Abstract
The Arabidopsis EMB30 gene is essential for controlling the polarity of cell growth and for normal cell adhesion during seedling development. In this article, we show that emb30 mutations also affect the growth of undifferentiated plant cells and adult tissues. EMB30 possesses a Sec7 domain and, based on similarities to other proteins, presumably functions in the secretory pathway. The plant cell wall depends on the secretory pathway to deliver its complex polysaccharides. We show that emb30 mutants have a cell wall defect that sometimes allows material to be deposited into the interstitial space between cells instead of being restricted to cell corners. In addition, pectin, a complex polysaccharide important for cell adhesion, appears to be abnormally localized in emb30 plants. In contrast, localization of epitopes associated with xyloglucan or arabinogalactan was similar in wild-type and emb30 tissues, and the localization of a marker molecule to vacuoles appeared normal. Therefore, emb30 mutations do not cause a general defect in the secretory pathway. Together, these results suggest that emb30 mutations result in an abnormal cell wall, which in turn may account for the defects in cell adhesion and polar cell growth control observed in the mutants.
INTRODUCTION
In Arabidopsis, the normal body pattern of a seedling is determined during embryogenesis. Mature embryos contain a shoot apical meristem, two cotyledons, a hypocotyl, and a root apical meristem. Several genes affecting this pattern formation have been cloned. One of these genes, EMB30, is required for the formation of roots and inflorescence stems and encodes a protein similar to the yeast Sec7 protein (Sec7p), which functions in the secretory pathway (Shevell et al., 1994). knolle mutants have an abnormal epidermal cell layer and are defective in cytokinesis. The KNOLLE protein sequence shares similarity with syntaxins, which are also involved in the secretory pathway (Lukowitz et al., 1996). The SHOOT MERISTEMLESS gene is required for the formation of a shoot apical meristem and encodes a homeodomain protein (Long et al., 1996). MONOPTEROS affects formation of the vascular system and the body pattern; it encodes a putative transcription factor (Hardtke and Berleth, 1998).
The fate of a cell in plants is largely defined by its position. Except during pollen tube formation, cell migration usually does not occur during plant development. Therefore, the position of a cell is determined by controlling patterns of cell division and cell expansion. Cell morphogenesis and differentiation, in turn, are affected by the interactions of a plant cell with its neighbors (Lyndon, 1990). Plant cells communicate with each other using cytoplasmic connections called plasmodesmata. In addition, plant cells adhere to each other via the middle lamella, a layer of the cell wall. The complex polysaccharide pectin is a major component of the middle lamella and is thought to be important in cell adhesion. Pectin is also found in other parts of the cell wall and can exist in various forms (McCann and Roberts, 1991). The majority of the plant cell wall, 86% in Arabidopsis (Zablackis et al., 1995), is composed of polysaccharides. Cell biology experiments have demonstrated that the major complex polysaccharides (pectin and hemicellulose) of the plant cell wall are synthesized in the Golgi complex and transported by vesicles to the cell wall (Driouich et al., 1993). Both the plant secretory pathway and the cell wall are altered in a developmentally- and tissue-specific fashion (Driouich et al., 1993; Freshour et al., 1996), and cell wall components must be delivered to particular sites of the wall for a cell to acquire the correct shape. Therefore, the control of cell wall formation by way of secretion plays an important role in plant cell–cell interactions and development.
emb30 (also called gnom) mutants (Meinke, 1985; Shevell et al., 1994; Mayer et al., 1993) display a range of phenotypes, but all lack a root. The most severe mutants are balls of tissue with little or no apical–basal polarity. The least severe emb30 plants have abnormally formed shoot apical meristems, cotyledons, and hypocotyls. A single emb30 allele gives rise to all these phenotypes, and all emb30 alleles show this phenotypic variation (Mayer et al., 1993; Shevell et al., 1994). The variation in emb30 phenotypes probably indicates a lack of control over determining the polarity of cell divisions and cell expansions. The more severe emb30 phenotypes could reflect an incorrect cell division or expansion that occurred very early in embryogenesis. In addition, emb30 cells separate from one another much more easily than do wild-type cells (Shevell et al., 1994).
The 163-kD EMB30 protein shares similarity to the yeast Sec7p (Shevell et al., 1994), which is required for directing COPII-dependent, endoplasmic reticulum–derived vesicles to the cis-Golgi compartment in vitro (Lupashin et al., 1996). Sec7p is not required for COPI or COPII vesicle budding, indicating that it is involved in the specific localization of vesicles to the Golgi (Lupashin et al., 1996). The most highly conserved region between EMB30 and Sec7p is the Sec7 domain, consisting of ∼200 amino acids (Shevell et al., 1994). Several emb30 alleles contain mutations in the Sec7 domain, indicating that this domain is important for EMB30 function (Shevell et al., 1994; Busch et al., 1996). Two recently identified yeast proteins, Gea1p and Gea2p, also contain Sec7 domains and are more similar in size and structure to EMB30 than is Sec7p (Peyroche et al., 1996). GEA1 was identified as a suppressor of a dominant negative mutation in the ADP–ribosylation factor2 (ARF2) protein, and Gea1p is part of a complex that has guanine nucleotide exchange factor (GEF) activity for ARF. ARFs are small GTP binding proteins that function in vesicle transport. In the GTP-bound form, ARF promotes vesicle budding. The Sec7 domains in Sec7p and the human protein ARNO have been shown to possess GEF activity for ARF1 (Chardin et al., 1996; Sata et al., 1998). The EMB30 protein also possesses ARF GEF activity, and EMB30 can complement a gea1-19 gea2Δ strain (Steinmann et al., 1999), further supporting a role for EMB30 in the secretory pathway.
