Abstract
Both induction of transcription of the ferric citrate transport genes and transport of ferric citrate by the Escherichia coli outer membrane receptor FecA require energy derived from the proton motive force (PMF) of the inner membrane. The energy is transduced to FecA by the inner membrane complex, TonB, ExbB, and ExbD. Region 160 of TonB and the conserved TonB box of other TonB-dependent receptors are implicated as sites of interaction. In the present study, the postulated TonB box (D80A81L82T83V84) of FecA was deleted in frame, with a subsequent loss of both FecA functions. DALTV of FecA could be functionally replaced with the core TonB boxes of FhuA (DTITV) and FepA (DTIVV). Each residue of the TonB box of FecA was sequentially replaced with cysteine residues, and only the D80C replacement showed a loss (reduction) of both FecA functions. A physical interaction between TonB and FecA was demonstrated using both in vivo site-specific disulfide bond cross-linking and nonspecific formaldehyde (FA) cross-linking. Pairwise combinations of FecA (DALTV)/Cys substitutions were cross-linked via disulfide bond formation with TonBQ160C, TonBQ162C, and TonBY163C. Unexpectedly, this cross-linking was not enhanced by substrate (ferric citrate). In contrast, the TonB-FecA interaction was enhanced by ferric citrate in the FA-cross-linking assay. Energy derived from the PMF was not required for the TonB-FecA interaction in either the disulfide- or FA-cross-linking assay. TonB/CysExbB/ExbD(D25N) was still able to cross-link with the FecA (DALTV)/Cys derivatives in a tonB tolQ background, even though ExbD25N renders the TonB/ExbBD complex nonfunctional (V. Braun, S. Gaisser, C. Herrmann, K. Kampfenkel, H. Killmann, and I. Traub, J. Bacteriol. 178:2836-2845, 1996). TonB cross-linked to FecA via FA was not inhibited by either carbonylcyanide-m-chlorophenylhydrazone or 1 mM 2,4-dinitrophenol, which dissipate the electrochemical potential of the cytoplasmic membrane and disrupt both FecA functions. The studies shown here demonstrate the significance of the TonB box for FecA functions and are consistent with the view that it is the structure and not the sequence of the TonB box that is important for activity. Demonstrated here for the first time is the physical interaction of TonB and FecA, which is enhanced by ferric citrate.
Escherichia coli has developed efficient active-transport systems for the uptake of substrates that are either too scarce or too large to simply diffuse through the porins of the outer membrane (OM). The TonB energy transduction system, consisting of TonB/ExbB/ExbD located in the cytoplasmic membrane, transduces energy from the proton motive force (PMF) of the cytoplasmic membrane to allow the TonB-dependent transport of iron siderophores and vitamin B12 (26, 31, 46). Analogs of the TonB/ExbB/ExbD system exist in many gram-negative bacteria, highlighting the significant role of this complex in the bacterial cell (6). Recently a second functional TonB-like complex has been identified in various gram-negative bacteria (13, 41, 44, 50, 64). As there is a limiting amount of available TonB for interactions with transporters, there is competition between the receptors for TonB (27, 40). The receptors are able to signal their occupancy to TonB efficiently, as TonB interacts preferentially with ligand-loaded receptors (39, 40).
The best-characterized TonB-dependent OM receptors are FecA, FhuA, FepA, and BtuB, which are required for the active transport of the iron siderophores ferric citrate, ferrichrome, and enterobactin and of vitamin B12, respectively. The crystal structures of FhuA (16, 34), FepA (10), and, more recently, FecA (15) reveal the basic structure of these transporters to consist of a monomeric β-barrel made of 22 β-strands, derived from the C terminus, and a globular cork, or plug, domain that sits inside the barrel, derived from the N terminus. The structure is stabilized by the presence of many hydrogen bonds that anchor the cork to the barrel shell. A unique feature of FecA is that external loops derived from the barrel close when dinuclear ferric citrate binds to FecA, preventing access to the extracellular environment (15). The closed loops effectively form a “second gate” in FecA, the first being the cork domain in the barrel. FhuA and FepA also possess external loops (16, 34).
The crystal structures of FhuA and FecA were obtained in both the presence and absence of substrate, which revealed that while binding of ligand to the receptor produces small conformational changes at the binding site, these changes are propagated through the cork domain, causing large changes in the conformation of the N-terminal domain exposed to the periplasm (15, 16, 34). This conformational change at the N terminus of ligand-loaded receptor is thought to be the molecular signal of occupancy from the receptor to TonB. Evidence for a direct physical interaction between TonB and TonB-dependent transporters has come from a range of in vivo and in vitro experiments (11, 30, 39, 40, 51).
Located at the N termini of all TonB-dependent OM transporters is the TonB box, a short stretch of amino acids shared by all the transporters and also by TonB-dependent B-group colicins (6, 26, 46). Mutations in the TonB box of some OM transporters led to the loss of TonB-dependent activity, and these mutations could be partially suppressed by mutations in region 160 of TonB (4, 26, 49), highlighting the relevance of the TonB box in the interaction with TonB. In addition, a physical interaction between the TonB box of BtuB and TonB region 160 was demonstrated by the specific disulfide cross-linking of the two regions (11).
While E. coli has seven TonB-dependent iron uptake systems, only the ferric citrate transport system is induced by its substrate. Transcription of the ferric citrate transport genes is induced by a novel mechanism, whereby binding of substrate to FecA on the outside of the cell triggers a molecular signal that is transmitted into the cell, which results in the induction of transcription of the ferric citrate transport genes fecABCDE (2, 3, 22, 65). This signal, a series of protein-protein interactions, is transmitted from FecA to the regulatory transmembrane protein FecR across the cytoplasmic membrane and activates the FecI sigma factor in the cytoplasm, whereby activated FecI complexes with the RNA polymerase core enzyme to transcribe the transport genes (14, 42, 43, 53, 59, 62). The ferric citrate transport complex consists of the ABC-like inner membrane transport component, FecBCDE, and the OM transport component, FecA (47, 52). FecA has a bifunctional role in this system, being required for both the transport of iron and the induction of transcription of the transport genes. Both functions require energy provided by the TonB/ExbB/ExbD complex (28).
FecA interacts with FecR through a 79-residue N-terminal region that is not contained in noninducing transporters. The extra N terminus places the predicted TonB box in the unusual position of residues 80 to 84, whereas in other transporters and B-group colicins it is located close to the N-terminal end. Since there is no evidence that demonstrates the involvement of the predicted TonB box of FecA (DALTV) with TonB interactions, this study was undertaken. We demonstrate the importance of the TonB box (DALTV) region of FecA in TonB-dependent activities by constructing a TonB box deletion mutant of FecA. In addition, the TonB box of FecA could be functionally replaced with the five-amino-acid core TonB boxes of both FhuA and FepA. It was also considered important to establish that a direct interaction between the TonB box of transporters and region 160 of TonB occurs, confirming the findings of Cadieux and Kadner (11), to ensure that the loss of function in TonB box mutations is not caused by an alteration of structure elsewhere in the protein. We demonstrate for the first time a physical interaction between the TonB box of FecA and the region at and around residue 160 of TonB, using in vivo site-specific disulfide bond formation between cysteine derivatives of FecA and TonB. Unexpectedly, this specific interaction was not enhanced by the presence of substrate (ferric citrate), indicating that perhaps FecA has specific requirements for an enhanced interaction to occur at this site. A physical interaction between TonB and FecA was also demonstrated using nonspecific in vivo formaldehyde (FA) cross-linking. In contrast to the findings of the site-specific interaction, FA cross-linking between TonB and FecA was enhanced by the presence of ferric citrate. Both the in vivo site-specific and nonspecific interactions between TonB and FecA were shown not to require TonB in an energized state.
MATERIALS AND METHODS
Bacteria, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Tables 1 and 2. All of the strains are E. coli K-12 derivatives, except for the E. coli B derivative BL21(DE3). Cells were grown in Luria-Bertani or nutrient broth (NB) minimal (M9) medium as previously described (38, 48). For induction assays, strains were grown in NB with low salt (2 g of NaCl/liter). Growth on ferric citrate as the sole iron source was tested on Fec agar plates containing NB medium, 1.5% nutrient agar, 0.2 mM 2,2′-dipyridyl, and various concentrations of citrate. Antibiotics were used at the following concentrations: ampicillin, 50 μg per ml; chloramphenicol, 40 μg per ml; and kanamycin, 25 μg per ml.
TABLE 1.
Strains of E. coli and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strain | ||
| AB2847 | aroB thi malT tsx | 21 |
| DH5α | F−hsdR17(rK−mK+) supE44 thi-1 gyrA relA1 recA1 endA1 Δ(argF lacZYA) U169 (φ80 ΔlacZ M15) λ− | Stratagene |
| AA93 | F−araD139 ΔlacU169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR thi aroB Δfec | 43 |
| WA1031 | AB2847 fepA fecA | 61 |
| CO1031 | WA1031 tonB | 22 |
| H2300 | AB2847 Δ(tonB trp) | K. Hankte |
| BL21 | F−hsd gal | 54 |
| HE2350 | GM1 tolQ exb::Tn10 [exbD] tonB | 5 |
| IS1031 | WA1031 lac::Tn10 | 22 |
| AS418 | AB2847 tonB fecB::lacZ | A. Sauter |
| Plasmids | ||
| pT7-7 | ori ColE1, phage T7 gene promoter; Ampr | 55 |
| pIS711 | pT7-7 fecA | 28 |
| pMON33 | pT7-7 fecA(D80C) | This study |
| pMON2 | pT7-7 fecA(A81C) | This study |
| pMON34 | pT7-7 fecA(L82C) | This study |
| pMON4 | pT7-7 fecA(T83C) | This study |
| pMON5 | pT7-7 fecA(V84C) | This study |
| pMON44 | pT7-7 fecAΔTonbox | This study |
| pMON26 | pT7-7 fecAFhubox | This study |
| pMON32 | pT7-7 fecAFepbox | This study |
| pMON37 | pT7-7 fecBCDE | This study |
| pMON28 | pT7-7 fecA fecBCDE | This study |
| pMON55 | pT7-7 fecAΔTonbox fecBCDE | This study |
| pHG575/6 | pSC101 derivative, Cmr | 56 |
| pMMO203 | pHSG576 fecIR | 53 |
| pLCIRA | pHSG576 fecIR fecA | This study |
| pMON52 | pHSG576 fecIR fecA(D80C) | This study |
| pMON7 | pHSG576 fecIR fecA(A81C) | This study |
| pMON8 | pHSG576 fecIR fecA(L82C) | This study |
| pMON9 | pHSG576 fecIR fecA(T83C) | This study |
| pMON10 | pHSG576 fecIR fecA(V84C) | This study |
| pMON45 | pHSG575 fecIR fecAΔTonbox | This study |
| pMON27 | pHSG575 fecI fecA with FhuA TonB box (DTITV) | This study |
| pMON29 | pHSG575 fecIR fecA with FepA TonB box (DTITVV) | This study |
| pBAD18 | ori pBR322 araC pBAD promoter; Ampr | 19 |
| pMON47 | pBAD18 fecA(D80C) | This study |
| pMON48 | pBAD18 fecA(A81C) | This study |
| pMON49 | pBAD18 fecA(L82C) | This study |
| pMON50 | pBAD18fecA(T83C) | This study |
| pMON51 | pBAD18fecA(V84C) | This study |
| pSU19 | ori P15A Cmr | 36 |
| pTonBC18A | pSU19 tonBC18A exbB exbD | 11 |
| pTonBQ160C | pSU19 tonBQ160C exbB exbD | 11 |
| pTonBQ162C | pSU19 tonBQ162C exbB exbD | 11 |
| pTonBY163C | pSU19 tonBY163C exbB exbD | 11 |
| pMON22 | pHSG575 tonBC18A | This study |
| pMON23 | pHSG575 tonBQ160C | This study |
| pMON24 | pHSG575 tonBQ162C | This study |
| pMON25 | pHSG575 tonBY163C | This study |
| pMON56 | pSU19 tonBQ160C exbB exbD(D25N) | This study |
| pMON57 | pSU19 tonBQ162C exbB exbD(D25N) | This study |
| pMON58 | pSU19 tonBY163C exbB exbD(D25N) | This study |
| pTonBC18ANP | pTonBC18A; without tonB native promoter | This study |
| pCH10 | pWSK29 exbB exbD(D25N) | 8 |
| pGFPA′ | GFP fused to fecA promoter | S. Enz |
TABLE 2.
