Abstract
We have isolated three Shewanella oneidensis mutants specifically impaired in trimethylamine oxide (TMAO) respiration. The mutations arose from insertions of an ISSo2 element into torA, torR, and torS, encoding, respectively, the TMAO reductase TorA, the response regulator TorR, and the sensor TorS. Although TorA is not the sole enzyme reducing TMAO in S. oneidensis, growth analysis showed that it is the main respiratory TMAO reductase. Use of a plasmid-borne torE′-lacZ fusion confirmed that the TorS-TorR phosphorelay mediates TMAO induction of the torECAD operon.
Trimethylamine oxide (TMAO) is a widespread osmoprotective constituent of aquatic animals (17) which can be used by many bacteria as an alternative terminal electron acceptor (3). Reduction of TMAO to the volatile compound trimethylamine generally involves molybdenum-containing enzymes of the same family (6). However, based on their substrate specificity, these enzymes can be divided into two subfamilies: the TMAO reductase and the dimethyl sulfoxide (DMSO) reductase groups. The TMAO reductase enzymes are highly specific, since they can reduce exclusively TMAO as a natural compound or related artificial compounds (13). These enzymes, called TorA, were found in particular in the periplasm of Escherichia coli and Shewanella species (6, 10, 20). The DMSO reductases, called DorA or DmsA, can reduce a wide range of N- and S-oxide compounds, including DMSO and TMAO (25, 26). They are present in many species, including enterobacteria and Rhodobacter species (19).
It has been recently shown in E. coli that the TorA protein binds to the pentaheme c-type cytochrome TorC (8). The latter is anchored to the inner membrane and allows electron transfer between the membranous quinone pool and the periplasmic terminal enzyme TorA. In E. coli and Shewanella species, the genes encoding the components of the TMAO reductase system are clustered in the torCAD and torECAD operons, respectively (6, 10, 20). Three regulatory genes called torR, torS, and torT are found near the tor operons. A detailed analysis of the Tor regulatory elements of E. coli revealed that TorS is the transmembrane sensor that detects the presence of TMAO in the medium and monitors the proper maturation of TorC (9, 15). TorS contains three phosphorylation sites and transphosphorylates the response regulator TorR by a four-step phosphorelay under inducing conditions (14). Once phosphorylated, TorR activates torCAD expression (2). TorT is a periplasmic protein essential for tor operon induction, but its precise role remains unclear (16).
Genes of Shewanella oneidensis encoding proteins homologous to TorS, TorR, and TorT of E. coli were identified and called torS, torR, and torT (10). However, as no S. oneidensis mutant was available, their regulatory functions were investigated by reconstitution experiments in E. coli. This approach showed that the three proteins seem to be essential for induction of the tor operon of S. oneidensis.
Isolation of S. oneidensis mutants specifically affected in TMAO respiration.