To understand the role of EMB30 in plant development, we analyzed the response of emb30 mutants to various stimuli and studied the effect of emb30 mutations on various plant tissues. We found EMB30 to be essential for the normal growth and organization of undifferentiated and adult tissues as well as for embryogenesis. Consistent with a defect in cell adhesion in emb30 mutations, our data indicate that EMB30 is required for the normal localization of pectin in the cell wall and that it plays a fundamental role in cell growth throughout plant development.
RESULTS
EMB30 Is Required for Organized Cell Growth of Undifferentiated Tissue
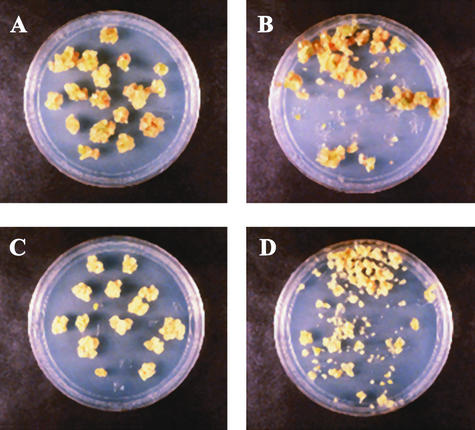
emb30 has been identified as a gene involved in embryogenesis and pattern formation (Meinke, 1985; Mayer et al., 1993; Shevell et al., 1994). Little is known, however, about how EMB30 functions in pattern formation and whether EMB30 is required in other stages of development. To investigate whether EMB30 is required for the growth of undifferentiated as well as differentiated tissue, we cultured emb30 mutants on callus-inducing medium (CIM). On CIM, wild-type Arabidopsis seedlings and most emb30 seedlings produced undifferentiated tissue or callus. As shown in Figures 1A and 1B, the wild-type and emb30 calluses appeared similar, except that the emb30 calluses grew more slowly and were extremely friable, crumbling and separating easily when handled with forceps. When we compared the friability of wild-type and mutant calluses by subjecting them to agitation, the emb30 calluses crumbled, whereas the wild-type calluses remained mostly intact (Figure 2).
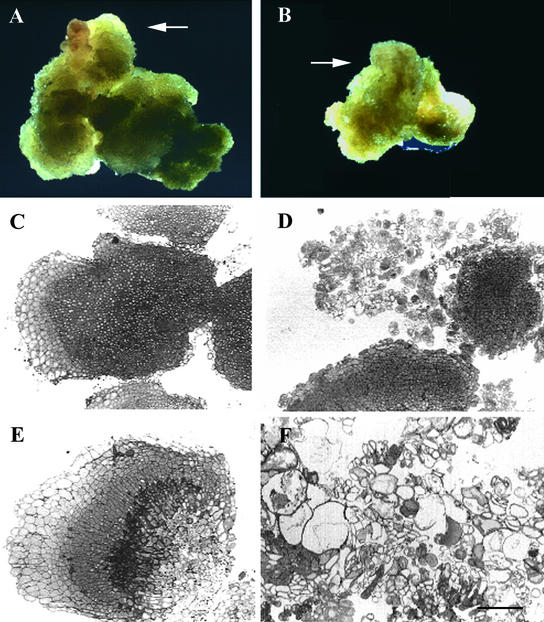
Figure 1.
EMB30 Is Required for Normal Growth of Callus.
Seeds were germinated on MS medium (see Methods); 14-day-old seedlings were transferred to CIM and harvested 29 days later. Results were similar for seedlings germinated directly on CIM (data not shown).
(A) and (B) Wild-type and emb30-3 calluses, respectively. Arrows point to one of several areas of callus growth.
(C) Wild-type Wassilewskija (Ws) callus.
(D) emb30-4 callus.
(E) Wild-type Ws callus.
(F) emb30-3 callus.
The emb30 sections shown have weak (D) or severe (F) phenotypes representative of all the emb30 alleles examined (emb30-1, emb30-3, and emb30-4). (A) and (B) are at the same magnification (×4); (C) to (F) are at the same magnification.  .
.
Figure 2.
emb30 Mutant Calluses Are More Friable Than Those of Wild Type.
Calluses were subjected to agitation as described in Methods.
(A) and (B) Wild-type Ws before and after treatment, respectively.
(C) and (D) emb30-3 before and after treatment, respectively.
Other differences between wild-type and emb30 calluses were very apparent in sections of the calluses. A wild-type callus usually consists of undifferentiated but organized cells, containing at least three zones of relatively small, compact cells that are distinguishable by different sizes (Figures 1C and 1E). Results from three different emb30 alleles showed that although some emb30 calluses consisted of mainly small, compact cells, the cells often were not organized into distinguishable zones (Figure 1D). These emb30 calluses also contained larger cells that separated from one another. We frequently observed emb30 calluses with cells ranging in size from small to gigantic (Figure 1F). This mutant tissue was completely disorganized; cells were not compact and often did not adhere to each other (Figure 1F). These extremely disorganized, abnormally shaped cells were never observed in wild-type calluses. These results demonstrate that EMB30 is essential for normal cell expansion and cell–cell adhesion in undifferentiated callus.
