptor-α. Mammary glands from mice expressing constitutively active T423E PAK1 (PAK-TE) developed widespread hyperplasia during lactation [22]. Additional work performed by this group revealed that estrogen rapidly activated PAK1 in a PI3-kinase-independent manner. Furthermore, estrogen induced the phosphorylation and perinuclear localization of the cell survival forkhead transcription factor, FKHR, in a PAK1-dependent process. PAK1 directly interacts with FKHR and phosphorylates it [23]. A further connection from PAK1 to mammary hyperplasia is that PAK1 activity stimulates cyclin D1 expression [17].
Detachment-induced apoptosis is suppressed in epithelial cells transformed by ras and src oncogenes [1]. Active forms of Ras protein are capable of protecting cells from anoikis by stimulating PI3-kinase through direct interaction with the catalytic p110 subunit, leading to the activation of Akt [24]. Ras transformation involves the synergistic effects of the Ras/Raf/MAPK pathway and the PI3-kinase/Akt pathway [25]. In studies of Ras transformation of Rat-1 fibroblasts, it has been shown that PAK is necessary for the cooperative transformation of Rat-1 fibroblasts by Ras, Rac, and Rho [26–29]. Akt may be a key intermediate between Ras and PAK1 in this pathway. In human breast cancer, suppression of anoikis by activated Ras has recently been reported to be independent of both ERK MAP kinases and PI3-kinase/Akt [30].
In this study, we were interested in whether PAK1 plays a role in cell survival in MCF10A human breast epithelial cells. MCF10A cells are derived from MCF10M human epithelial cells, which were obtained from a woman with fibrocystic disease [31]. The first spontaneous immortalization of the MCF10M cells resulted in MCF10A and MCF10F cells, which are routinely used as normal immortalized breast epithelium controls for studies of human breast cancer cells lines [32]. We are able to show that, within 24 hours of growth in suspension, MCF10A cells begin undergoing apoptosis as evidenced by cleavage of caspase 3 and poly(ADP-ribose) polymerase (PARP). Overexpression of active PAK1 or active Akt blocks cleavage of caspase 3 and PARP, revealing a protective role for these kinases in the prevention of anoikis. These results indicate that inappropriate activation of PAK1 could play a role in aberrant cell survival in human breast epithelial cells.
Materials and Methods
Plasmids and Antibodies
Antibodies to the C-terminus of PAK1 (c19) and to caspase 3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to phosphoPAK1 (Thr-423)/PAK2 (Thr-402), phosphoAkt (Ser-423), total Akt, total BAD, phosphoBAD (Ser-112 and Ser-136), and phospho-p70 S6 kinase (Thr-421 and Ser-424) were purchased from Cell Signalling Technology (Beverly, MA). The caspase 9 antibody was purchased from Stressgen (Victoria, BC, Canada). PARP antibody was purchased from Biomol Research Laboratories (Plymouth Meeting, PA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GADPDH) monoclonal antibody was purchased from Calbiochem (San Diego, CA). Lipofectamine 2000 Plus was purchased from Invitrogen (Carlsbad, CA). Plasmids used in this study include pRK7 and pRK7mycPAK1 [10]. Expression vectors pRK7mycPAK-299R and pRK7mycPAK-TE were prepared from pCMV6mycPAK-299R and pCMV6mycPAK-TE, respectively (kind gifts from Jonathan Chernoff), by subcloning BamHI/EcoRI fragments into pRK7. Dominant-negative Akt, pCMV6.HA.Akt-K/M, and active Akt, pCMV6.myr-Akt.HA, plasmids were kind gifts from Alex Toker.