Deoxyoligonucleotides used in this study
| Deoxyoligonucleotide | Sequencea |
|---|---|
| MOAA4 | 5′-CCGTTAGAATTCAGTCTATTA-3′ |
| MOfecBpu | 5′-AGAGGTTGGGCTGAGC-3′ |
| MO6 | 5′-CGCCCGCACCAAAAGAAGTCGGCGACTGGCTGGGT-3′ |
| MO7 | 5′-CAGCCAGTCGCCGACTTCTTTTGGTGCGGGCG-3′ |
| MOBox1 | 5′-GAAGATACCATCACCGTGGTCG-3′ |
| MOBox2 | 5′-ACCACGGTGATGGTATCTTCTTTTG-3′ |
| MOBoxFep1 | 5′-GATACCATCGTTGGTGTCGGCG-3′ |
| MOBoxFep2 | 5′-GCCGACCACAACGATGGTATCTT-3′ |
| MODC1 | 5′-AAAGAATGCGCCCTGACCGTGGTCG-3′ |
| MODC2 | 5′-GGTCAGGGCGCATTCTTTTGGTGCGGGCGC-3′ |
| MOAC1 | 5′-AAAGAAGATTGCCTGACCGTGGTCGGC-3′ |
| MOAC2 | 5′-CGGTCAGGCAATCTTCTTTTGGTGCGGG-3′ |
| MOLC1 | 5′-AGATGCCTGCACCGTGGTCGGCGACT-3′ |
| MOLC2 | 5′-CCACGGTGCAGGCATCTTCTTTTGGTG-3′ |
| MOTC1 | 5′-GCCCTGTGCGTGGTCGGCGACTGG-3′ |
| MOTC2 | 5′-CGACCACGCACAGGGCATCTTCTTTTGGT-3′ |
| MOVC1 | 5′-CTGACCTGCGTCGGCGACTGGCTGG-3′ |
| MOVC2 | 5′-CGCCGACGCAGGTCAGGGCATCTTCTTTT-3′ |
| FecANoPro1 | 5′-ACGTGAATTCCAACAAAAATGATGATGGGGA-3′ |
| FecANoPro3 | 5′-AGACTTCTAGAAGGCCTGCAAAAAGAAAACG-3′ |
| MOfecBam | 5′-CGATGGATCCTGCCGGGCTTTTAGCTGGA-3′ |
| MOfecHind2 | 5′-GATCAAGCTTTTTGGTTCTTACGGCCTGTGC-3′ |
| tonB1 | 5′-TCGTACTGCAGCGAGACCTGGTTTTTCTACTGA-3′ |
| tonB2 | 5′-CACGTGTCGACGGTCGGAGGCTTTTGACTTTC-3′ |
Altered nucleotides are shown in boldface, and restriction enzyme sites are underlined.
Construction of plasmids.
All mutations of the TonB box of FecA were constructed using the PCR overlap extension technique described previously by Horton et al. (23). Each mutant construction required three separate PCRs. The first two PCRs utilized plasmid pIS711 as the DNA template unless otherwise stated. Plasmid pIS711 carries fecA on a 2.4-kb EcoRI/BamHI fragment (28). PCR 1 generated a 500-nucleotide (nt) fragment and utilized a 5′ oligonucleotide, MOAA4, in combination with a 3′ oligonucleotide specific for each mutation. PCR 2 generated a 450-nt fragment and utilized a 5′ oligonucleotide specific for the mutation in combination with the 3′ oligonucleotide MOFecBpu. The oligonucleotides MOAA4 and MOFecBpu incorporate an EcoRI and a Bpu1102I site, respectively. PCR products 1 and 2 were purified and mixed at a 1:1 ratio. This PCR fragment mixture served as the template for the third and final PCR, in which the oligonucleotides MOAA4 and MOFecBpu were used to generate a 950-nt product. The resultant PCR fragment, which incorporates mutations in the TonB box of FecA, was then digested with EcoRI/Bpu1102I and used to replace the equivalent fragment carrying the wild-type TonB box in pIS711. Site-specific mutagenesis was confirmed by sequencing.
The construction of the FecA ΔTonB box mutant required oligonucleotides MOAA4 and MO7 for PCR 1 and oligonucleotides MOfecBpu and MO6 for PCR 2. The FecA ΔTonB box has an in-frame deletion of residues D80A81L82T83V84. The oligonucleotides MO6 and MO7 are complementary and span the region flanking (but not including) the DALTV region of FecA. The conversion of the TonB box of FecA (DALTV) to that of FhuA (DTITV) required a combination of oligonucleotides MOAA4 and MOBox2 for PCR 1 and oligonucleotides MOBox1 and MOfecBpu for PCR 2. The conversion of the TonB box of FecA (DALTV) to that of FepA (DTIVV) required a combination of oligonucleotides MOAA4 and MOBoxFep2 for PCR 1 and oligonucleotides MOBoxFep1 and MOfecBpu for PCR 2. In this instance, the DNA template for reactions 1 and 2 was pIS711fecA (Fhubox).
Residues 80 to 84 incorporating the TonB box (DALTV) of FecA were sequentially replaced with cysteine using the PCR overlap extension described above. Construction of pIS711fecA(DC), pIS711fecA(AC), pIS711fecA(LC), pIS711fecA(TC), and pIS711fecA(VC) utilized oligonucleotide MOAA4 in combination with MODC2, MOAC2, MOLC2, MOTC2, and MOVC2, respectively, for PCR 1. PCR 2 utilized oligonucleotide MOfecBpu in combination with one of the oligonucleotides MODC1, MOAC1, MOLC1, MOTC1, and MOVC.
For the purpose of phenotypic assays, fecA-Cys and tonB-Cys derivatives were cloned into low-copy-number vectors. The 2.6-kb EcoRI/BamHI fragment of pIS711 carrying fecA or fecA mutant derivatives was cloned into the EcoRI/BamHI sites of the low-copy-number vector pMMO203 (pHSG576fecIR) such that fecA lay upstream of fecIR. The 0.988-kb PstI/SalI fragment of pSU19 carrying tonB or the tonB cysteine derivatives was cloned into the PstI/SalI sites of pHSG575 such that tonB lay in the opposite direction to the lacZ promoter and therefore relied on its own promoter for expression (thus closely resembling the chromosomal situation).
To clone fecA and fecA cysteine derivatives into pBAD18, the fecA gene was PCR amplified using the oligonucleotides FecANoPro1 and FecANoPro3, which incorporate EcoRI and XbaI sites, respectively. PCR-amplified fecA was cloned into the EcoRI/XbaI sites of pBAD18. pBAD18 does not have a unique BamHI site in the polylinker, and therefore fecA could not be cloned into pBAD18 on an EcoRI/BamHI fragment. The 1.63-kb Bpu1102I/XbaI fragment derived from the 3′ end of fecA in the pBAD18 constructs was then exchanged with the equivalent 1.6 kb of pIS711. This was done to reduce the amount of fecA derived from PCR amplification that may have generated unintentional errors. The 0.82-kb EcoRI/Bpu1102I fragment derived from the 5′ end of fecA was sequenced for each of the FecA DALTV/Cys derivatives. Unintentional errors introduced via PCR amplification of this region were removed via cloning (details not provided). Expression of fecA from pBAD18 was repressed by the addition of 0.2% glucose and induced by the addition of 0.2% arabinose.
The ferric citrate transport genes fecBCDE were PCR amplified from the chromosome of E. coli strain AB2847, using a combination of oligonucleotides MOfecBAM and MofecHind2, which contain BamHI and HindIII restriction sites, respectively. The resultant 4.7-kb PCR fragment carrying fecBCDE was digested with enzymes BamHI/HindIII and cloned into the BamHI/HindIII sites of the pT7-7 polylinker to generate plasmid pMON37. Derivatives of pMON37 (pMON35 and pMON55) were constructed by cloning the 2.6-kb EcoRI/BamHI fragment including either fecA or fecA mutant derivatives into the EcoRI/BamHI polylinker sites of pMON37 so that fecA lay upstream of fecBCDE.
To examine the effect of energy on TonB-FecA cross-linking, the wild-type exbBD of the pTonB/Cys clones was replaced with exbB exbD(D25N). The pTonB/Cys derivatives were digested with AvaI/EcoRI to remove the 1.32-kb fragment including exbBD, and the remainder of the construct was end filled using Klenow polymerase. A 1.6-kb XhoI/EcoRI fragment including exbB exbD(D25N), isolated from pCH10 and end filled, was ligated to the pTonB/Cys derivatives from which exbBD had been removed.