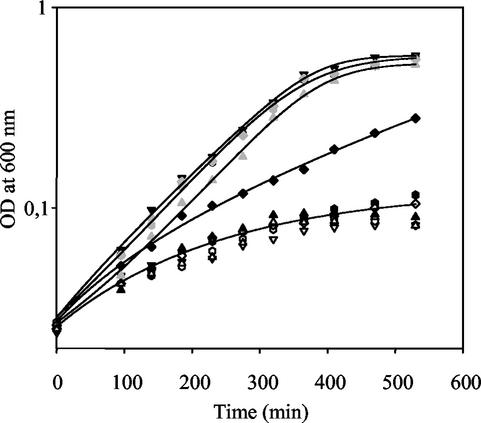
To isolate mutations affecting the main TMAO respiratory system of S. oneidensis, we plated MR1-R cells (Table 1), potentially mutagenized by Tn5 random insertions (4), onto solid rich medium containing 50 mM TMAO and the appropriate antibiotics, and the cells were incubated anaerobically for 36 h at 30°C. Among thousands of clones, we isolated 22 small colonies. To distinguish between pleiotropic and specific mutations, we tested whether these potential mutants were still able to grow normally on rich medium either in aerobiosis or in anaerobiosis in the presence of nitrate (20 mM). Of the 22 clones, only 3 formed large colonies in the presence of oxygen or nitrate. The other 19 clones were not studied further, and we focused our analysis on the three clones that were potentially defective for the main TMAO respiratory system. As shown in Fig. 1, the three mutant strains (SOA-1, SOS-2, and SOR-3) grew more slowly than reference strain MR1-R in anaerobiosis with TMAO, whereas all four strains grew at the same low rate in the absence of TMAO. Interestingly, SOS-2 grew significantly more rapidly than mutants SOA-1 and SOR-3 in the presence of TMAO, while the latter two grew at almost the same low rate with or without TMAO. These results show that SOA-1 and SOR-3 are almost deficient for TMAO respiration, whereas SOS-2 is affected to a lesser extent.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic | Reference or source |
|---|---|---|
| S. oneidensis MR1 | Wild type; formerly Shewanella putrefaciens MR1 ATCC 700550 | 23 |
| MR1-R | Rifampin-resistant derivative of MR1 | G. De Luca |
| SOA-1 | torA::ISSo2 mutant | This work |
| SOS-2 | torS::ISSo2 mutant | This work |
| SOR-3 | torR::ISSo2 mutant | This work |
| Plasmids | ||
| pACYC184 | Cloning vector with a p15A origin of replication | 5 |
| pGE593 | Operon fusion vector with a pBR origin of replication | 7 |
| pBAD33 | Vector containing pBAD promoter with a p15A origin of replication | 11 |
| pSTRSO | torSTR sequence from S. oneidensis cloned into pBAD33 | 10 |
| pBTorASO | torA sequence from S. oneidensis cloned into pBAD33 | This work |
| pPTorSO7 | torE promoter (−84 to +119)a from S. oneidensis cloned into pGE593 | 10 |
| pElacZ | torE′-lacZ fusion of pPTorSO7 cloned into pACYC184 | This work |
Nucleotide positions relative to the transcription start site of torE.
FIG. 1.
Anaerobic growth profiles of S. oneidensis MR1-R and tor mutants carrying different plasmids. Strains MR1-R (▿ and ▾), SOA-1 (▵ and ▴), SOS-2 (◊ and ⧫), and SOR-3 (□ and ▪), all carrying pBAD33, were grown at 30°C in Luria-Bertani rich medium complemented with 40 mM l-lactate and 20 mM HEPES (24), in either the absence (white symbols) or presence (black symbols) of 50 mM TMAO. The grey symbols correspond to SOS-2 (diamonds) and SOR-3 (squares), both carrying pSTRSO, and SOA-1, carrying pBTorASO (triangles), grown in the presence of TMAO. For the latter, arabinose (0.001%) was added. Growth was monitored at 600 nm. Data are typical of at least three independent experiments.
Location and nature of the mutations.