Cell Culture and Transfection Assays
Immortalized nonmalignant human breast epithelial MCF10A cells were cultured as described previously [33]. Briefly, MCF10A cells were incubated in 100-mm dishes in DMEM/F-12 medium supplemented with 5% horse serum, 0.5 µg/ml hydrocortisone, 10 µg/ml insulin, 20 ng/ml epidermal growth factor, 0.1 µg/ml cholera enterotoxin, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine. MCF10A cells were transfected with Lipofectamine 2000 Plus reagent with 20 µg of pRK7myc-PAK1 plasmid, 20 µg of pRK7mycPAK-299R, 20 µg of pRK7mycPAK-TE, 10 µg of pCMVHA.Akt-K/M, 10 µg of pCMVHA.Akt, 10 µg of pCMVHA.myr-Akt, or with 20 µg of pRK7 plasmid alone (as a negative control). Control transfections using a pRK7 plasmid with an enhanced green fluorescent protein insert demonstrated that approximately 20% of the cells was strongly fluorescent and a similar additional proportion exhibited much weaker fluorescence (data not shown).
Anoikis Assay
Cells were trypsinized 48 hours posttransfection, transferred to 15-ml conical tubes, washed, collected by centrifugation for 5 minutes at 500g, and counted. A total of 1.5 x 106 cells were transferred to either 100-mm culture dishes or 100-mm polyhema-coated Petri dishes, and cultured as previously described [33]. At the indicated times, the suspended cells were washed twice with phosphate-buffered saline (PBS) and then lysed with 200 µl of boiling 2x Laemmli buffer. The medium from the attached cells was reserved to a 15-ml conical tube. The attached cells were harvested by trypsinization, combined with the appropriate reserved medium, and centrifuged. The cells were washed twice with PBS and then lysed with 200 µl of boiling 2x Laemlli buffer.
Agonist Treatment and Detection of Active Endogenous PAK1
MCF10A cells were grown to confluency on 100-mm culture dishes and treated as previously described [10,11].
Immunoblot Analysis
Cells lysates in 2x Laemlli buffer were boiled for 10 minutes and then subjected to SDS-PAGE. After electrophoresis, the proteins were transferred to nitrocellulose membranes. Western blot analysis with enhanced chemiluminescent detection was performed as previously described [10]. All results shown are representative of at least three independent experiments.
Results
Time Course of PARP Cleavage in MCF10A Cells Grown in Suspension
To establish a system in which the induction of anoikis in MCF10A cells could be assayed, we measured the cleavage of PARP with time following suspension. PARP is a substrate of caspase 3 and its cleavage occurs in the late stage of apoptosis. PARP cleavage in MCF10A cells has been reported in cells grown for 24 hours in suspension [33]. We therefore tested cells that were grown in suspension or attached in enriched media from 0 to 48 hours. In the cells that were attached, there was minimal PARP cleavage detected. However, in cells grown in suspension for 8 to 48 hours, there was detectable PARP cleavage (Figure 1). To confirm the fraction of cells that undergo anoikis following suspension, we monitored general cellular morphology by phase microscopy and nuclear morphology by 4,6-diamidino-2-phenylindole (DAPI) fluorescent microscopy. Within 48 hours of suspension, the majority of the cells exhibited a shrunken and blebbed morphology with condensed and fragmented nuclei (data not shown).
Figure 1.
Time course of PARP cleavage in MCF10A cells grown in suspension. Cells were grown either attached on regular culture dishes or in suspension on polyhema-coated Petri dishes for times ranging from 0 to 48 hours. PARP cleavage was detected by Western blot analysis of cell lysates for the appearance of the 85-kDa fragment.