Cellular Morphogenesis and Organ Differentiation in emb30 Mutants
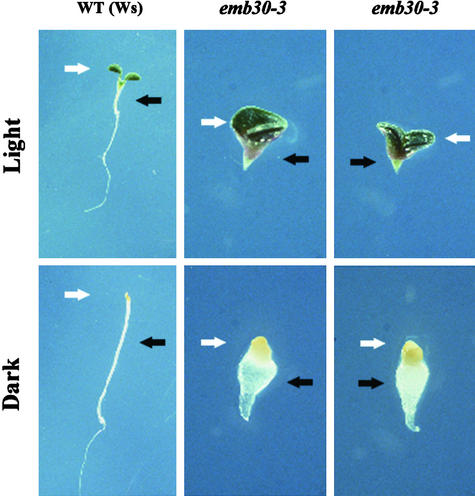
Several seedling-lethal developmental mutants are defective in photomorphogenesis (Castle and Meinke, 1994; Mayer et al., 1996). Therefore, we compared the growth of light- and dark-grown wild-type and emb30 seedlings. The light-grown wild-type seedlings had large, expanded, green cotyledons and short hypocotyls, whereas the wild-type cotyledons grown in the dark remained small and unexpanded, and the hypocotyls were specifically elongated (Figure 3). Comparing light- and dark-grown emb30 mutants clearly showed much less cotyledon growth in dark-grown emb30 mutants (Figure 3). Previously, we noted that the region beneath the cotyledons in light-grown emb30 mutants did not resemble a wild-type hypocotyl and that at the cellular level, emb30 hypocotyl epidermal and cortex cells were abnormally shaped and disorganized (Shevell et al., 1994). Yet strikingly, compared with the light-grown seedlings, dark-grown emb30 mutants had greatly enlarged hypocotyl regions (Figure 3). The expansion of tissue in darkness is a well-defined feature of hypocotyls (Gendreau et al., 1997). In the dark, wild-type hypocotyls specifically elongated longitudinally, but dark-grown emb30 hypocotyl cells expanded in both width and length. This difference between wild-type and emb30 hypocotyls may reflect a lack of control over the direction of cell expansion in emb30 mutants. Overall, light perception appears to be normal in emb30 seedlings, but execution of the precise cellular morphogenic response is impaired.
Figure 3.
Response of emb30 Mutants to Light or Darkness.
Wild-type and emb30-3 seedlings grown in 16-hr-light/8-hr-dark periods or in the dark for 7 days. The light-grown seedlings are green; the dark-grown seedlings are white-yellow. Magnification of wild-type Ws seedlings is ×2; that of emb30-3 seedlings is ×4. The white arrows point to the cotyledons; the black arrows point to the hypocotyls. Similar results were obtained with emb30-1 (data not shown).
We also observed that below the expanded hypocotyl of the dark-grown emb30 mutants was a region of plant tissue that did not expand (Figure 3). This tissue was positioned at where the root should have been formed, but its cellular and tissue morphology was completely different from that of roots (Figure 3; data not shown). Thus, emb30 mutants appear to have the ability to differentiate all of the apical–basal regions but are defective in root formation.
Position-Dependent Tissue Differentiation Is Affected in emb30 Leaves
To determine whether emb30 seedlings could be induced to form organs such as roots, leaves, and flowers, we grew the mutants on root-inducing medium and shoot-inducing medium (SIM) and compared them with wild-type seedlings grown under the same conditions. Previously, Baus et al. (1986) had shown that emb30 mutants could not be induced to form roots; our results were similar (data not shown). Without any hormones in the medium, a few emb30 mutants formed a few leaflike structures (data not shown). On SIM, the less severe mutants formed many more of these leaflike structures but no inflorescence stems or flowers (Figure 4B). The leaflike structures were rounder and much smaller than wild-type leaves, but they produced trichomes, which occur only on true leaves in Arabidopsis and not on cotyledons. The emb30 trichomes were fewer than wild-type trichomes, were found primarily on the edge of the leaf blade, and were unbranched (data not shown), whereas wild-type trichomes usually have three branches.
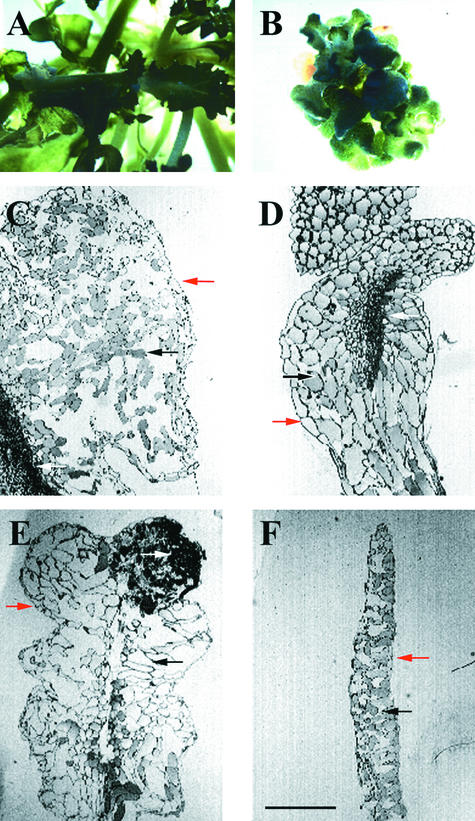
Figure 4.
Wild-Type and emb30 Leaves on SIM.
Phenotypes similar to those shown were observed in all emb30 alleles examined (emb30-1, emb30-2, emb30-3, and emb30-4). Red arrows point to epidermal cells; black arrows point to mesophyll cells; white arrows point to vascular-like cells.
(A) and (B) Wild-type and emb30-1 leaves, respectively, grown on SIM.
(C) emb30-2 leaf.
(D) emb30-1 leaf.
(E) emb30-4 leaf.
(F) Wild-type leaf.
(A) and (B) are at the same magnification (×3); (C) to (F) are at the same magnification. 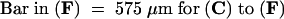 .
.
emb30 leaves (Figures 4C to 4E) were much thicker than wild-type leaves (Figure 4F); sometimes the expanded leaf space was filled with greatly enlarged mesophyll cells (Figures 4D and 4E). emb30 mesophyll cells were often two to four times larger than the largest wild-type mesophyll cells. In other specimens, the mesophyll cell density was much less than normal, and a large amount of space was present between emb30 mesophyll cells (Figure 4C). In addition, vascular cells in emb30 leaves were abnormally shaped, very disorganized, and much more numerous. In some cases, parts of the leaflike structures were composed primarily of vascular-like cells (Figure 4E) that contained tracheid-like cells (as distinguished by their characteristic cell wall). These data indicate that in plants grown on SIM, position-dependent cell differentiation and organ formation can be uncoupled in emb30 leaves.