To clone tonBC18A without its native promoter and Fur binding box (tonBC18ANP), the tonB gene was PCR amplified using oligonucleotides tonB1 and tonB2, which incorporate PstI and SalI restriction sites, respectively. The 0.78-kb PstI/SalI fragment generated from this reaction was used to replace the 0.99-kb PstI/SalI fragment carrying tonBC18A from clone pTonBC18A.
Recombinant DNA techniques.
Isolation of plasmids, use of restriction enzymes, ligation, agarose gel electrophoresis, and transformation were done by standard techniques (48). DNA was sequenced by MWG-Biotech (Ebersberg, Germany), using the dideoxy chain termination method.
PCR techniques.
PCR amplification was performed using the Expand High Fidelity PCR system (Roche, Mannheim, Germany) in accordance with the manufacturer's instructions. DNA was initially denatured by heating it to 94°C for 3 min, followed by 30 cycles of denaturing at 94°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 1 min per kb of DNA.
Immunoblot analysis.
Cultures of bacteria were grown to an A600 of 0.6 (unless otherwise stated) and were adjusted to comparable optical densities prior to gel loading. Whole-cell lysates were prepared by centrifugation of 1 ml of the culture and resuspending it in 100 μl of sodium dodecyl sulfate (SDS) sample buffer. Protein samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (29), and the proteins were transferred to nitrocellulose for Western blot analysis as described elsewhere (14). The filters were probed with anti-TonB polyclonal antisera (diluted 1:20,000), followed by goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase. The production of anti-TonB antisera has been described elsewhere (24). Detection of proteins was done with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium in the detection buffer (100 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2 [pH 9.5]).
Phenotypic assays.
Phenotypic assays were performed in triplicate. Induction assays to measure the abilities of FecA mutants to induce the fec transport genes utilized a plasmid carrying a fecA promoter-gfp fusion (pGFPA′). The assay was performed with freshly transformed E. coli strain WA1031 aroB fecA. Cells were grown in low-salt NB (2 g of NaCl/liter). Green fluorescent protein (GFP; encoded by gfp) was quantified by fluorometry in a Bio-Tek FL500 microplate fluorescence reader (Bio-Tek Instruments Inc., Winooski, Vt.). The specific activity of GFP in bacterial cultures was expressed as a relative fluorescence intensity at 530 nm of cells adjusted to an optical density at 578 nm of 0.5 in phosphate-buffered saline (58). To measure the abilities of TonB mutants to induce the fec transport genes, freshly transformed E. coli AS418 tonB fecB::lacZ grown in NB was used. Induction ability in this case was determined by measuring β-galactosidase activity, according to the methods of Miller (38) and Giacomini (18).
Growth promotion by citrate was achieved by overlaying NB agar plates containing 2,2′-dipyridyl with 3 ml of NB soft agar containing 20 μl of the log-phase bacterial culture to be tested. Paper filter disks were soaked in 1 M or 100, 10, or 1 mM citrate and then placed on the agar plate, and the diameter of growth and growth density around the disk were measured following an overnight incubation. Growth promotion of either plasmid-encoded FecA or TonB was determined in strain WA1031 (AB2847 aroB fecA) or H2300 (AB2847 ΔtonB), respectively.
The sensitivities of cells to TonB- and TolQ-dependent ligands (colicin B, colicin E1, colicin M, φ80, and albomycin) were tested by spotting 4 μl of 10-fold-diluted solutions on tryptone-yeast extract agar plates overlaid with 3 ml of tryptone-yeast extract soft agar containing 108 cells of HE2350 carrying various plasmids.
Transport assays.
The transport ability of plasmid-encoded FecA or TonB was determined in freshly transformed strain IS1031 or H2300, respectively. Bacterial cultures were grown overnight at 37°C in NB medium supplemented with 0.4% glucose and were then suspended in NB- 0.4% glucose- 1 mM citrate and grown for 3 h to an A578 of 0.5. The cells were harvested by centrifugation and suspended in 5 ml of transport medium {NaH2PO4 · 2H2O, 2.07 g liter−1; KH2PO4, 0.69 g liter−1; (NH4)2SO4, 0.66 g liter−1; MgCl2, 0.047 g liter−1; CaCl2, 0.022 g liter−1; glucose, 1 g liter−1; adjusted to pH 6.9 with 5 M NaOH- 5 M KOH (3:1 [vol/vol])} to an A578 of 0.5. The 5 ml of cells were incubated with 50 μl of 10 mM trisodium salt of nitrilotriacetic acid for 5 min at 37°C. After the addition of 50 μl of radioactive iron citrate (10.6 μM 55Fe3+, 96.6 μM FeCl3 in 0.02 M HCl, 966 mM sodium citrate, pH 6.8), 0.8-ml samples were taken after 1, 6, 11, 16, 21, and 26 min of incubation at 37°C. The samples were washed twice on filters with 5 ml of 0.1 M LiCl and dried, and the radioactivity was determined in the liquid scintillation counter. Experiments using pBAD18fecA derivatives were carried out as described above with the following exceptions: overnight cultures were grown in M9 medium (0.1% aroB additions- Casamino Acids- 0.4% glucose) and subcultured into M9 medium (aroB additions- 0.1% Casamino Acids- 0.2% arabinose) to induce fecA production. After the addition of 50 μl of radioactive iron citrate (10.6 μM 55Fe3+, 96.6 μM FeCl3 in 0.02 M HCl, 966 mM sodium citrate, pH 6.8), 0.8-ml samples were taken after 1, 7, 13, 19, 25, and 31 min of incubation at 37°C.
Site-specific in vivo cross-linking assays.
This method is a variation of that described by Cadieux and Kadner (11). Pairwise combinations of E. coli CO1031 (tonB fecA) carrying the pBAD18 FecA (DALTV)/Cys mutants and the TonB-Cys mutants were grown overnight in NB (0.2% glucose plus chloramphenicol and ampicillin). One milliliter of the overnight culture was then subcultured (in duplicate) into 5 ml of NB (0.2% arabinose plus chloramphenicol and ampicillin). The cells were grown for 3 to 4 h at 37°C with shaking. A final concentration of 1 mM sodium citrate was added to one set of the duplicate flasks 15 min prior to harvesting the cells. The cells were centrifuged for 5 min, and the pellet was suspended in 3 ml of 1× phosphate-buffered saline. The cultures were adjusted to an A578 of 1.0. Whole-cell lysates were harvested by centrifugation of 1 ml of the cell suspension and suspending the resultant pellet in 100 μl of SDS nonreducing buffer containing 50 mM iodoacetamide. Samples were boiled for 5 min, and 5-μl aliquots were then subjected to SDS-PAGE followed by Western immunoblotting. The duplicate Western blots were probed with anti-TonB (1/20,000) antisera, kindly provided by P. Howard (24), and anti-FecA, provided by S. Enz.
Nonspecific in vivo cross-linking assays.
Cells were grown overnight in NB (0.2% glucose plus chloramphenicol and ampicillin). One milliliter of the overnight culture was then subcultured into 10 ml of NB (0.2% arabinose plus chloramphenicol and ampicillin). One mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added 1 h postinoculation to induce TonB production from pTonBC18ANP. The cells were grown for a total of 4 h at 37°C with shaking and then washed and resuspended at an A578 of 1.0 in 1 ml of 10 mM sodium phosphate buffer (pH 6.8) and incubated for 15 min in the presence or absence of 1 or 10 mM sodium citrate premixed with 100 μM FeCl3, as specified. One percent FA was added, and the mixture was incubated for a further 15 min, after which the cells were solubilized in 100 μl of 2× SDS sample buffer for 5 min at 60°C and analyzed further by Western immunoblotting. Incubations with 10 or 50 μM carbonylcyanide-m-chlorophenylhydrazone (CCCP) or 1 mM 2,4-dinitrophenol (DNP), added either before or after the citrate iron mix (as indicated in the text), were done at 37°C for 5 min.
RESULTS
Construction of FecA TonB box mutants.
To date, there is no direct evidence for the involvement of the predicted TonB box of FecA (D80A81L82T83V84) with TonB interactions. Therefore, various mutations of the TonB box of FecA were constructed in pIS711 (pT7-7fecA), using overlap PCR extension (described in Materials and Methods), to assess the role of the TonB box of FecA in activity. Initially, an in-frame deletion removing the DALTV region of FecA (ΔTonbox) was constructed. To examine the sequence requirement of the TonB box of FecA, DALTV was replaced with the five-amino-acid “core sequences” of the TonB boxes of FhuA (DTITV) (12) and FepA (DTIVV) (35). To determine whether there is a direct physical interaction between TonB and FecA, cysteine residues were sequentially incorporated into the TonB box of FecA for use in site-specific disulfide bond cross-linking assays (see below).
All pIS711 fecA mutant derivatives were transformed into E. coli strain BL21, which enabled the overexpression of FecA mutant proteins whose expression is controlled by the T7 promoter. BL21 produces chromosomally encoded T7 RNA polymerase, whose expression is inducible upon the addition of IPTG (55). Following IPTG induction, OM preparations from BL21 strains carrying fecA mutant derivatives were isolated and examined to ensure that the mutagenesis did not greatly affect either protein stability or the proper insertion of FecA into the OM. The mutagenized FecA proteins were present in amounts similar to that of the wild-type FecA in the OM fraction (data not shown).
Phenotypic assessment of the FecA TonB box mutants.
For accurate phenotypic assessment of fecA mutants, fecA should be present in the cell at levels comparable to the normal chromosomal situation. Therefore, fecAΔTonbox, fecAFhubox, fecAFepbox, and the fecA (DALTV)/cysteine derivatives were recloned from pT7-7 into the low-copy-number plasmid pMMO203, which also carries the regulatory genes fecIR to ensure that the levels of FecA, FecI, and FecR in the cell are similar to the chromosomally encoded ratios.