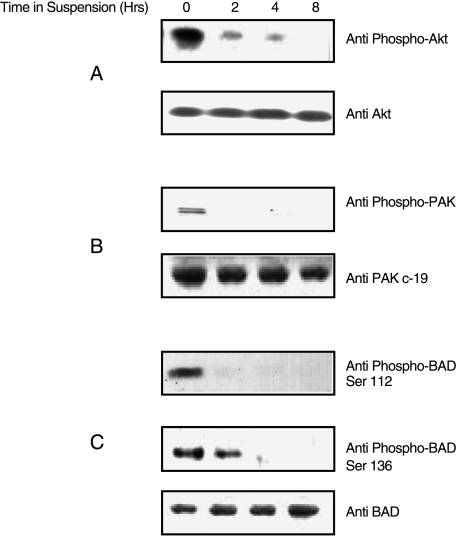
Endogenous PAK1, Akt, and BAD Phosphorylation Decreases in MCF10A Cells Grown in Suspension
Our hypothesis is that anoikis is regulated by the activity of critical serine-threonine kinases such as PAK1 and Akt. We therefore determined whether the activation/phosphorylation status of endogenous PAK1 and Akt differed in suspended versus attached MCF10A breast epithelial cells during the time period when anoikis is induced. The proapoptotic factor, BAD, is known to be phosphorylated by PAK1 and Akt [15]; thus, we also assayed BAD phosphorylation levels under the same conditions. PAK1, Akt, and BAD were all phosphorylated in MCF10A cells that were attached and grown in media-containing serum (Figure 2). After 2 hours in suspension, no PAK1 Thr-423 or BAD Ser-112 phosphorylation was detected, and Akt phosphorylation at Ser-423 and BAD phosphorylation at Ser-136 was reduced. After 8 hours in suspension, neither Akt Ser-423 nor BAD Ser-136 phosphorylation was detected (Figure 2). These results are therefore consistent with a system in which the unphosphorylated forms of PAK1, Akt, and BAD may contribute to the induction of anoikis in MCF10A cells.
Figure 2.
Decrease in endogenous PAK1, Akt, and BAD phosphorylation in MCF10A cells grown in suspension. MCF10A cells were grown either attached (t = 0) or suspended for 2, 4, or 8 hours. (A) Western blots of whole cell lysates were probed with anti-phospho Akt (Ser-423) to detect active Akt, and then stripped and reprobed with anti-Akt to detect total Akt. (B) Western blots of whole cells lysates were probed with anti-phospho PAK1 (Thr-423) to detect active PAK1 (the doublet observed may be due to the fact that the antibody will also react with any endogenous PAK2 that may be present and phosphorylated on residue Thr-402), and then stripped and reprobed with anti-Pak c-19 to detect total PAK1. (C) Western blots of whole cell lysates were probed with anti-phospho-BAD (Ser-136) to detect phosphorylated BAD, then stripped and reprobed with anti-phospho-BAD (Ser-112) to detect phosphorylated BAD, and then stripped and reprobed with anti-BAD to detect total BAD.
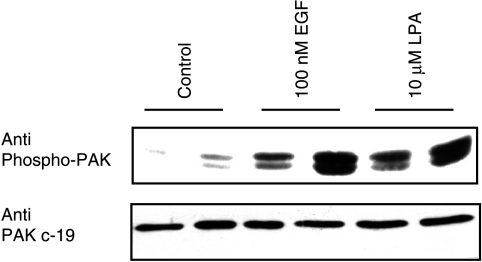
Endogenous PAK1 Is Activated in MCF10A Cells Treated with EGF or LPA
We have previously shown that PAK1 can be activated by several agonists, such as LPA and EGF, in NIH3T3 cells [10]. Because growth factor regulation of PAK1 activity may be important in breast epithelial cells, we determined if endogenous PAK1 would be activated following treatment of MCF10A cells with LPA or EGF. PAK1 activation, as assayed by its phosphorylation at Thr-423, was increased in MCF10A cells treated with LPA or EGF (Figure 3).
Figure 3.
Endogenous PAK1 is activated by EGF and LPA in MCF10A cells. MCF10A cells were treated with 100 nM EGF or 10 µM LPA for 5 minutes and lysates were prepared. The results shown are for two independent isolates of each condition that were analyzed on the same gel. After detection of active phosphorylated PAK1 (top panel), the blots were stripped and reprobed for total PAK1 (bottom panel).