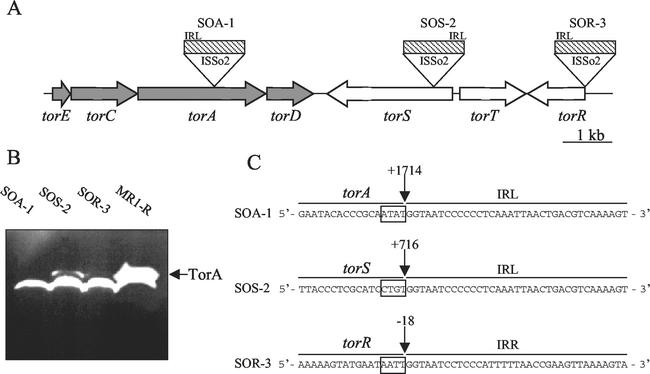
As shown previously (10), the putative TMAO reductase of S. oneidensis is a large molybdoenzyme called TorA, and the encoding gene is located in the torECAD operon of the tor locus (Fig. 2A). To check whether TorA was still present in the three mutant strains, we prepared crude extracts (6) from the mutant and the reference strains anaerobically grown overnight in the presence of TMAO. Equivalent amounts of nonheated samples were then loaded on a sodium dodecyl sulfate-polyacrylamide gel, and the TMAO reductase active bands were revealed after electrophoresis (Fig. 2B). As expected, an intense band was present for strain MR1-R, and, based on its mobility, it probably corresponds to the TorA enzyme. Strikingly, this active band was missing in SOA-1 and was very faint in SOR-3. Although a band corresponding to TorA was clearly present in SOS-2, its intensity was very low compared to that of strain MR1-R. These results are consistent with the growth properties of the mutant strains, since mutants SOA-1 and SOR-3, which produce at best a very small amount of TorA, grew very slowly in anaerobiosis with TMAO, whereas, under the same conditions, SOS-2, which contains small but significant amounts of TorA, grew more rapidly than SOA-1 or SOR-3. Figure 2B also shows the presence of a second TMAO reductase enzyme migrating just underneath TorA in the four strains. Since this enzyme displayed strong DMSO reductase activity (data not shown), we propose that it is a DMSO reductase able to reduce N- and S-oxide substrates, including TMAO. However, it did not allow efficient TMAO respiration under our experimental conditions.
FIG. 2.
(A) Schematic representation of the positions of the ISSo2 insertion in torA, torS, and torR. The large arrows show the location and orientation of the tor genes. The hatched boxes symbolize the insertion elements; the position of the left inverted repeat (IRL) is indicated. (B) In-gel TMAO reductase activity. Nonheated crude extracts (75 μg) of SOA-1, SOS-2, SOR-3, and MR1-R were loaded on a sodium dodecyl sulfate-7.5% polyacrylamide gel. After electrophoresis, the gel was stained for methyl viologen-TMAO reductase activity (6). (C) Insertion sites of the ISSo2 element in torA, torS, and torR. The vertical arrows indicate the insertion sites, and boxes indicate the four nucleotides which were duplicated during ISSo2 insertion. Left and right inverted repeats (IRL and IRR) show the orientation of the mobile element. Numbering is relative to the translation start point of each gene.
To check the possibility that the mutations arose from a Tn5 insertion within tor genes, we amplified by PCR with appropriate primers each of the seven genes of the tor locus (data not shown). Surprisingly, this simple approach revealed that an insertion of about 1.2 kb was present within the torA, torS, and torR genes for SOA-1, SOS-2, and SOR-3, respectively. Although it proved that torA, torS, and torR were disrupted in these strains, this result was unexpected, since the size of Tn5 (5.8 kb) is larger than that observed in the mutants. To define the precise positions and the nature of the insertions, we sequenced each of the mutated genes. The three insertions correspond to the same ISSo2 element (Fig. 2), which belongs to the IS3 family (18), and this element was found in four intact copies and one partly deleted copy on the chromosome of strain MR1 (12). The insertions of this mobile element within the tor genes led to a duplication of four nucleotides of the target DNA. As indicated in Fig. 2C, torA, torS, and torR were disrupted at positions +1714, +716, and −18 from the start codon in SOA-1, SOS-2, and SOR-3, respectively. The fact that the mutations in the tor genes occurred by insertions of the ISSo2 endogenous element rather than by Tn5 transposition is puzzling, since Tn5 mutagenesis has been used successfully in S. oneidensis (4).
To confirm that the phenotypes of the mutants came from the tor gene disruptions, we amplified the torA gene and the torSTR gene cluster by PCR and cloned them into pBAD33, a replicative plasmid for S. oneidensis (22). Plasmids pBTorASO and pSTRSO were then introduced by electrotransformation into strains SOA-1 and SOS-2 or SOR-3, respectively. Electrotransformation was performed as previously described (22), except that the solution containing 1 M sorbitol was buffered with Tris-HCl, pH 7.6. As shown in Fig. 1, presence of the hybrid plasmids complemented the defect of the mutant strains in anaerobic growth with TMAO, confirming that the disrupted genes are involved in the main TMAO respiratory pathway of S. oneidensis.