Localization of Pectin and Other Polysaccharide Markers in emb30 Mutants
Analysis of emb30 callus, seedlings, and leaves indicates a broader role for emb30 in maintaining cell shape and cell–cell adhesion. Because pectin is thought to be extremely important for cell adhesion (McCann and Roberts, 1991), we used several methods to examine pectin in emb30 mutants. Sections of 5-day-old wild-type and emb30 seedlings were stained with Ruthenium red (RR), a dye commonly used to stain pectin (Jensen, 1962). RR stains tissues pink (less pectin) to red (more pectin). In wild-type cotyledons, most of the RR staining was seen in the vascular cells, which were pink (Figure 5A). In contrast, in emb30 mutants, the mesophyll tissue as well as the vascular tissue was stained (Figure 5A), and the staining was much stronger (bright red).
Figure 5.
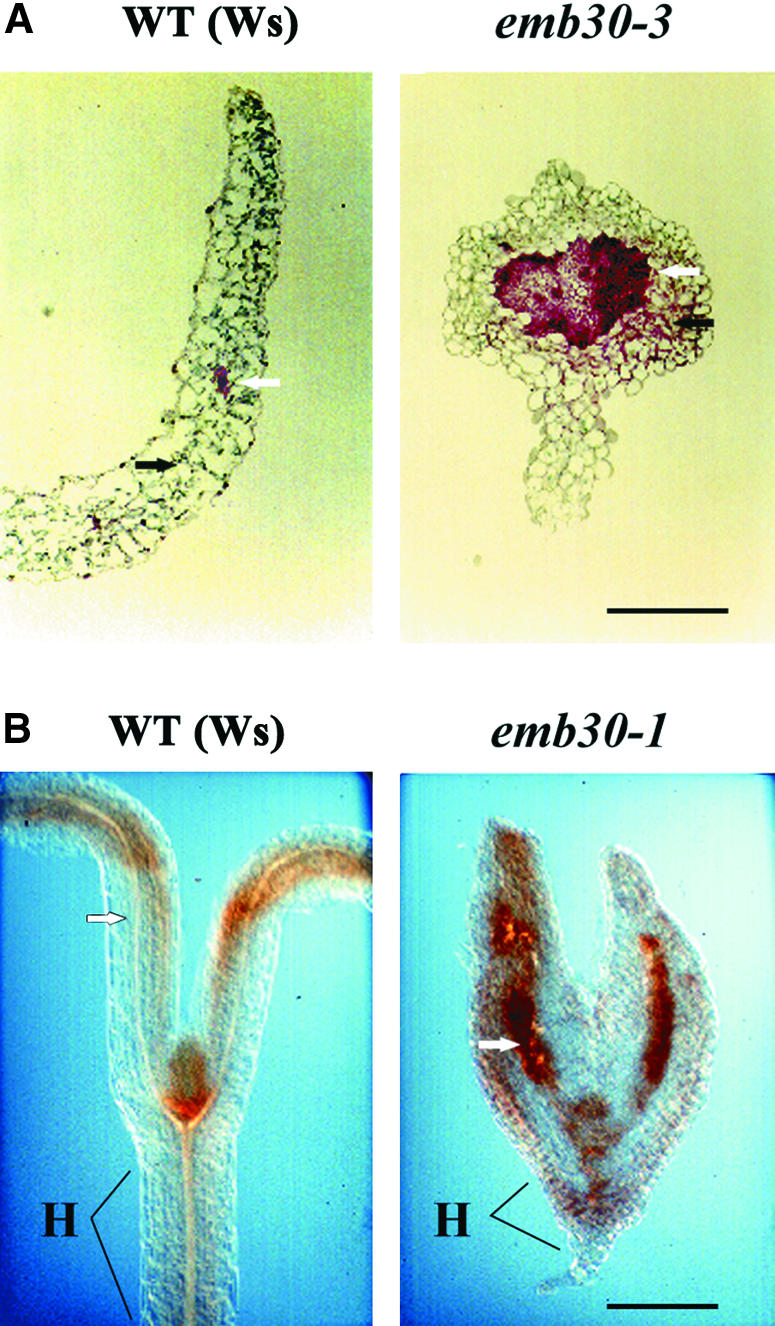
Histochemical Localization of Pectin in Wild-Type and emb30 Seedlings.
(A) RR-stained sections.
(B) HF-AC–stained sections of tissue pretreated with hot acidic methanol (see Methods).
Black arrows point to mesophyll cells; white arrows point to vascular cells. emb30-1 and emb30-3 seedlings were each examined with both types of stains (this figure and data not shown).
H, hypocotyl; WT, wild type. 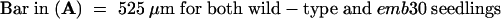 ; b
; b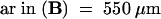 for both wild-type and emb30 seedlings.
for both wild-type and emb30 seedlings.
RR, however, may not be entirely specific for pectin, and it stains only relatively large amounts of the polysaccharide. To confirm the above results, therefore, we used a hydroxylamine–ferric chloride (HA-FC) solution that reacts with methylated pectins to produce a brown stain (Reeve, 1959). Because this reaction recognizes only methylated pectins, we examined both untreated seedlings and those that had been pretreated with a solution to methylate pectic acids. Both untreated and methylated seedlings showed the same pattern of staining, although staining was much stronger in the methylated tissues (data not shown). HA-FC stained the cell walls in both the wild-type and mutant seedlings, but in every case, staining was more intense in the emb30 seedlings (Figure 5B). In particular, vascular tissue and the hypocotyl region of emb30 mutants stained much more strongly than did the same tissue in wild-type seedlings.