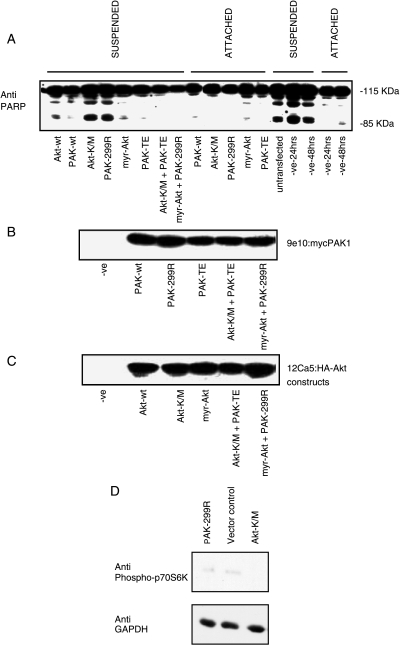
Inhibition of PARP Cleavage Occurs in Cells Overexpressing Active PAK1 or Active Akt
Our hypothesis is that PAK1 plays a key role in cell survival mechanisms in MCF10A breast epithelial cells, and that its action should therefore be compared to that of Akt, which is known to be important in mammary carcinoma [34]. To test whether PAK1 could be involved in rescuing cells from anoikis, we overexpressed dominant-negative PAK-299R, active PAK-TE, wild-type PAK1, wild-type Akt, dominant-negative Akt-K/M, or active myr-Akt in MCF10A cells. After allowing the cells to grow for 48 hours posttransfection, cells were split and plated on culture dishes or polyhema-coated Petri dishes for 24 hours. In negative control, vector-transfected cells, and cells overexpressing dominant-negative PAK-299R or dominant-negative Akt-K/M, the suspended cells exhibited PARP cleavage (Figure 4). Expression of dominant-negative PAK1 or Akt did not induce PARP cleavage in cells that retained attachment. In cells overexpressing active forms of PAK1 or Akt, this cleavage was inhibited (Figure 4). To a lesser extent, overexpression of wild-type PAK1 or Akt also reduced PARP cleavage. The coexpression of dominant-negative PAK1 with active myr-Akt did not reverse the protective role of active Akt, nor did the coexpression of dominant-negative Akt-K/M with active PAK-TE reverse the protective role of PAK1 in these cells (Figure 4). These results suggest that active PAK1 and active Akt protect MCF10A cells from undergoing anoikis.
Figure 4.
Cleavage of PARP is inhibited by overexpression of active PAK1 or active Akt. MCF10A cells were transfected with plasmids encoding wild-type (wt), active (PAK-TE or myr-Akt), or dominant-negative (PAK-299R or Akt-K/M) forms of PAK1 and Akt as shown. Cells were grown for 48 hours posttransfection, counted, and split into two plates: one regular culture dish and one polyhema-coated Petri dish for a further 24 hours. Cell lysates were then processed for PARP cleavage (A), the presence of the myc tag on the transfected PAK1 constructs (B), and the presence of the HA tag on the transfected Akt constructs (C). Lysates from attached cells were also immunoblotted for phospho-p70S6K as a downstream marker of cellular Akt activity, followed by anti-GAPDH as a loading control (D).
To confirm that the dominant-negative Akt construct was inhibiting Akt activity when transfected into MCF10A cells, despite its failure to induce PARP cleavage in the attached cells and its lack of effect to block protection by active PAK1, we assayed the phosphorylation of p70 S6 kinase as a marker of cellular PI3-kinase activity [35]. Phosphorylation of p70S6K was inhibited by transfection with dominant-negative Akt, but not by dominant-negative PAK1 (Figure 4D).