Regulation of torECAD operon expression.
Based on reconstitution experiments in E. coli (10) and on the results described above, we suspected that the torR and torS gene products play a key role in the induction of the tor structural operon. To study expression of the tor operon directly in S. oneidensis, we amplified and cloned the entire torE′-lacZ DNA fragment of pPTorSO7 (from position −29 to +3335 relative to the EcoRI cloning site) into pACYC184. The resulting plasmid (pElacZ) was introduced into the mutant and reference strains, and β-galactosidase activities were measured on whole cells by the method of Miller (21). As shown in Table 2, in anaerobiosis, the β-galactosidase activities of the plasmid-borne torE′-lacZ fusion increased almost 40-fold when TMAO was added for the reference and the SOA-1 strains. In contrast, the activities of the fusion were very low and did not significantly increase upon TMAO addition for SOS-2 and SOR-3. These results confirm that TMAO strongly induces tor operon expression and that this induction requires the torS and torR gene products. No torA autoregulation was observed, since the activity levels were similar in the reference and the torA strains under inducing conditions. Interestingly, although expression of the plasmid-borne torE′-lacZ fusion was very low in SOS-2, it was even lower in SOR-3. This last point supports the idea that expression of the tor operon is not entirely locked in SOS-2 and agrees with the fact that a small amount of TorA enzyme was still present in the torS strain (Fig. 2). An explanation is that TorS, like many sensors, can dephosphorylate its partner in the absence of any inducer (27) and can thus remove phosphate of any origin from TorR. As in E. coli (1), dephosphorylation of TorR could involve a reverse phosphotransfer from TorR to TorS.
TABLE 2.
Expression of the plasmid-borne torE′-lacZ fusion in S. oneidensis strains
| Strain carrying pElacZa | β-Galactosidase activityb
|
|
|---|---|---|
| + TMAO | − TMAO | |
| MR1-R | 6,240 ± 430 | 157 ± 23 |
| SOA-1 | 6,030 ± 290 | 153 ± 14 |
| SOS-2 | 350 ± 28 | 340 ± 17 |
| SOR-3 | 215 ± 30 | 166 ± 22 |
All strains were grown anaerobically in the presence (+) or absence (−) of TMAO.
β-Galactosidase activities are expressed in Miller units. Values are averages of at least three independent experiments. Standard deviations were <15%.
In conclusion, isolation and characterization of various mutations in the tor genes of S. oneidensis allowed us to confirm that the TorS-TorR phosphorelay is involved in the control of torECAD in response to TMAO availability and to establish that TorA is the major TMAO reductase respiratory enzyme of S. oneidensis.
Acknowledgments
We thank H. Quinson for reviewing the manuscript and M. Ansaldi, C. Jourlin-Castelli, and an anonymous reviewer for helpful suggestions. We are grateful to G. De Luca for providing strains and to the Institute for Genomic Research for genome sequence data.
This work was supported by grants from the Centre National de la Recherche Scientifique and the Université de la Méditerranée. C.B. was supported by grants from the MENRT.