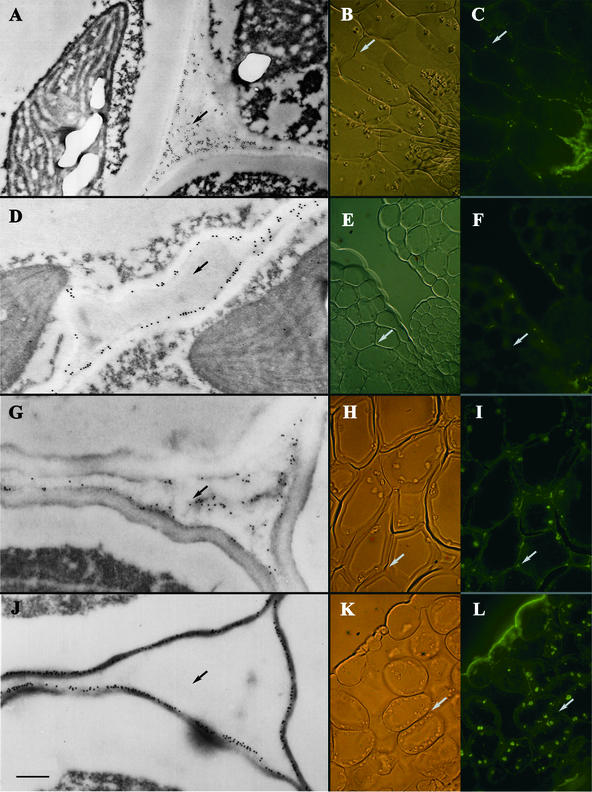
To further confirm that we were observing pectin staining and to more carefully examine the localization of pectin in emb30 cells, we utilized polyclonal and monoclonal antibodies. In all the experiments described below, we focused on comparing mesophyll cells in cotyledon tissue because mesophyll cells are abundant and have a relatively simple staining pattern with low background. Given space limitations, only a few pictures that represent the key results for each antibody experiment are shown. The conclusions, however, are based on the analysis of many cells from each antibody experiment. Even without antibody staining, electron microscopy immediately revealed a difference between wild-type and emb30 tissues. In wild-type tissues, cells secreted substances only into the corner junctions between cells (cell corners) (Figures 6J and 8C). Most of the interstitial space between the wild-type cells remained clear. In emb30 tissues, however, the entire interstitial space, as well as the cell corners, often contained secreted material (Figures 6G and 8D). The PGA/RG-I antibody is a polyclonal antibody that reacts with both rhamnogalacturonan I (RG-I) and polygalacturonic acid (PGA), structural domains of acetic pectic polysaccharides found in the middle lamella (Moore et al., 1986; Lynch and Staehelin, 1992). In immunofluorescence (IF) and immunoelectron microscopy (IEM) experiments, the staining pattern with PGA/RG-I antibody clearly differed between emb30 and wild-type seedlings (Figures 6G to 6L). This antibody primarily stained the cell wall in wild-type seedlings, but in emb30 seedlings it heavily stained not only the cell wall but also the cell corners and the material in the interstitial space between the cells.
Figure 6.
Immunostaining of emb30-3 and Wild-Type Cotyledon Sections with JIM5 and PGA/RG-I Antibodies.
(A) to (C) JIM5 staining of emb30-3 section. JIM5 stains the cell wall and often, in emb30-3 mutants, the interstitial space between cells.
(D) to (F) JIM5 staining of wild-type sections.
(G) to (I) PGA/RG-I antibody staining of emb30-3 sections. PGA/RG-I antibody also stains the cell wall and, in emb30-3 sections, the interstitial space between cells.
(J) to (L) PGA/RG-I antibody staining of wild-type sections.
(A), (D), (G), and (J) show IEM of the sections. (B), (E), (H), and (K) show Nomarski optics and (C), (F), (I), and (L) show IF microscopy of the same sections, respectively. Arrows point to the interstitial space between cells. 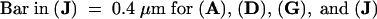 .
.
The monoclonal antibody JIM5 recognizes de-esterified pectin with decreasing sensitivity to pectin containing as much as 50% esterification (Knox et al., 1990). The IF and IEM results with JIM5 were similar to those obtained with the PGA/RG-I antibody (Figures 6A to 6F). In wild-type cotyledons, JIM5 primarily stained the cell wall, whereas in emb30 cotyledons, it also strongly stained the interstitial spaces between the cell walls. JIM7, a monoclonal antibody that reacts well with pectins containing 35 to 90% esterification (Knox et al., 1990), stained neither wild-type nor emb30 cotyledon tissue well (data not shown). Interestingly, neither JIM5 nor JIM7 reacts with RG-I or RG-II (Knox et al., 1990).
The monoclonal antibody CCRC-M2 reacts with RG-I, but the epitope structure is not yet known (Freshour et al., 1996). Previous analysis of CCRC-M2 indicated that it stains certain cell types in roots but does not stain shoots. CCRC-M2 did not react well with wild-type or mutant tissue (data not shown).
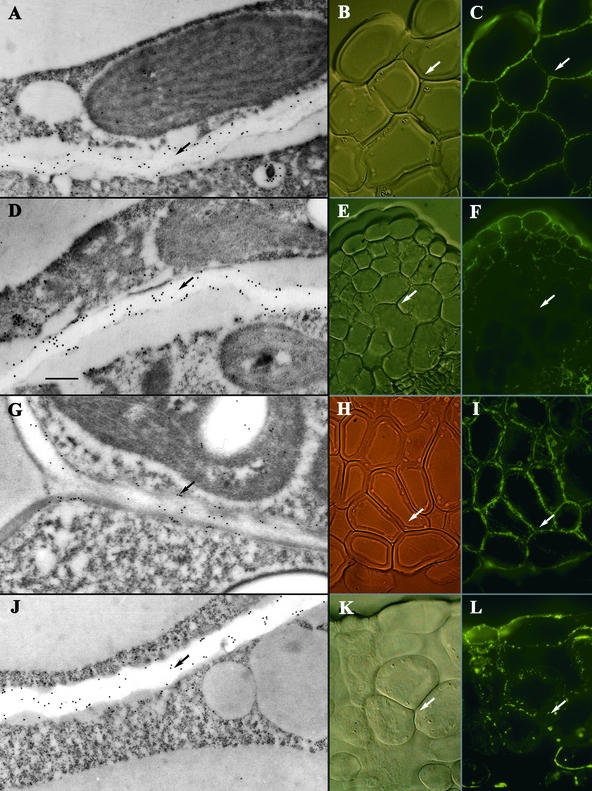
CCRC-M1 is a monoclonal antibody that reacts with a terminal (1→2)-linked α-fucosyl residue, which exists in most dicot cell wall xyloglucans and is also present to a small extent in some species of RG-I (Puhlmann et al., 1994). In IEM studies, CCRC-M1 stained Golgi vesicles, vesicles in transit to the cell wall, and to a lesser extent, the cell wall itself. The localization of CCRC-M1 epitopes was similar in wild-type and emb30 mutants in that staining was observed in the cell wall in both IF and IEM experiments (Figures 7A to 7F). No obvious difference in the amount of staining was detected in IEM experiments.