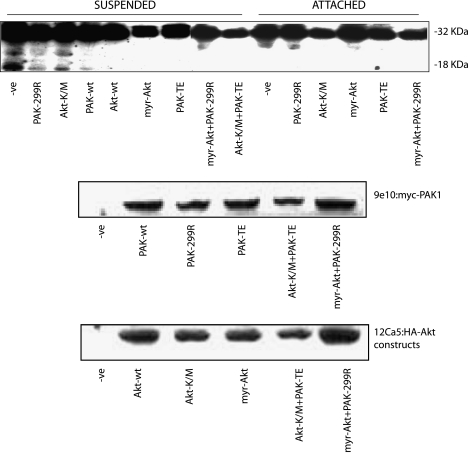
Caspase 3 Cleavage Is Inhibited by Overexpression of Active PAK1 and Active Akt
To further investigate the role of PAK1 in cell survival, we assayed the cleavage of caspase 3 in MCF10A cells grown in suspension. Caspase 3 cleavage was detected in control transfections of cells and in cells overexpressing dominant-negative PAK1 or dominant-negative Akt (Figure 5). Expression of dominant-negative PAK1 or Akt did not induce caspase 3 cleavage in cells that retained attachment. The cleavage of caspase 3 in cells grown in suspension could be inhibited when active PAK1 or Akt was overexpressed (Figure 5). Interestingly, coexpression of active PAK1 with dominant-negative Akt still inhibited caspase 3 cleavage, as did coexpression of active Akt with dominant-negative PAK1 (Figure 5). These results indicate a role for both PAK1 and Akt in cell survival through the suppression of anoikis. Furthermore, these results suggest that this protection could take place by two pathways that are at least partially independent.
Figure 5.
Cleavage of caspase 3 is inhibited by overexpression of active PAK1 and active Akt. MCF10A cells were transfected with plasmids encoding wild-type (wt), active (PAK-TE or myr-Akt), or dominant-negative (PAK-299R or Akt-K/M) forms of PAK1 and Akt as shown. Cells were grown for 48 hours posttransfection, counted, and split into two plates: one regular culture dish and one polyhema-coated Petri dish for a further 24 hours. Cell lysates were then processed for detection of caspase 3 cleavage, which is revealed by the appearance of an 18-kDa fragment (upper panel). Blots were also probed with 9e10 to detect myc-tagged PAK1 constructs (middle panel) and with 12Ca5 to detect HA-Akt constructs (lower panel).
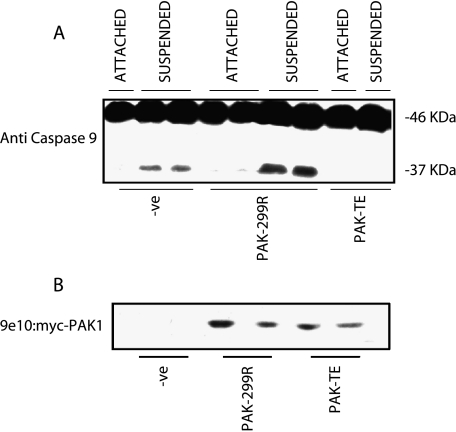
Caspase 9 Cleavage Is Inhibited in MCF10A Cells Overexpressing Active PAK1
To further define the effect of PAK1 on inhibiting the apoptotic cascade, we assayed the cleavage of caspase 9. Caspase 9 is a more upstream element that is capable of cleaving caspase 3 [36,37]. In MCF10A cells grown in suspension, there is cleavage of caspase 9 (Figure 6). Expression of active PAK1 inhibited caspase 9 cleavage, whereas expression of dominant-negative PAK1 in suspended-MCF10A cells increased caspase 9 cleavage (Figure 6). Once again, dominant-negative PAK1 did not induce cleavage of caspase 9 in cells that retained attachment. These results indicate that PAK1 activation can regulate caspase 9 activation in detached MCF10A cells.
Figure 6.
Cleavage of caspase 9 is regulated by the activation of PAK1. MCF10A cells were transfected with wild-type, active, or dominant-negative PAK1 constructs. Cells were grown for 48 hours posttransfection, counted, and split into two plates: one regular culture dish and one polyhema-coated Petri dish for a further 24 hours. Cell lysates were then processed for cleavage of caspase 9, as revealed by appearance of a 37-kDa fragment (A). Blots were also probed with 9e10 to detect myc-tagged PAK1 constructs (B).