REFERENCES
- 1.Ansaldi, M., C. Jourlin-Castelli, M. Lepelletier, L. Théraulaz, and V. Méjean. 2001. Rapid dephosphorylation of the TorR response regulator by the TorS unorthodox sensor in Escherichia coli. J. Bacteriol. 183:2691-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansaldi, M., G. Simon, M. Lepelletier, and V. Méjean. 2000. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 182:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, E. L., and H. S. Kwan. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39:131-149. [DOI] [PubMed] [Google Scholar]
- 4.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos, J. P., C. Iobbi-Nivol, C. Couillault, G. Giordano, and V. Méjean. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. J. Mol. Biol. 284:421-433. [DOI] [PubMed] [Google Scholar]
- 7.Eraso, J. M., and G. M. Weinstock. 1992. Anaerobic control of colicin E1 production. J. Bacteriol. 174:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gon, S., M. T. Giudici-Orticoni, V. Méjean, and C. Iobbi-Nivol. 2001. Electron transfer and binding of the c-type cytochrome TorC to the trimethylamine N-oxide reductase in Escherichia coli. J. Biol. Chem. 276:11545-11551. [DOI] [PubMed] [Google Scholar]
- 9.Gon, S., C. Jourlin-Castelli, L. Théraulaz, and V. Méjean. 2001. An unsuspected autoregulatory pathway involving apocytochrome TorC and sensor TorS in Escherichia coli. Proc. Natl. Acad. Sci. USA 98:11615-11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gon, S., J. C. Patte, J. P. Dos Santos, and V. Méjean. 2002. Reconstitution of the trimethylamine oxide reductase regulatory elements of Shewanella oneidensis in Escherichia coli. J. Bacteriol. 184:1262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, et al. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 13.Iobbi-Nivol, C., J. Pommier, J. Simala-Grant, V. Méjean, and G. Giordano. 1996. High substrate specificity and induction characteristics of trimethylamine-N-oxide reductase of Escherichia coli. Biochim. Biophys. Acta 1294:77-82. [DOI] [PubMed] [Google Scholar]
- 14.Jourlin, C., M. Ansaldi, and V. Méjean. 1997. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J. Mol. Biol. 267:770-777. [DOI] [PubMed] [Google Scholar]
- 15.Jourlin, C., A. Bengrine, M. Chippaux, and V. Méjean. 1996. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol. Microbiol. 20:1297-1306. [DOI] [PubMed] [Google Scholar]
- 16.Jourlin, C., G. Simon, J. Pommier, M. Chippaux, and V. Méjean. 1996. The periplasmic TorT protein is required for trimethylamine N-oxide reductase gene induction in Escherichia coli. J. Bacteriol. 178:1219-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly, R. H., and P. H. Yancey. 1999. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fishes, skate, and decapod crustaceans. Biol. Bull. 196:18-25. [DOI] [PubMed] [Google Scholar]
- 18.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDevitt, C. A., P. Hugenholtz, G. R. Hanson, and A. G. McEwan. 2002. Molecular analysis of dimethyl sulphide dehydrogenase from Rhodovulum sulfidophilum: its place in the dimethyl sulphoxide reductase family of microbial molybdopterin-containing enzymes. Mol. Microbiol. 44:1575-1587. [DOI] [PubMed] [Google Scholar]
- 20.Méjean, V., C. Iobbi-Nivol, M. Lepelletier, G. Giordano, M. Chippaux, and M. C. Pascal. 1994. TMAO anaerobic respiration in Escherichia coli: involvement of the tor operon. Mol. Microbiol. 11:1169-1179. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 22.Myers, C. R., and J. M. Myers. 1997. Replication of plasmids with the p15A origin in Shewanella putrefaciens MR-1. Lett. Appl. Microbiol. 24:221-225. [DOI] [PubMed] [Google Scholar]
- 23.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 24.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh, T., and F. N. Kurihara. 1987. Purification and properties of dimethylsulfoxide reductase containing a molybdenum cofactor from a photodenitrifier, Rhodopseudomonas sphaeroides f.s. denitrificans. J. Biochem. 102:191-197. [DOI] [PubMed] [Google Scholar]
- 26.Simala-Grant, J. L., and J. H. Weiner. 1996. Kinetic analysis and substrate specificity of Escherichia coli dimethyl sulfoxide reductase. Microbiology 142:3231-3239. [DOI] [PubMed] [Google Scholar]
- 27.Stock, J. B., M. G. Surette, M. Levit, and P. Park. 1995. Two-component signal transduction systems: structure-function relationships and mechanism of catalysis, p. 25-51. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.