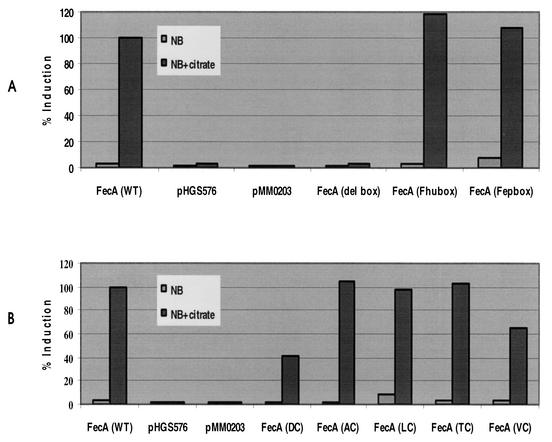
The FecA TonB box mutants were assessed for the ability to induce the expression of fec transport genes in the presence of citrate. Plasmids encoding FecA and FecA mutant derivatives were transformed into E. coli WA1031, an AB2847 aroB derivative with a fecA missense mutation (22) carrying plasmid pGFPA′ (with the fecA promoter fused to the promoterless GFP gene). The level of fluorescence measured with wild-type fecA (pLCIRA) in the presence of citrate was taken as 100% expression. In the presence of citrate, FecA(ΔTonbox) failed to induce expression of the fec transport genes, which supports the prediction that the TonB box of FecA has an important role in the TonB-dependent activities of FecA (Fig. 1A). FecA carrying the core TonB box of FhuA or FepA [FecA(Fhubox) or FecA(Fepbox), respectively] were fully inducible in the presence of citrate (Fig. 1A). The FecA (DALTV)/Cys mutants were all able to induce transcription; however, the induction abilities of FecAD80C and FecAV84C were reduced, with 40 and 63% of the ability of wild-type FecA, respectively (Fig. 1B).
FIG. 1.
Induction of transcription of the fec transport genes by FecA TonB box mutants. The ability of strain WA1031 (AB2847 aroB fecA) carrying FecA TonB box mutants to induce transcription of FecA′-GFP was measured in the presence or absence of 1 mM sodium citrate, as described in Materials and Methods. The induction ability of WA1031 carrying pMMO203fecA (wild type [WT]), pMMO203, and pHG576 was compared to those of WA1031 carrying pMMO203fecA (del box), pMMO203fecA (Fhubox), and pMMO203fecA (Fepbox) (A) and WA1031 carrying pMMO203fecA and D80C (DC), A81C (AC), L82C (LC), T83C (TC), and V84C (VC) substitutions (B).
The FecA TonB box mutant derivatives were assessed for the ability to transport citrate into the cell, using a growth promotion assay. Cells expressing the FecA mutants were used to seed nutrient broth-dipyridyl agar plates devoid of available iron, in which iron is trapped by dipyridyl. The only source of iron was filter disks soaked in various concentrations of sodium citrate, which forms ferric citrate in the medium. Growth around the disks was then measured to determine transport ability. WA1031 was transformed with pMMO203fecA mutant derivatives, and the growth promotion ability was determined.
FecA(ΔTonbox) was unable to promote the growth of WA1031 (Table 3). Growth was not observed at either 1, 10, or 100 mM citrate; however, low-level growth was observed at 1 M citrate, which occurs independently of FecA, as it was also seen with WA1031, WA1031(pHSG576), and WA1031(pMMO203). High levels of ferric citrate are known to diffuse through the porins into the periplasm of E. coli (22). However, as FecA(ΔTonbox) was also unable to induce the production of the transport genes that are needed for iron transport, it is difficult to assess transport ability in this case. Therefore, fecA and fecAΔTonbox were cloned in conjunction with the fecBCDE transport genes into the medium-copy-number plasmid pT7-7. Expression of the transport genes from a medium-copy-number vector should bypass the need for FecIR-mediated induction of expression of the fecBCDE transport genes. The growth promotion abilities of these constructs were determined in strain AA93 Δfec, in which all of the ferric citrate transport genes have been deleted, and are shown in Table 3. AA93(pIS711)(pT7-7 fecA) was unable to transport, which was expected, as the transformant lacks the inner membrane Fec transport machinery. Some growth was observed with AA93(pT7-7 fecBCDE) at high citrate concentrations (100 mM and 1 M), which can be attributed to the FecA-independent diffusion of ferric citrate into the periplasm, described earlier. Ferric citrate that has diffused into the periplasm can then be transported into the cytoplasm via the inner membrane FecBCDE citrate transport system (52). A wild-type transport phenotype was restored with AA93(pT7-7 fecA fecBCDE), in which all the fec transport components are expressed. AA93 expressing FecA(ΔTonbox) combined with FecBCDE from pT7-7 was unable to transport citrate in this system. Zones of growth were observed only at high citrate concentrations due to citrate diffusion into the periplasm, as seen with AA93 (fecBCDE).
TABLE 3.
FecA-, FecA(ΔTonB box)-, FecA(Fhubox)-, and FecA(Fepbox)-mediated growth promotion by ferric citrate as the sole iron source
| Straina | Plasmidb | TonB box sequence in FecA | Genes present | Growth zone (mm)c
|
|||
|---|---|---|---|---|---|---|---|
| 1 mM | 10 mM | 100 mM | 1 M | ||||
| AB2847 | 13 | 23 | 34 | 45 | |||
| WA1031 | 0 | 0 | 0 | (24) | |||
| pHSG576 | 0 | 0 | 0 | (22) | |||
| pMMO203 | 0 | 0 | 0 | (20) | |||
| pLCIRA | FecA (DALTV) | 13 | 24 | 34 | 46 | ||
| pMON45 | FecA (ΔTonB box) | 0 | 0 | 0 | (35) | ||
| pMON27 | FhuA (DTITV) | 13 | 23 | 33 | 49 | ||
| pMON29 | FepA (DTIVV) | 14 | 24 | 34 | 50 | ||
| AB2847 | 11 | 19 | 28 | 40 | |||
| AA93 | 0 | 0 | 0 | 0 | |||
| pT7-7 | 0 | 0 | 0 | 0 | |||
| pIS711 | fecA(WT)d | 0 | 0 | 0 | |||
| pMON37 | fecBCDE | 0 | 0 | 14 | 28 | ||
| pMON28 | fecABCDE | 10 | 19 | 29 | 44 | ||
| pMON44 | fecA(ΔTonB box) | 0 | 0 | 0 | 0 | ||
| pMON55 | fecA(ΔTonB box) fecBCDE | 0 | 0 | 13 | 29 | ||
Growth measurements of plasmids were determined in strain WA1031 (AB2847 fecA) or AA93 (AB2847 Δfec) as indicated.
The plasmids listed also carry the fecIR regulatory genes.
Growth was measured as the diameters of the growth zones around 6-mm-diameter filter disks that had been saturated with 10 μl of the indicated concentrations of sodium citrate. Numbers in parentheses indicate weak growth.
WT, wild type.
Both FecA(FhuBox) and FecA(FepBox) were able to transport iron using this system (Table 3). All the FecA (DALTV)/Cys derivatives were able to promote growth in strain WA1031; however, FecAD80C showed a great reduction in growth promotion ability (Table 4), which is similar to its reduced ability to induce expression of the transport genes. In this assay, FecAV84C was able to transport to wild-type levels, even though it showed lower induction (Fig. 1B). As growth promotion assays are performed overnight, it is probable that during that time sufficient Fec transport machinery to support ferric citrate transport is synthesized.
TABLE 4.
FecA and FecA (DALTV)/Cys mutant-mediated growth promotion by ferric citrate as the sole iron source
| Straina | Plasmidb | FecA TonB box sequence | Growth zone (mm)c
|
|||
|---|---|---|---|---|---|---|
| 1 mM | 10 mM | 100 mM | 1 M | |||
| AB2847 | 14 | 24 | 33 | 44 | ||
| WA1031 | 0 | 0 | 0 | (22) | ||
| pHSG576 | 0 | 0 | 0 | (22) | ||
| pMMO203 | 0 | 0 | 0 | (23) | ||
| pLCIRA | DALTV | 14 | 23 | 33 | 43 | |
| pMON1 | CALTV | 0 | 15 | 24 | 30 | |
| pMON2 | DCLTV | 13 | 22 | 30 | 39 | |
| pMON3 | DACTV | 11 | 22 | 32 | 40 | |
| pMON4 | DALCV | 13 | 23 | 30 | 37 | |
| pMON5 | DALTC | 12 | 22 | 31 | 28 | |
Growth measurements of plasmids were determined in strain WA1031 (AB2847 fecA).
The plasmids listed also carry the fecIR regulatory genes.
Growth was measured as the diameters of the growth zones around 6-mm-diameter filter disks which had been saturated with 10 μl of the indicated concentrations of sodium citrate.
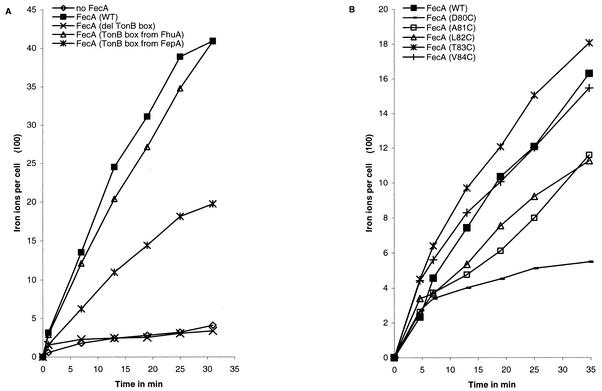
Transport of 55Fe3+-citrate was measured in E. coli IS1031 cells carrying pT7-7-encoded FecA or FecA TonB box mutant derivatives (Fig. 2A). FecA ΔTonB box was unable to transport 55Fe3+-citrate, like cells with no FecA. The rate of transport of 55Fe3+-citrate by FecA(Fhubox) was comparable to that by wild-type FecA, while FecA(Fepbox) showed a reduced transport rate. The ability of the FecA (DALTV)/Cys derivatives to transport 55Fe3+-citrate was determined using pBAD18, on which FecA production was induced by the addition of 0.2% arabinose. The most significant defect in transport ability caused by the TonB box cysteine substitutions was seen with FecAD80C, which had a greatly reduced transport rate (Fig. 2B). FecAD80C also displayed a reduced ability to induce expression of the Fec transport complex; therefore, it was coexpressed with FecBCDE from a high-copy-number plasmid, but it was still unable to transport at a significant level (not shown). The transport rate of FecAV84C is comparable to that of the wild-type, while FecAT83C shows a slightly increased rate. Both FecAA81C and -L82C show slight decreases in the transport rate (Fig. 2B).
FIG. 2.
Transport of 55Fe3+ into E. coli IS1031 aroB fecA by plasmid-encoded FecA and FecA TonB box derivatives. (A) Transport mediated by citrate of plasmid pT7-7-encoded wild-type FecA (FecA WT), FecA with a deletion in the DALTV TonB box (del TonB box), FecA with the TonB box of FepA or FhuA, or no FecA. (B) Transport of plasmid pBAD18-encoded wild-type FecA (WT) or FecA with cysteine substitutions in the TonB box (as indicated). Production of FecA and FecA derivatives was induced by the addition of 0.2% arabinose.