Discussion
A balance between cell proliferation, cell differentiation, and cell death in the stem cell population and throughout the mammary gland is critical for normal development. In contrast, perturbations in this balance can contribute to carcinogenesis. Conditions that upregulate cell proliferation or downregulate apoptosis can lead to accumulation of mutations, which contribute to the subsequent development of breast cancer. Atypical ductal hyperplasia is an abnormal ductal epithelial proliferative stage that can become more aggressive and eventually fill the lumen of the duct; thus, it is considered a precursor of development of ductal carcinoma in situ [38–41]. Presumably a failure of anoikis is required for the early stages of mammary epithelial transformation. The breakdown of anoikis is expected to confer a selective advantage on precancerous epithelial cells, affording them an increased survival time in the absence of matrix attachment, and facilitating eventual reattachment and colonization of secondary sites.
Detachment of endothelial and epithelial cells from the ECM induces the cells to undergo a form of apoptosis known as anoikis [1,42]. Many of the signals from the ECM, including those for cell survival, are relayed to the intracellular signaling machinery by integrins—heterotrimeric transmembrane receptors with overlapping specificity toward ECM components [42–44]. Matrix binding to cell surface integrins can trigger survival signaling for cells through the activation of PI3-kinase and FAK [24,45]. Akt, a downstream effector of PI3-kinase, is responsible for transmitting the matrix-induced survival signal [46]. It has also been reported that FAK, in conjunction with fibronectin, is capable of suppressing an apoptotic pathway regulated by the tumor-suppressor p53 in fibroblasts and endothelial cells [47,48].
We have shown that active PAK1 and active Akt are involved in cell survival as they prevent caspase activation and PARP cleavage in MCF10A cells grown in suspension. The protective effect is most pronounced for constitutively activated forms of Akt and PAK1, but overexpression of wild-type Akt and PAK1 also reduced the cleavage of PARP and caspase 3. It may well be relevant to consider the potential role of growth factors in the stimulation of Akt and PAK1 and whether this may also contribute to the suppression of apoptosis by growth factors. Inadequate growth factor signaling, for example, leads to apoptosis, and several lines of evidence suggest that this death may be due to loss of mitochondrial homeostasis [49]. Our results show that PAK1 can be activated in MCF10A cells by both EGF and LPA.
Several antiapoptotic pathways have been defined for Akt. For example, it is known that Akt translocates to the nucleus and is capable of phosphorylating transcription factors such as CREB, forkhead, E2F, and NFκB [50]. Akt can also phosphorylate and thereby inactivate the proapoptotic Bcl-2 member BAD, which leads to protection from apoptosis. Caspase 9 and forkhead are capable of inducing apoptosis, which can be inhibited by Akt-mediated phosphorylation of both proteins. In addition, Akt is linked to the activation of NFκB, which promotes expression of antiapoptotic genes. Notably, Akt is also required for the activation of PAK1 by Ras [29]. This result implies that PAK1 or some PAK activator may be a critical Akt substrate. It has been shown that Akt phosphorylates PAK1 at Ser-21 and that expression of Akt can release PAK1 from Nck [51]. In our study, expression of the dominant-negative PAK-299R in cells overexpressing active Akt did not reverse the inhibition of caspase 3 or PARP cleavage in MCF10A cells (Figures 4 and 5). It is possible that, in addition to PAK1, Akt mediates cells survival through a different downstream effector. It is notable, therefore, that Akt, PAK1, and PAK4 are all capable of phosphorylating BAD [14,15,52], and that the inhibition of anoikis that was conferred by active PAK1 was also not blocked by dominant-negative Akt. Therefore, one conclusion from our studies is that active PAK1 and Akt can both suppress anoikis in MCF10A cells and that they act to suppress apoptosis through mechanisms that are at least partially independent. It is important to note, therefore, that suppression of anoikis in human breast cancers induced by Ras activation has recently been shown to be independent of both Akt and ERK [30]. Our data also show that expression of dominant-negative PAK1 or Akt is insufficient to trigger apoptosis in MCF10A cells if they remain attached.