Figure 7.
Immunostaining of emb30-3 and Wild-Type Cotyledon Sections with CCRC-M1 and CCRC-M7 Antibodies.
(A) to (C) CCRC-M1 staining of emb30-3 sections.
(D) to (F) CCRC-M1 staining of wild-type sections. emb30-3 and wild-type sections show a similar pattern of staining with CCRC-M1.
(G) to (I) CCRC-M7 staining of emb30-3 sections.
(J) to (L) CCRC-M7 staining of wild-type sections. emb30-3 and wild-type sections show a similar pattern of staining with CCRC-M7.
(A), (D), (G), and (J) show IEM of sections. Arrows point to the cell wall. (B), (E), (H), and (K) show Nomarski optics and (C), (F), (I), and (L) show IF microscopy of the same sections, respectively. In (B), (C), (E), (F), (H), (I), (K), and (L), arrows point to the interstitial space between cells. 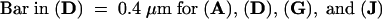 .
.
Studies with the CCRC-M7 monoclonal antibody primarily showed staining of vesicles in transit to the cell wall and in the area of the plasma membrane and cell wall. CCRC-M7 recognizes an arabinosylated (1→6)-linked β-galactan epitope that exists in both RG-I and arabinogalactan proteins (Puhlmann et al., 1994). The pattern of staining was similar in wild-type and emb30 cotyledons (Figures 7G to 7L).
To determine whether emb30 had an unusual cell wall polysaccharide structure, the total cell wall carbohydrate composition of emb30 and wild-type seedlings was analyzed by preparing both alditol acetate and trimethylsilyl derivatives of the cell wall sugars. No obvious difference was observed (E. Zablackis, personal communication). These analyses, however, would not have detected subtle differences in polysaccharide composition.
Localization of Lectin Reporter Molecules in emb30 Mutants
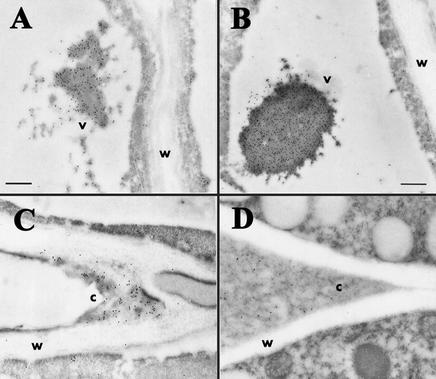
Several factors—the phenotype of emb30 mutants, our results showing that EMB30 can complement a sec7 mutant (data not shown), and the findings of Steinmann et al. (1999) that EMB30 can complement a gea1 gea2 double mutant—suggest that EMB30 may function in the plant secretory pathway, which is vital for the biosynthesis and localization of glycoproteins and cell wall complex polysaccharides. To determine whether the lack of EMB30 has a general effect on the transport or localization of glycoproteins, we examined the localization of two marker molecules (Matsouka et al., 1995): barley lectin fused to a C-terminal propeptide (CTPP) sequence known to direct proteins to vacuoles (referred to as CTPP+ lectin), and barley lectin without a vacuolar targeting signal sequence (CTPP− lectin). The CTPP− lectin molecules are often secreted, although some are still transported to the vacuole. IEM experiments showed that in wild-type plants, the CTPP+ lectin is localized to the vacuole, whereas the CTPP− lectin is both secreted outside of the cells and found in vacuoles, as illustrated in Figures 8A and 8C. Likewise, in plants homozygous for emb30-3 and a marker gene, the CTPP+ lectin is primarily localized to the vacuole, and the CTPP− lectin is found both in vacuoles and outside of the plant cells (Figures 8B and 8D). As expected, CTPP− lectin was secreted to the cell corners in wild-type seedlings but was sometimes secreted throughout the interstitial space between emb30 cells. The finding that the CTPP− lectin is found not only in cell corners but also in interstitial spaces between cells in emb30 mutants probably reflects the general defect of the mutants to restrict secreted material to cell corners, whereas secretion per se of the glycoprotein outside of the cell is not affected.
Figure 8.
IEM with an Antibody That Recognizes Lectin.
(A) Wild-type Ws seedling expressing CTPP+ lectin.
(B) emb30-3 seedling expressing CTPP+ lectin.
(C) Wild-type seedling expressing CTPP− lectin.
(D) emb30-3 seedling expressing CTPP− lectin.
c, cell corner; v, vacuole; w, cell wall. (A), (C), and (D) are at the same magnification. 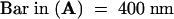 ;
; 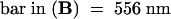 .
.
DISCUSSION
EMB30 Is Required for the Normal Growth of Callus
We have shown here that emb30 is required for the normal growth of undifferentiated tissue. Despite reports that emb30 mutations do not affect the growth of callus (Busch et al., 1996), our studies of three different emb30 alleles in two different ecotypes clearly show that EMB30 is essential for normal cell expansion and cell adhesion in callus. Cell division, expansion, and adhesion are also abnormal in emb30 leaves in comparison with wild-type leaves, when both were induced on SIM. In many emb30 leaves, the mesophyll cell density was reduced, and sometimes a lobe of a leaf was filled entirely with vascular tissue, indicating a loss of position-dependent tissue differentiation. These findings suggest that EMB30 plays a vital role in normal cell growth and organization throughout plant development.