Phenotypic assessment of TonB cysteine mutants in the Fec system.
The site-specific disulfide bond cross-linking assay (see below) required the incorporation of Cys residues in TonB around residue 160 (the implicated site for interaction with FecA). TonB/Cys mutants (pTonBQ160C, pTonBQ162C, and pTonBY163C) and a TonB derivative devoid of its normal Cys residue at position 18 (pTonBC18A) were kindly provided by Robert Kadner. These TonB/Cys mutants and TonB without Cys are encoded on the multicopy plasmid pSU19, and so for the purpose of phenotypic assessment, all were recloned into the low-copy-number plasmid pHSG575. tonB was cloned in the opposite orientation from the lacZ promoter of the vector to ensure that expression of tonB was solely from its own promoter. The abilities of the TonB/Cys derivatives to support the TonB-dependent functional induction and transport abilities of FecA were then assessed in a manner similar to that for the FecA mutants. Cadieux and Kadner (11) showed that all three TonB/Cys mutants supported growth on vitamin B12 in E. coli.
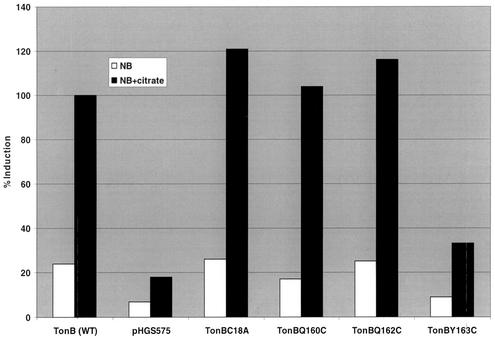
Induction measurements were performed in E. coli strain AS418 tonB fecB::lacZ. As this strain carries chromosomal fecB fused to the lacZ reporter gene, induction of the transport genes was determined by measuring β-galactosidase activity. Induction experiments showed that TonBC18A, TonBQ160C, and TonBQ162C were able to support the induction of the citrate transport system (ranging from 100 to 120% of that with the wild-type) in the presence of citrate (Fig. 3). However, the amino acid substitution at position 163 of TonB was less tolerated, as TonBY163C induced expression in response to the addition of citrate to less then 40% of that induced by wild-type TonB.
FIG. 3.
Induction of transcription of fec transport machinery by TonB/Cys mutants. Strain AS418 (tonB fecB::lacZ) was transformed with pHGS575, pHGS575tonB (wild type [WT]), pHGS575tonB (C18A), pHGS575tonB (Q160C), pHGS575tonB (Q162C), and pHGS575tonB (Y163C), and the ability to induce fecB::lacZ was measured in the presence and absence of 1 mM sodium citrate. Induction ability was determined by measuring β-galactosidase activity by the methods of Miller (38) and Giacomini et al. (18).
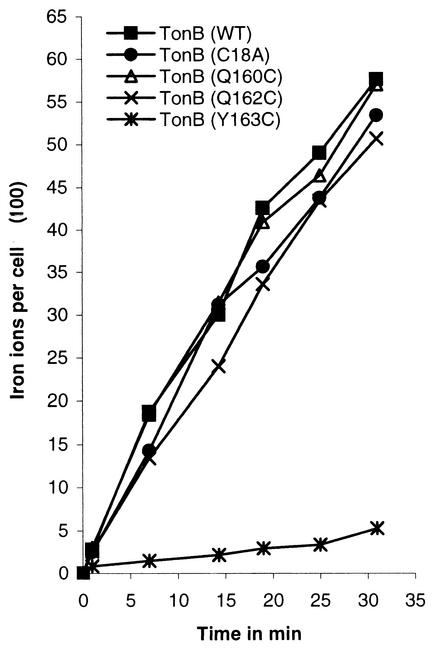
To determine the abilities of the TonB cysteine mutants to support ferric citrate transport, E. coli strain H2300 (AB2847 ΔtonB) was transformed with the pHSG575 tonB mutant derivatives. TonBC18A, TonBQ160C, and TonBQ162C were all able to promote growth with ferric citrate as the sole iron source; however, TonBY163C was unable to promote growth at a significant level (Table 5). Transport of 55Fe3+-citrate was measured in H2300 carrying pSU19-encoded TonB or TonB cysteine mutant derivatives. As shown in Fig. 4, TonBQ160C and TonBQ162C were able to transport 55Fe3+-citrate at rates comparable to that of wild-type TonB; however, TonBY163C was unable to transport. As TonBY163C also had a reduced ability to induce the production of the Fec transport machinery, FecABCDE was expressed from a medium-copy-number vector together with TonBY163C in strain CO93 (Δfec tonB), but it was still unable to support ferric citrate transport (not shown).
TABLE 5.
Abilities of TonB and TonB/Cys to support FecA-mediated growth promotion where ferric citrate is the sole iron source
| Straina | Plasmid | TonB sequence | Growth zone (mm)b
|
|||
|---|---|---|---|---|---|---|
| 1 mM | 10 mM | 100 mM | 1 M | |||
| AB2847 | 13 | 20 | 34 | 45 | ||
| WA1031 | 0 | 0 | (15) | (23) | ||
| H2300 | 0 | 0 | (12) | (25) | ||
| pHSG575 | 0 | 0 | (12) | (23) | ||
| pMON22 | C18A | 14 | 24 | 35 | 50 | |
| pMON23 | Q160C | 15 | 20 | 35 | 52 | |
| pMON24 | Y162C | 15 | 26 | 39 | 53 | |
| pMON25 | Y163C | 0 | 0 | 14 | 25 | |
Growth measurements of plasmids were determined in strain H2300 (AB2847 ΔtonB).
Growth was measured as the diameters of the growth zones around 6-mm-diameter filter disks that had been saturated with 10 μl of the indicated concentrations of sodium citrate. Numbers in parentheses indicate growth zones with low cell densities.
FIG. 4.
Transport of 55Fe3+, mediated by citrate, into E. coli H2300 ΔtonB producing plasmid-encoded wild-type TonB (WT) or cysteine derivatives of TonB (as indicated).
Site-specific in vivo disulfide cross-linking between FecA and TonB.
The loss of TonB-related activities in the TonB box deletion derivative of FecA suggests that the TonB box of FecA is required for TonB interactions. Therefore, we sought to demonstrate a physical interaction between the TonB box of FecA and a region at and around region 160 of TonB via site-specific cross-linking studies. Site-specific cross-linking of two proteins via disulfide bond formation is a technique that determines if proteins are closely associated in the cell. This approach demonstrated an interaction between BtuB and TonB (11).
Pairwise combinations of E. coli CO1031 tonB fecA carrying the FecA (DALTV)/Cys mutants (encoded on pT7-7) and the TonB/Cys mutants (encoded on pUS19) were examined in a cross-linking assay. The constructs carrying the TonB/Cys mutants carried in addition the genes encoding the proteins ExbB and ExbD, as both proteins are required for the activity and stability of TonB (1, 17). CO1031 expressing the various cysteine derivatives of TonB and FecA was grown in NB, and cultures were collected and adjusted to an A578 of 1.0. Whole-cell samples were resuspended in 1× nonreducing SDS buffer containing no β-mercaptoethanol (which reduces disulfide bonds) but containing iodoacetamide (which prevents further formation of disulfide bonds). Samples were then subjected to SDS-PAGE in duplicate, followed by Western immunoblotting with both anti-TonB and anti-FecA antisera.
Figure 5A shows full-length TonB, indicated at 35 kDa (while the calculated mass of TonB is 26 kDa, TonB displays aberrant mobility due to a proline-rich region [33, 57]). In addition, a breakdown product of TonB (TonB′) was detected at ∼25 kDa, as previously reported (11, 30, 32). Curiously, TonB appeared as a doublet in nonreducing buffer but appears as a single band in reducing buffer (not shown). The doublet formation of TonB does not depend on the presence of cysteine residues, as TonBC18A (which contains no cysteines) was also present as a doublet in nonreducing buffer (Fig. 5). Identification of FecA by FecA antiserum is indicated in Fig. 5B.
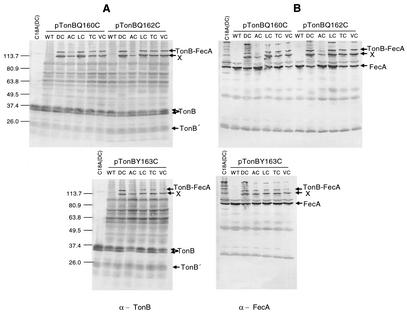
FIG. 5.
Site-specific in vivo disulfide cross-linking between FecA (DALTV)/Cys and TonB/Cys as detected by anti-FecA and anti-TonB antisera. Pairwise combinations of strain CO1031 (tonB fecA) carrying each member of the series of pT7-7 FecA/Cys substitutions (D80C [DC], A81C [AC], L82C [LC], T83C [TC], and V84C [VC]) and TonBQ160C, TonBQ162C, and TonBY163C were grown in duplicate in NB. The cultures were adjusted to an A578 of 1.0, whole-cell lysates were collected, and samples were electrophoresed on duplicate SDS-polyacrylamide gels followed by Western immunoblotting using either anti-TonB (A) or anti-FecA (B) antiserum. As negative controls, pairwise combinations of TonBC18A (containing no cysteine) together with FecA (DC) and TonB/Cys together with FecA (containing no cysteine) (WT) were included. The protein species detected are indicated on the right of each gel, and the molecular mass markers (in kilodaltons) are indicated on the left. An additional cross-linked species (X), which is likely to be a truncated TonB disulfide cross-linked to FecA (TonB′-FecA), was detected by both TonB and FecA antisera.
TonB-FecA heterodimers running at ∼117 kDa (the additive molecular mass for TonB and FecA) were detected with both TonB antiserum (Fig. 5A) and FecA antiserum (Fig. 5B), indicating disulfide bond formation (cross-linking) between the two proteins. FecAA81C displayed a weak interaction with both TonBQ160C and TonBQ162C. An additional cross-linked species which, based on molecular weight, is likely to be a truncated TonB disulfide cross-linked to FecA (TonB′-FecA) was detected by both TonB and FecA antisera, similar to the findings of Cadieux and Kadner, in which TonB′-BtuB species were detected (11). Controls that demonstrate a requirement for the presence of cysteines to produce cross-linked TonB-FecA included a combination of FecAD80C expressed with TonBC18A (and therefore devoid of Cys) that shows no TonB-FecA cross-linked species (Fig. 5, lanes 1). Coexpression of TonB/Cys with wild-type FecA, devoid of Cys, also demonstrated no TonB-FecA cross-linking.