The readily apparent differences between the cytoskeletal structures of attached versus suspended cells suggest that survival signaling in anoikis is likely to be extensively regulated by the cytoskeleton. Such regulation may be affected by the multiple cytoskeletal changes in transformed cells [53]. PAK1 is a downstream effector of Rac and Cdc42, which are both known to be involved in cytoskeletal rearrangements [54–56]. Active PAK1 itself is believed to alter the cytoskeleton, and localization of PAK1 to the plasma membrane has been shown to induce neurite outgrowth in PC12 cells [57–59]. PAK1 may therefore play a central role in cell survival signaling pathways through modulation of the cytoskeleton. We have shown that active PAK1 expression in MCF10A cells inhibits suspension-induced anoikis. PAK1 may very well be complexed at focal adhesions with other proteins. A fibronectin-dependent matrix survival pathway has been proposed, in which fibronectin supports a prosurvival signaling complex based on focal adhesion kinase and the adaptor protein p130 Cas located at focal adhesions [60]. Paxillin is thought to participate in the binding of focal adhesion kinase to focal adhesion sites [61]. The fibronectin/focal adhesion kinase-associated survival signals activate Ras and are propagated further through Rac/PAK1/MKK4/JNK signaling, with phosphorylated JNK detected at the focal adhesion as well as in the nucleus. This pathway is independent of the serum-dependent pathway that is activated when matrix is withdrawn. This serum-dependent pathway requires FAK and PI3-kinase [60]. In the future, determination of the downstream targets of PAK1 in survival would give insights as to the exact role PAK1 plays in cell survival and anoikis.
Acknowledgements
We thank Gary Bokoch for the original PAK1 plasmid, Jonathan Chernoff for the dominant-negative and active PAK1 plasmids, and Alex Toker for the Akt plasmids. We would also like to thank John J. Reiners, Jr., Hyeong-Reh Choi Kim, and David Kessel for providing information and reagents pertaining to the caspase assays; So Hee Kim and Raymond F. Novak for advice on p70S6K phosphorylation; and Quanwen Li for critical reading of the manuscript.
Abbreviations
- EGF
epidermal growth factor
- ECM
extracellular matrix
- GPCR
G protein-coupled receptor
- HA
hemagglutinin
- LPA
lysophosphatidic acid
- PAK
p21-activated kinase
- PARP
poly(ADP-ribose) polymerase
- PI3-kinase
phosphatidylinositol 3-kinase
Footnotes
This work was supported, in part, by grants from the Wilson Foundation, RO1-CA81150 (to R.R.M.), T32-CA09531 (to R.E.M.), and National Institutes of Health center grants P30 ES06639 and P30 CA22453.
Currently at University of Chicago Ben May Institute for Cancer Research, 5741 South Maryland, Chicago, IL 60637, USA.
References
- 1.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruoslahti E, Reed JC. Anchorage dependence, integrins, apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 3.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50:273–279. doi: 10.1046/j.1440-1827.2000.01047.x. [DOI] [PubMed] [Google Scholar]
- 7.Streuli CH, Gilmore AP. Adhesion-mediated signaling in the regulation of mammary epithelial cell survival. J Mammary Gland Biol Neoplasia. 1999;4:183–191. doi: 10.1023/a:1018729308878. [DOI] [PubMed] [Google Scholar]
- 8.Yawata A, Adachi M, Okuda H, Naishiro Y, Takamura T, Hareyama M, Takayama S, Reed JC, Imai K. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681–2686. doi: 10.1038/sj.onc.1201792. [DOI] [PubMed] [Google Scholar]
- 9.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 10.Menard RE, Mattingly RR. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell Signal. 2003;15:1099–1109. doi: 10.1016/s0898-6568(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 11.Menard RE, Mattingly RR. G beta gamma subunits stimulate p21-activated kinase 1 (PAK1) through activation of PI3-kinase and Akt but act independently of Rac1/Cdc42. FEBS Lett. 2004;556:187–192. doi: 10.1016/s0014-5793(03)01406-6. [DOI] [PubMed] [Google Scholar]
- 12.Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- 13.Rudel T, Zenke FT, Chuang TH, Bokoch GM. p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J Immunol. 1998;160:7–11. [PubMed] [Google Scholar]
- 14.Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem. 2001;276:14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 15.Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist BAD and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Yin X, Zhu X, Yan J, Ji S, Chen C, Cai M, Zhang S, Zong H, Hu Y, et al. The C-terminal kinase domain of the p34cdc2-related PITSLRE protein kinase (p110C) associates with p21-activated kinase 1 and inhibits its activity during anoikis. J Biol Chem. 2003;278:20029–20036. doi: 10.1074/jbc.M300818200. [DOI] [PubMed] [Google Scholar]