Cell Differentiation, Morphogenesis, and Tissue Organization in emb30 Mutants
We showed that emb30 mutants contain a region below the cotyledons that does not resemble a hypocotyl in terms of overall tissue or cellular morphology but responds like a hypocotyl to darkness by expanding. The expansion of tissue in the dark is one of the best defined traits of hypocotyls (Gendreau et al., 1997), and this result indicates that in the hypocotyls of Arabidopsis seedlings, cellular differentiation can be uncoupled from cellular and tissue morphogenesis. This finding is consistent with the analysis of the raspberry mutant, which also showed that cell differentiation can occur in the absence of organ formation during early embryogenesis in Arabidopsis (Yadegari et al., 1994).
The one morphological characteristic that all emb30 mutants share is the lack of a root. emb30 mutants also fail to produce an inflorescence bolt in tissue culture–grown plants. The defect in polarized cell growth and the lack of a well-developed vascular system in emb30 mutants may prevent the formation of these organs. Alternately, EMB30 could be required mainly for root development, and another protein could provide an activity similar to that of EMB30 in cotyledons and hypocotyls but not in roots. We previously reported that DNA gel blot hybridization studies suggest the EMB30 family may have a possible second member (Shevell et al., 1994). Recently, a search of Arabidopsis genes using BLAST (Altschul et al., 1997) revealed a putative protein on chromosome 5 that has 57 to 74% similarity to EMB30 over the length of the protein and 60 to 72% similarity at the DNA level. The putative EMB30 homolog was sequenced as part of the Arabidopsis Genome Project (GenBank accession number AB009054).
Pectin Localization in emb30 Mutants
Experiments examining the regeneration of cell walls on carrot protoplasts have indicated that a correctly assembled pectin network is important for the subsequent deposition of cellulose. In addition, the local organization of the cell wall may depend in part on the amounts of pectin and of pectin methylesterase activity (McCann and Roberts, 1991; Stephenson and Hawes, 1994). Interestingly, Liners et al. (1994) observed that the friability of sugar beet callus is correlated with an increase in acetylation of its pectin.
Electron microscopy experiments showed that emb30 mutants often fail to restrict secreted material to cell corners. In addition, IF and IEM experiments with a polyclonal antibody that recognizes PGA and RG-I (PGA/RG-I antibody) and a monoclonal antibody against de-esterified pectin but not RG-I or RG-II (JIM5) showed staining not only of cell walls (the wild-type phenotype) but also of the cell corners and sometimes of the interstitial spaces of emb30 tissues, suggesting that pectin was being secreted outside of the cell wall in emb30 mutants. The abnormal localization of pectin could explain the cell adhesion defect observed in emb30 mutants. The fact that EMB30 can substitute for some of the Sec7p and Gea functions and has ARF GEF activity suggests that EMB30 may play a role in the secretory pathway. Pectin is sequentially synthesized in different parts of the Golgi, and the disruption of vesicle transport between the different Golgi compartments could result in the production of altered forms of pectin that might not function correctly in the cell wall or might tend to be secreted. Alternately, EMB30 could be involved in the proper localization of vesicles containing pectin or pectin-altering enzymes. Pectins delivered to the cell wall at the wrong place or time or in abnormal amounts might not integrate correctly into the cell wall. Interestingly, this defect in localization was not observed with a monoclonal antibody (CCRC-M1) recognizing a terminal (1→2)-linked α-fucosyl residue primarily associated with xyloglucans or with a monoclonal antibody (CCRC-M7) reacting with an arabinosylated (1→6)-linked β-galactan epitope found on RG-I and arabinogalactan proteins, which suggests that this defect does not extend to all polysaccharides. The finding that the lectin marker molecules for transport to the vacuole and for transport outside of the cell were correctly localized in emb30 mutants indicates that the emb30 mutation does not result in a general secretory defect. In yeast, evidence for specificity in transport pathways is provided by yeast Ts sec21 mutants and biochemical experiments, which show there can be cargo-selective blocks in protein transport (Campbell and Schekman, 1997; Gaynor and Emr, 1997).
In contrast to a previous hypothesis suggesting that EMB30 plays a specific role in establishing the asymmetric division of the zygote (Mayer et al., 1993), our results indicate that EMB30 has a broader but critically important function in the polarity and organization of cell growth. Recently, the polar localization of the putative auxin efflux carrier PIN1 has been shown to be defective in emb30 embryos (Steinmann et al., 1999). Also, emb30 embryos show variable expression of the apical marker gene LTP (Vroemen et al., 1996). Whether these defects are directly or indirectly due to emb30 mutations is not clear. EMB30 could be directly required for the vesicular trafficking of PIN1. However, the polar localization of PIN1 first occurs at the midglobular stage of embryogenesis, whereas emb30 mutants already show a defect at the zygote stage, indicating that EMB30 performs a role in polarity independently of PIN1.
The simplest model based on our studies is that a cell wall defect may be the primary cause of the emb30 mutant phenotypes, although we cannot rule out the possibility of other secretory-related defects in emb30 mutants. The abnormal synthesis or mislocalization (or both) of pectin could explain the increase in emb30 friability. A lack of cell–cell communication brought about by the emb30 defect in cell adhesion could account for other emb30 phenotypes. If the cells are not adhering to each other properly, a cell may not receive all the necessary signals for normal cell division and position-dependent tissue differentiation. Alternately, the emb30 abnormalities could be explained by a general structural defect in emb30 cell walls. Targeted secretion is essential for the proper formation of the plant cell wall, which in turn is critical for normal cell division, cell expansion (polar and nonpolar), and cell adhesion. In either case, an incorrectly formed cell wall might interfere with the normal asymmetric division of the zygote and later prevent polar localization of PIN1. The subsequent lack of auxin transport could affect bolt and root formation and result in the increased number of vascular cells observed in emb30 mutants. Indeed, a defect in auxin transport in emb30 mutants has been proposed, based on the ability of inhibitors of auxin transport to phenocopy the emb30 morphogenesis defects in Brassica juncea embryos (Liu et al., 1993; Shevell et al., 1994).