The subsequent aim was to determine whether the presence of ferric citrate enhances the TonB-FecA interaction. Crystal structure data show that binding of dinuclear ferric citrate to FecA produces strong conformational changes in the domain exposed to the periplasm, which might then interact with TonB (15). Initially, cross-linking in the presence and absence of ferric citrate was performed using the pT7-7 FecA (DALTV)/Cys derivatives in strain CO1031. However, in this system, the levels of FecA were substantially increased upon the addition of sodium citrate (not shown), presumably resulting from the induction pathway via the FecIR regulatory proteins. The levels of FecA and TonB must remain constant for a clear interpretation of ferric citrate-enhanced cross-linking. It was therefore decided to reclone fecA (DALTV)/Cys without its native promoter to eliminate any interference with iron regulation via FecIR. Cloning fecA on a high-copy-number plasmid resulted in deletions and point mutations in the fecA gene, suggesting that continuous high-level expression of FecA may be deleterious to the cell. PCR-amplified fecA and fecA cysteine derivatives were therefore cloned into vector pBAD18 (19) behind the pBAD arabinose promoter. Expression from the pBAD promoter is repressed in the presence of glucose and induced by the addition of arabinose (19). The −10, −35, and Fur box regions of the fecA promoter were removed to eliminate any interference by iron regulation via FecIR and Fur; however, the Shine-Dalgarno region remained. The optimal concentration of arabinose required for visible cross-linked TonB-FecA species was found to be 0.2%.
Cross-linking assays were then performed using pairwise combinations of E. coli CO1031 tonB fecA carrying the FecA (DALTV)/Cys mutants (encoded on pBAD18) and the TonB/Cys mutants in the presence and absence of ferric citrate. The assays were performed in duplicate as described earlier; however, 0.2% arabinose was added 1 h postinoculation for the expression of fecA. Fifteen minutes prior to harvesting the cells, sodium citrate was added to one set of the flasks. Cultures were collected and adjusted to an A578 of 1.0, and whole-cell samples were harvested as described earlier, subjected to SDS-PAGE followed by Western immunoblotting, and probed with anti-TonB antisera.
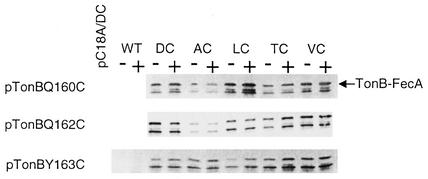
Figure 6 shows pairwise combinations of the FecA (DALTV)/Cys mutants together with TonBQ160C, TonBQ162C, and TonBY163C. Based on previous findings with TonB-dependent transporters, it was expected that ferric citrate would enhance interaction between TonB and FecA. The addition of citrate to the cells did not significantly enhance the interaction between TonB and FecA in this assay. The only increase in cross-linking was observed between TonBQ160C and FecAL82C (Fig. 6). Cross-linking here is slightly more intense than that in Fig. 5, presumably because FecA is made in higher amounts using pBAD18 than using pT7-7.
FIG. 6.
Site-specific in vivo disulfide cross-linking between FecA and TonB is not enhanced by the presence of sodium citrate. Pairwise combinations of strain CO1031 (tonB fecA) carrying each member of the series of pBADFecA/Cys substitutions (D80C [DC], A81C [AC], L82C [LC], T83C [TC], and V84C [VC]) and TonBQ160C, TonBQ162C, and TonBY163C were grown in duplicate in NB (0.2% arabinose). Sodium citrate (1 mM) was added to one set (+) 15 min prior to harvesting. The cultures were adjusted to an A578 of 1.0, and whole-cell lysates were collected and subjected to SDS-PAGE followed by Western immunoblotting using anti-TonB antisera. As negative controls, pairwise combinations of TonBC18A together with FecA (DC) and TonB/Cys together with FecA (wild type [WT]) were included. TonB cross-linked to FecA is indicated on the right.
To avoid the possibility that NB may contain low levels of citrate that could cause TonB to interact prematurely with FecA, the cross-linking assays were also performed in NB media supplemented with dipyridyl (to chelate free iron) and in minimal media supplemented with Casamino Acids. Cross-linking of FecA to TonB was not enhanced in the presence of citrate when cells were grown under these conditions (data not shown). It is possible that higher levels of citrate and/or longer incubation periods with substrate are needed. However, the addition of increased levels of iron for extended periods resulted in decreased levels of TonB in this assay (data not shown). This is most likely attributable to the presence of the native tonB promoter in the pTonB/Cys constructs, which contains the Fur box, to which iron-loaded Fur binds and represses tonB transcription (63). Therefore, all the TonB/Cys derivatives were PCR amplified, removing the native promoter of tonB, and recloned into pSU19exbBD. TonB was cloned downstream of the lacZ promoter so that production of TonB from these constructs relied on the addition of IPTG. Cross-linking assays using these promoterless derivatives of tonB-Cys and fecA-Cys were repeated in the presence of 1, 10, and 100 mM sodium citrate and also citrate in combination with FeCl3 (to ensure sufficient ferric citrate was produced in the assay). Under all conditions examined, no increase in TonB-FecA interaction was observed. Citrate addition to the cultures 1 h after subculturing the overnight cultures to extend the time of exposure to ferric citrate did not affect TonB-FecA cross-linking (data not shown).
Nonspecific in vivo FA cross-linking between TonB and FecA is enhanced by citrate.
The interaction between TonB and FecA determined by disulfide bonding was not enhanced by the presence of sodium citrate. However, studies based on other TonB-dependent OM transporters of E. coli show that TonB preferentially interacts with ligand-loaded transporters. In view of these findings, it was decided to analyze the interaction of ligand-loaded FecA with TonB using in vivo FA cross-linking. The disulfide bond cross-linking assay relies on the interaction between FecA and TonB at specific sites and may require significant movement of the TonB box region of FecA in order to be detected. In contrast, the targets for cross-linking by FA are more general and include lysine, cysteine, tyrosine, and, to a lesser extent, tryptophan, histidine, aspartate, and arginine (9, 37).
For the FA-cross-linking studies, strain CO1031 was transformed with a plasmid encoding TonBC18A without its native promoter (pTonBC18ANP), so that expression of tonBC18ANP is from the vector lacZ promoter and requires the addition of IPTG. Plasmid pBAD18 or pBAD fecA (wild type) was introduced into CO1031(pTonBC18ANP), and cultures were grown in arabinose NB supplemented with 1 mM IPTG. Citrate was added to the cultures, and they were incubated for 15 min, after which cross-linking with FA was performed. The cells suspended in sample buffer were heated to 60°C for 5 min, so as not to disrupt FA-induced cross-links, prior to SDS-PAGE and Western immunoblotting using anti-TonB antisera.
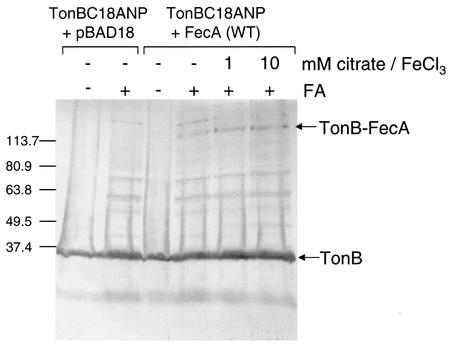
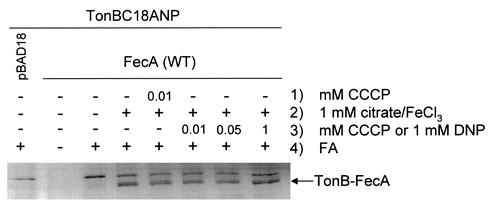
CO1031 expressing both TonBC18ANP (produced from pTonBC18A, from which the TonB native promoter was removed) and wild-type FecA produced a protein species that was detected only in the presence of FA (Fig. 7). This protein species is thought to be TonB cross-linked with FecA, as it is not detected in cells carrying pTonBC18ANP and the vector control pBAD18. Note, however, that by overloading this sample on a gel it is possible to detect very low levels of this band, since CO1031 still produces low levels of nonfunctional FecA that could cross-link to TonB. This cross-linked band was, however, completely absent when the assay was performed in a strain from which fecA was deleted, E. coli AA93 Δfec, supporting the hypothesis that it is TonB cross-linked to FecA (data not shown). In addition, FA-cross-linked FecA-TonB disappeared following treatment at 95°C for 15 min, which disrupts bonds between proteins created by FA. Because the FA cross-links are heat labile, samples were not boiled and the proteins were not completely denatured. Hence, it was not possible to unequivocally deetermine the molecular weights of protein species based on mobility in SDS-PAGE. The FA-induced cross-linking between TonB and FecA increased in the presence of 1 and 10 mM citrate. Other FA-cross-linked species were detected with anti-TonB antisera; however, this nonspecific cross-linking was not dependent on FecA and was not enhanced by sodium citrate. Therefore, in vivo FA-cross-linking assays suggest that the interaction between TonB and FecA is enhanced by the presence of substrate.
FIG. 7.
Nonspecific in vivo cross-linking between TonB and FecA is enhanced in the presence of sodium citrate. Cross-linking experiments were performed in E. coli strain CO1031 carrying TonBC18ANP and either pBAD18 or pBADfecA (wild type [WT]). The cells were grown in NB (0.2% arabinose) for 4 h, adjusted to an A578 of 1.0, and resuspended in 10 mM sodium phosphate buffer (pH 6.8). The cells were incubated for a further 15 min in the presence (+) or absence (−) of 1 or 10 mM sodium citrate premixed with 100 μm FeCl3. One percent FA was added to some of the cultures (+), and the cultures were incubated for a further 25 min. Whole-cell lysates were collected and subjected to SDS-PAGE followed by Western immunoblotting using anti-TonB antisera. The molecular mass markers (in kilodaltons) are indicated on the left. However, as FA cross-links are heat labile, samples were not boiled, so it is not possible to designate the exact molecular masses of protein species based on mobility in SDS-PAGE. TonB cross-linked with FecA is indicated.
Interaction between TonB and FecA does not require energy derived from the PMF.