- 17.Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 18.Salh B, Marotta A, Wagey R, Sayed M, Pelech S. Dysregulation of phosphatidylinositol 3-kinase and downstream effectors in human breast cancer. Int J Cancer. 2002;98:148–154. doi: 10.1002/ijc.10147. [DOI] [PubMed] [Google Scholar]
- 19.Bekri S, Adelaide J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, Kelley PM, Pebusque MJ, Theillet C, Birnbaum D, et al. Detailed map of a region commonly amplified at 11q13→q14 in human breast carcinoma. Cytogenet Cell Genet. 1997;79:125–131. doi: 10.1159/000134699. [DOI] [PubMed] [Google Scholar]
- 20.Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–12050. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- 21.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 22.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. p21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 24.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Marwaha S, Rutkowski JL, Tennekoon GI, Phillips PC, Field J. A role for Pak protein kinases in Schwann cell transformation. Proc Natl Acad Sci USA. 1998;95:5139–5144. doi: 10.1073/pnas.95.9.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol. 1999;19:1881–1891. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 30.Eckert LB, Repasky GA, Ulku AS, McFall A, Zhou H, Sartor CI, Der CJ. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 31.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 32.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92:1185–1186. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- 34.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SK, Woodcroft KJ, Khodadadeh SS, Novak RF. Insulin signaling regulates gamma-glutamylcysteine ligase catalytic subunit expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther. 2004;311:99–108. doi: 10.1124/jpet.104.070375. [DOI] [PubMed] [Google Scholar]
- 36.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 37.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, Roy SK, Shull JD. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci USA. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvell DM, Strecker TE, Xie B, Buckles LK, Tochacek M, McComb RD, Shull JD. Diet-gene interactions in estrogen-induced mammary carcinogenesis in the ACI rat. J Nutr. 2001;131:3087S–3091S. doi: 10.1093/jn/131.11.3087S. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RG, Bhat KP, Hawthorne ME, Kopelovich L, Mehta RR, Christov K, Kelloff GJ, Steele VE, Pezzuto JM. Induction of atypical ductal hyperplasia in mouse mammary gland organ culture. J Natl Cancer Inst. 2001;93:1103–1106. doi: 10.1093/jnci/93.14.1103. [DOI] [PubMed] [Google Scholar]
- 41.Shull JD, Pennington KL, Reindl TM, Snyder MC, Strecker TE, Spady TJ, Tochacek M, McComb RD. Susceptibility to estrogen-induced mammary cancer segregates as an incompletely dominant phenotype in reciprocal crosses between the ACI and Copenhagen rat strains. Endocrinology. 2001;142:5124–5130. doi: 10.1210/endo.142.12.8530. [DOI] [PubMed] [Google Scholar]
- 42.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 44.Hynes RO. Specificity of cell adhesion in development: the cadherin superfamily. Curr Opin Genet Dev. 1992;2:621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- 45.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999;1:E33–E35. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- 47.Frisch SM. Anoikis. Methods Enzymol. 2000;322:472–479. doi: 10.1016/s0076-6879(00)22043-0. [DOI] [PubMed] [Google Scholar]
- 48.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 49.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 50.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 51.Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol. 2003;23:8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–47. doi: 10.1016/s0959-437x(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 54.Nobes CD, Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- 55.Ridley A. Rho GTPases. Integrating integrin signaling. J Cell Biol. 2000;150:F107–F109. doi: 10.1083/jcb.150.4.f107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley AJ. Signalling by Rho family proteins. Biochem Soc Trans. 1997;25:1005–1010. doi: 10.1042/bst0251005. [DOI] [PubMed] [Google Scholar]
- 57.Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 60.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tachibana K, Sato T, D'Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1099. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]