Because energized TonB is required for both FecA transport and induction (28), it was necessary to determine whether the FecA-TonB interaction requires energy. We examined whether the site-specific cross-linking of TonB to the TonB box of FecA is energy dependent. One approach was to perform cross-linking experiments in the presence and absence of CCCP, a chemical known to dissipate the electrochemical potential of the cytoplasmic membrane (45). Such an experiment was difficult to perform because disulfide bond formation between FecA/Cys and TonB/Cys occurs in the absence of substrate (Fig. 5 and 6) and is therefore likely to occur early in the assay, when the proteins are produced. Thus, the addition of CCCP late in the reaction could have no affect on disulfide cross-linking. The addition of CCCP earlier in the assay inhibited the production of FecA from pBAD18. Therefore, an alternative approach was employed.
Cross-linking studies were performed using the plasmid-encoded TonB/Cys derivatives in combination with ExbD, which has an amino acid substitution of asparagine for aspartate at position 25 (ExbD25N). Asp25 is the only charged amino acid in the membrane-spanning region of ExbD, and its replacement with Asn renders ExbD inactive (8). Plasmid-encoded ExbD25N is unable to restore the Ton activities of a strain carrying a chromosomal mutant of ExbD and negatively complemented ExbD+ strains of E. coli (8). Strain HE2350 (GM1 tolQ exb::Tn10 [exbD] tonB) does not produce ExbBD, TonB, or TolQ. TolQ was absent because the TolQR complex can partially complement the ExbBD system (5, 7). HE2350 carrying the pBAD FecA/Cys derivatives was transformed with either pTonB/CysExbBD or pTonB/CysExbB/ExbD(D25N), and cross-linking assays were performed. All three TonB/Cys derivatives were able to cross-link with FecA in the absence of a functional TonB/ExbB/ExbD complex (Fig. 8). Interestingly, the ability of the FecA-TonB cross-linked derivatives that are presumed to be TonB′-FecA (Fig. 5) were unable to cross-link when expressed with TonB/ExbB/ExbD(D25N) (Fig. 8). It should be noted that the overall pattern of cross-linking here differs slightly from that seen in Fig. 6. The overall intensity of cross-linking is reduced, and most noticeably, FecAL82C showed a reduced capacity to cross-link to TonBQ162C and TonBY163C. This variation in cross-linking probably results from strain variation. The cross-linking shown in Fig. 5 and 6 was performed in an AB2847 derivative, while the cross-linking in Fig. 8 utilized a GM1 derivative.
FIG. 8.
Site-specific disulfide cross-linking of FecA (DALTV)/Cys and TonB/Cys can occur without a functional TonB/ExbB/ExbD complex. Cross-linking experiments were performed in E. coli strain HE2350 (GM1 tolQ exb::Tn10 [exbD] tonB) expressing one of the FecA/Cys series (D80C [DC], A81C [AC], L82C [LC], T83C [TC], and V84C [VC]) and either TonBQ160C, TonBQ162C, or TonBY163C (as indicated on the left) together with either a functional ExbBD (+) or nonfunctional ExbB/ExbD(D25N) (−) complex. Cross-linking experiments were performed as described in the legend to Fig. 4, but sodium citrate was not added. TonB and TonB′ cross-linked with FecA are indicated on the right.
To ensure that HE2350 expressing plasmid-encoded TonB/ExbB/ExbD(D25N) is truly devoid of TonB and TolQ functions, the energy status was determined by examining the sensitivity of the strain to colicin B, colicin E1, colicin M, φ80, and albomycin. A tolQ strain of E. coli K-12 is resistant to group A colicins, including colicin E1, while a tonB strain is resistant to the group B colicins B and M and is also resistant to φ80 and albomycin (5). HE2350[pTonBQ160CExbB/ExbD(D25N)] was resistant to colicins B, M, and E1, φ80, and albomycin, while HE2350(pTonBQ160CExbBD) showed susceptibility comparable to that of the wild-type strain AB2847 (not shown).
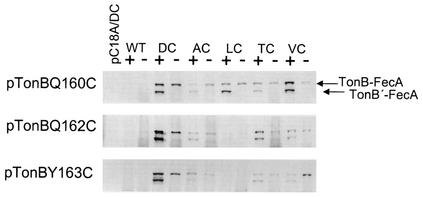
The interaction between TonB and FecA as determined by nonspecific in vivo FA cross-linking was also examined for a requirement for PMF-derived energy. FA-cross-linking assays were performed as described earlier; however, following the 15-min incubation with sodium citrate, 10 or 50 μm CCCP or 1 mM DNP was added to the samples, which were incubated for a further 5 min prior to the addition of FA. The addition of either CCCP or DNP did not affect TonB-FecA interaction (Fig. 9). Addition of 10 μm CCCP 5 min prior to the addition of sodium citrate also did not affect the formation of cross-linked TonB and FecA (Fig. 9). The 10 μm CCCP used here inhibited citrate-mediated induction of pGFPA′ by FecA when added simultaneously with citrate and reduced transcription to 30% of wild type when added 120 min after citrate (data not shown). This confirms the previous findings of Kim et al. (28), who showed that CCCP was able to inhibit the FecA-mediated citrate-induced transcription of a fecA-lacZ fusion. Taken together, these findings show that the physical interaction between TonB and FecA does not require energy from the PMF, and they correlate with the finding that the site-specific interaction between the two proteins does not require a functional TonB/ExbBD complex.
FIG. 9.
Citrate-enhanced nonspecific cross-linking between TonB and FecA does not require energy derived from the PMF. Nonspecific FA-cross-linking experiments were prepared as described in the legend to Fig. 6. The experiments were performed in E. coli strain CO1031 carrying TonBC18ANP and either pBAD18 or pBADfecA (wild type [WT]). Following the resuspension of the cells in 10 mM sodium phosphate buffer (pH 6.8), 10 or 50 μm CCCP or 1 mM DNP was added either before or after the addition of 1 mM sodium citrate- 100 μm FeCl3. The order in which various substrates or chemicals were added is indicated in ascending order by numbers 1 to 4 on the right. The leftmost lane is TonBC18ANP with pBAD18. TonB cross-linked with FecA is indicated. +, present; −, absent.
DISCUSSION
The OM of gram-negative bacteria is relatively impermeable and therefore must rely on the active transport of large or scarce substrates through dedicated active transporters located in the OM. These active transporters extract energy from the PMF of the inner membrane via the TonB/ExbB/ExbD energy-transducing complex. While much attention has been focused on the interactions between TonB and other E. coli K-12 OM TonB-dependent transporters, very little work has been done on TonB-FecA interactions.
The ferric citrate transporter FecA differs from the other TonB-dependent transporters by its role in the induction of transcription of the ferric citrate transport genes in addition to the transport of ferric citrate. Both FecA activities require energy provided by the TonB complex, and it is therefore of particular interest to know how FecA interacts with the TonB protein. It is conceivable that FecA interacts with TonB differently, depending on the FecA function requiring energy. For example, while it is known that OM TonB-dependent transporters require the TonB box for transport, it is not known if this same region is required for FecA induction. Previous work has identified the TonB box, situated adjacent to the switch helix, as important for TonB-receptor interactions. Another feature of FecA that differentiates it from the other E. coli TonB-dependent transporters is the position of the TonB box. The TonB box is usually located at the extreme N terminus; however, with FecA, the TonB box is preceded by a unique N-terminal extension (28). This N-terminal extension of FecA interacts with FecR and is required for the induction pathway that leads to the expression of the fec transport genes (14, 28).
The present studies showed that deletion of the TonB box of FecA (D80A81L82T83V84) renders the protein inactive for both induction and transport. The DALTV region of FecA could be functionally replaced with the core sequences of the TonB boxes of FhuA (DTITV) and FepA (DTIVV), retaining its ability to induce transcription of the transport genes and to transport ferric citrate. However, FecA carrying the FepA box transported iron at a lower rate than the wild type. The residues of the TonB box of FecA were sequentially replaced with cysteine residues, and all but one of the cysteine residue replacements in the DALTV of FecA were tolerated. The FecAD80C change was poorly tolerated, as it showed strongly reduced induction and transport. These data support the generally accepted notion that it is the secondary structure of the TonB box that is important for function rather than the sequence (11). Although the TonB box is conserved among TonB-dependent transporters, it is quite tolerant of amino acid substitutions. There are examples in which the residues of the TonB box can be replaced with other amino acids; however, the introduction of amino acids in certain positions that alter conformation can disrupt function (11, 49). While the substitution of cysteine residues in the TonB box of BtuB did not disrupt function, the introduction of proline or glycine residues at certain positions produced an uncoupled phenotype and showed an altered pattern in cross-linking (4, 11). A single mutation in the TonB box of FepA (I14P) prevented the chemical cross-linking of FepA to TonB (30). The substitutions L82P and V84G in the TonB box of FecA eliminate both the induction and transport functions of FecA (20).
A physical interaction between the TonB box of FecA and region 160 of TonB was demonstrated here, using in vivo site-specific disulfide bond formation. While it was previously known that region 160 of TonB is important for the interaction with the TonB boxes of other TonB-dependent OM transporters (4, 11, 26, 49), it was not known if this region interacts with the TonB box of FecA. Cysteine substitutions at residues 160, 162, and 163 of TonB were coincubated with the FecA TonB box cysteine derivatives and were able to form disulfide cross-links. The abilities of all three cysteine replacements to grow on vitamin B12 via BtuB were unaffected (11); however, only TonBQ160C and TonBQ162C could support FecA activities. The TonBY163C substitution was not tolerated, as it had a greatly reduced ability to support either of the FecA functions, identifying residue tyrosine 163 as a critical residue for FecA function.
Region 160 of TonB cross-linked via disulfide bridges to the TonB box of FecA showed no enhancement in the presence of substrate (ferric citrate). This is somewhat puzzling, as the crystal structure of FecA shows that the TonB box becomes disordered in the presence of ferric citrate. There is an unwinding and repositioning of both the switch helix and the TonB box (15). Enhanced interaction of the same TonB/Cys derivatives with TonB box cysteine derivatives of BtuB was demonstrated in the presence of vitamin B12 (11). The lack of ferric citrate enhancement of TonB-FecA interaction here may be a limitation of the assay system used. Alternatively, it may reflect a unique interaction of TonB with FecA compared to other TonB-dependent transporters. In contrast, a clear ferric citrate-enhanced interaction between TonB and FecA was demonstrated using in vivo nonspecific FA cross-linking. This supports the notion that ligand-loaded transporters are preferentially sampled by TonB. FA cross-links can occur between various regions of the proteins and so, unlike the disulfide assay, do not rely on specific sites of interaaction.
The physical interaction between TonB and FecA was able to occur in the absence of energy derived from the PMF of the cytoplasmic membrane. Functional ExbD was replaced with the nonfunctional ExbD25N mutant derivative in the TonB/CysExbBD complex in E. coli strain HE2350 tonB tolQ. TonBQ160C, TonBQ162C, and TonBY163C were able to cross-link with the FecA cysteine derivatives when produced as part of the TonB/ExbB/ExbD(D25N) complex. Interestingly, the ExbD(D25N) mutation prevented formation of the second cross-linked product, assigned to TonB′-FecA. Although the meaning of this finding is not clear, it nevertheless shows that TonB′-FecA disulfide formation requires a functional TonB/ExbB/ExbD complex. PMF independence of TonB-FecA interaction was confirmed by FA, which cross-linked TonB and FecA in the presence of either CCCP or DNP, chemicals known to dissipate the PMF of the cytoplasmic membrane.
The cross-linking studies described here show that TonB is able to physically interact with FecA in vivo in the absence of both substrate and the PMF of the cytoplasmic membrane. This is in agreement with previous findings that the in vivo production of the periplasmic domain of TonB was able to inhibit FecA- and FhuA-mediated transport (24), and similarly, that anchorless TonB produced in vivo was able to form a complex with FepA (25). Both full-length TonB (40) and a membrane anchorless derivative of TonB were able to bind FhuA in vitro in the absence of an energy source and in the absence of ligand (39). FecA must extract energy from TonB for both the induction and transport processes. The citrate-induced conformational changes of FecA, including movement of the gating extracellular loops, does not require energy, as the crystal structure of ligand-loaded FecA was obtained in the absence of TonB-derived energy (15). The ligand-loaded state of FecA was obtained by exposing the apo-FecA crystals overnight in dinuclear ferric citrate and ferric citrate. Therefore, it is likely that energy extracted by FecA from TonB is required for subsequent steps, such as the transport of ferric citrate through the FecA molecule into the periplasm. It is not yet known how the substrate passes through the barrel to the periplasm; either the cork is rearranged and moves to one side of the barrel, or it moves out of the barrel into the periplasm. Either process would require breaking the many hydrogen bonds that hold the cork in place, which is an energy-consuming process. It has previously been shown both in vitro and in vivo that FecA and FecR are able to physically interact in the absence of TonB-derived energy (14), so presumably the energy-dependent step of induction of transcription occurs after the proteins have made contact.
Recent completion of microbial genome sequences has revealed the existence of at least 50 FecAIR-like regulatory systems in ∼20 different genera (60). It appears, therefore, that the ferric citrate transport system of E. coli is a paradigm for this type of regulatory and transport system. Of these homologue systems, only a few have been examined experimentally, and none to the extent of the Fec system. It is essential, therefore, to understand the complete pathway of this type of regulatory and transport system at the molecular level.
Acknowledgments
We thank Robert. J. Kadner for providing the TonB/Cys clones, Christina Herrman for technical assistance, and Alex Lech and Uwe Stroeher for useful discussions.
This work was supported by the Deutsche Forschungsgemeinschaft (BR330/9-1 and -2). M. Ogierman was the recipient of an Alexander von Humboldt Research Fellowship.
REFERENCES
- 1.Ahmer, B. M., M. G. Thomas, R. A. Larsen, and K. Postle. 1995. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB functions and stability. J. Bacteriol. 177:4742-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169:483-490. [DOI] [PubMed] [Google Scholar]
- 3.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of σ70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 4.Bell, P. E., C. D. Nau, J. T. Brown, J. Konisky, and R. J. Kadner. 1990. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J. Bacteriol. 172:3826-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, V. 1989. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J. Bacteriol. 11:6387-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, V. 1995. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16:295-307. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., and C. Herrmann. 1993. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol. Microbiol. 8:261-268. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V., S. Gaisser, C. Herrmann, K. Kampfenkel, H. Killmann, and I. Traub. 1996. Energy-coupled transport across the outer membrane of Escherichia coli: ExbB binds ExbD and TonB in vitro, and leucine 132 in the periplasmic region and aspartate 25 in the transmembrane region are important for ExbD activity. J. Bacteriol. 178:2836-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkley, M. 1992. A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjug. Chem. 3:2-13. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 11.Cadieux, N., and R. J. Kadner. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl. Acad. Sci. USA 96:10673-10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulton, J. W., P. Mason, D. R. Cameron, G. Carmel, R. Jean, and H. N. Rode. 1986. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 165:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P. J., E. Garges, and C. A. Genco. 2000. Pathogenic neisseriae can use hemoglobin, transferrin, and lactoferrin independently of the tonB locus. J. Bacteriol. 182:5586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. Van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diedericns, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, E., K. Günter, and V. Braun. 1989. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J. Bacteriol. 711:5127-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomini, A., B. Corich, F. J. Ollero, A. Squartini, and M. P. Nuti. 1992. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol. Lett. 100:87-90. [DOI] [PubMed] [Google Scholar]
- 19.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habeck, M. 1998. Ph.D. thesis. University of Tübingen, Tübingen, Germany.
- 21.Hantke, K., and V. Braun. 1978. Functional interaction of the TonA/TonB receptor system in Escherichia coli. J. Bacteriol. 135:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Härle, C., I. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 24.Howard, S. P., C. Herrmann, C. W. Stratilo, and V. Braun. 2001. In vivo synthesis of the periplasmic domain of TonB inhibits transport through the FecA and FhuA iron siderophore transporters of Escherichia coli. J. Bacteriol. 183:5885-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaskula, J. C., T. E. Letain, S. K. Roof, J. T. Skare, and K. Postle. 1994. Role of the TonB amino terminus in energy transduction between membranes. J. Bacteriol. 176:2326-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadner, R. J. 1990. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol. Microbiol. 4:2027-2033. [DOI] [PubMed] [Google Scholar]
- 27.Kadner, R. J., and K. J. Heller. 1995. Mutual inhibition of cobalamin and siderophore uptake systems suggest their competition for TonB function. J. Bacteriol. 177:4829-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, R. A., D. Foster-Hartnett, M. A. McIntosh, and K. Postle. 1997. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J. Bacteriol. 179:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen, R. A., P. S. Myers, J. T. Skare, C. L. Seachord, R. P. Darveau, and K. Postle. 1996. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J. Bacteriol. 178:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen, R. A., M. G, Thomas, and K. Postle. 1999. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31:1809-1824. [DOI] [PubMed] [Google Scholar]
- 33.Larsen, R. A., G. E. Wood, and K. Postle. 1993. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol. Microbiol. 10:943-953. [DOI] [PubMed] [Google Scholar]
- 34.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 35.Lundrigan, M. D., and R. J. Kadner. 1986. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane transporters that interact with TonB. J. Biol. Chem. 261:10797-10801. [PubMed] [Google Scholar]
- 36.Martinez, E., B. Bartolome, and F. De la Cruz. 1988. pACY184-derived cloning vectors containing the multiple cloning site and lacZ reporter gene of pUC8/9 and pUC18/19 plasmids. Gene 68:159-162. [DOI] [PubMed] [Google Scholar]
- 37.Means, G. E., and R. E. Feeney. 1971. Chemical modifications of proteins. Holden-Day Publishers, San Francisco, Calif.
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 39.Moeck, G. S., and L. Letellier. 2001. Characterization of in vitro interactions between a truncated TonB protein from Escherichia coli and the outer membrane receptors FhuA and FepA. J. Bacteriol. 183:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moeck, G. S., J. W. Coulton, and K. Postle. 1997. Cell envelope signalling in Escherichia coli: ligand binding to the ferrichrome-iron receptor FhuA promotes interaction with the energy-transducing protein TonB. J. Biol. Chem. 272:28391-28397. [DOI] [PubMed] [Google Scholar]
- 41.Occhino, D. A., E. E. Wyckoff, D. P. Henderson, T. W. Wrona, and S. M. Payne. 1998. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol. Microbiol. 29:1493-1507. [DOI] [PubMed] [Google Scholar]
- 42.Ochs, M., A. Angerer, S. Enz, and V. Braun. 1996. Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. [DOI] [PubMed]
- 43.Ochs, M., S. Veitinger, K. InSook, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 44.Paquelin, A., J. M. Ghigo, S. Bertin, and C. Wandersman. 2001. Characterisation of HasB, a Serratia marcescens TonB-like protein specifically involved in the haemophore-dependent haem acquisition system. Mol. Microbiol. 42:995-1005. [DOI] [PubMed] [Google Scholar]
- 45.Possot, O., L. Letellier, and A. Pugsley. 1997. Energy requirement for pullulanase secretion by the main branch of the general secretory pathway. Mol. Microbiol. 24:457-464. [DOI] [PubMed] [Google Scholar]
- 46.Postle, K. 1993. TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 25:591-601. [DOI] [PubMed] [Google Scholar]
- 47.Pressler, U., H. Staudenmaier, L. Zimmermann, and V. Braun. 1988. Genetics of the iron dicitrate transport system of Escherichia coli. J. Bacteriol. 170:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schöffler, H., and V. Braun. 1989. Transport across the outer membrane of Escherichia coli via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol. Gen. Genet. 217:378-383. [DOI] [PubMed] [Google Scholar]
- 50.Seliger, S. S., A. R. Mey, A.-M. Valle, and S. M. Payne. 2001. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol. Microbiol. 39:801-812. [DOI] [PubMed] [Google Scholar]
- 51.Skare, J. T., B. M Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 52.Staudenmaier, H., B. van Hove, Z. Yaraghi, and V. Braun. 1989. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J. Bacteriol. 171:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stiefel, A., S. Mahren, M. Ochs, P. T. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studier, J. T., and B. A. Moffat. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 55.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeshita, S., S. Masahiro, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 57.Traub, I., S. Gaisser, and V. Braun. 1993. Activity domains of the TonB protein. Mol. Microbiol. 8:409-423. [DOI] [PubMed] [Google Scholar]
- 58.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 59.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcriptional control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signaling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 61.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welz, D., and V. Braun. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. 180:2387-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young, G. M., and K. Postle. 1994. Repression of tonB transcription during anaerobic growth requires Fur binding at the promoter and a second factor binding upstream. Mol. Microbiol. 11:943-954. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, Q., and K. Poole. 2000. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol. Lett. 184:127-132. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann, L., K. Hantke, and V. Braun. 1984. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J. Bacteriol. 159:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]