The cell wall plays a key role in determining cell polarity and cell fate in the brown alga Fucus, where targeted secretion of molecules into the rhizoid cell wall may provide positional information for determining the orientation of the plane of the first cell division (reviewed in Belanger and Quatrano, 2000). In other studies on multicellular Fucus embryos, cell wall contact between cells was necessary for maintaining cell polarity (Bouget et al., 1998). The function of the cell wall in higher plant development is not as well elucidated but is likely to play a similar role. Our studies were conducted on seedlings. Although difficult, looking for a cell wall defect in the emb30 zygote or egg would be interesting. Further studies, including the subcellular localization of EMB30, should provide additional molecular insights into the role of EMB30 in cell wall formation and development.
METHODS
Plant Lines and Growth Conditions
The emb30-1 and emb30-2 (both ecotype Columbia [Col-0]) and emb30-3 and emb30-4 (both ecotype Wassilewskija [Ws]) mutants were described previously (Meinke, 1985; Shevell et al., 1994). The plants containing the C-terminal propeptide (CTPP)+ lectin or CTPP− lectin constructs were provided by Natasha Raikhel and were crossed with plants heterozygous for emb30-3. Subsequently, plants homozygous for both the marker constructs and the emb30-3 mutation were assayed for lectin localization. All plants were grown as described by Shevell et al. (1994), except for those in the photomorphogenesis experiments, in which sterilized seed was plated on MS medium (Murashige and Skoog, 1962) with 3% sucrose and exposed to 30 min of light. The plates were then placed in the dark for 7 days (dark-grown) or on a 16-hr-light/8-hr-dark cycle for 7 days (light-grown). To compare the responses of wild-type (Col-0 and Ws) and emb30 plants to hormones, we germinated seeds directly on media containing hormones, and we transferred 10- to 14-day-old seedlings that had been germinated on MS medium to media containing hormones. To induce callus formation, plants were grown on callus-inducing medium (CIM), which consisted of MS medium with 1% sucrose, 2% glucose, 0.5 mg/L (2,4-dichlorophenoxy)acetic acid, 0.3 mg/L kinetin, and 5 mg/L indole acetic acid. Shoot-inducing medium (SIM) contained MS medium with 1% sucrose, 2% glucose, 0.15 mg/L indole acetic acid, and 5.0 mg/L 2-isopentenylaminopurine. Root-inducing medium contained MS medium with 1% sucrose, 2% glucose, 12 mg/L 4-(3-indolyl)butyric acid, and 0.1 mg/L kinetin.
Assay for Friability
To qualitatively assess the difference in friability between emb30 and wild-type calluses, Petri dishes in which the calluses were growing were taped to a rack and the rack was then manually struck against a firm surface three to five times. Wild-type and mutant calluses were tested together on the same rack, and only Petri dishes attached to the same rack were compared, to control for any variation in the amount of agitation. The experiment was repeated three times, and the results were similar in all experiments.
Microscopy
Wild-type (Col-0 and Ws) and emb30 seedlings grown under different conditions, as described above, were carefully harvested, fixed, postfixed with osmium tetroxide, and further processed according to the electron microscopy protocol described by Patton and Meinke (1990). The Ruthenium red (RR) staining was performed on paraffin sections of seedlings, as described by Jensen (1962), except that sections were stained for 2 to 3 hr. The hydroxylamine–ferric chloride reaction (HA-FC) was performed with fresh seedlings that had been fixed and sectioned by hand, as described by Reeve (1959). Some of the sections were pretreated with hot acidified methanol before staining with HA-FC, also described by Reeve (1959). In addition to relying on different chemical reactions, the RR and HA-FC staining procedures use very different fixation techniques and cannot be compared directly. The antibody against Triticum vulgaris lectin was from Sigma; the polyclonal antibody against PGA/RG-I was provided by Andrew Staehelin; the JIM5 and JIM7 monoclonal antibodies by Keith Roberts; and the CCRC-M1, CCRC-M2, and CCRC-M7 monoclonal antibodies by Michael Hahn. As controls, the primary antibodies were omitted from immunolabeling experiments. Immunoelectron microscopy experiments with all of these antibodies followed the procedures described by Moore et al. (1986). Although other fixation procedures were tried, the emb30 sections were poorly preserved under other conditions that well preserved the wild-type samples (D.E. Shevell and N.-H. Chua, unpublished results).
For immunofluorescence studies, 5-day-old seedlings were fixed in 4% paraformaldehyde/0.2% glutaraldehyde and embedded in HistoResin (Jung, Germany), according to the manufacturer's instructions. Sections 5 to 8 μm thick were attached to slides. The slides containing the sections were incubated for 15 min at 42°C, and the uncovered glass area was silanized. The following steps were performed at room temperature with constant agitation. Samples were incubated in blocking solution (PBS with 1% BSA [w/v; Sigma]) for 1 hr and then washed two times for 5 min with PBS. The slides were then incubated with the primary antibody (diluted 1:10 in blocking solution) for 90 min and washed three times with PBS. The biotinylated secondary antibody (anti–rat or anti–mouse antibody; Vector Laboratories, Burlingame, CA) was dissolved in PBS at 10 μg/mL and incubated with the slides for 2.5 hr; the slides were then washed twice with PBS and once with a solution of 0.1 M sodium bicarbonate and 0.15 M NaCl, pH 8.2 (SB). To detect the antibody–antigen complexes, the slides were keep in the dark and incubated with fluorescein aviden D (Vector Laboratories; dissolved in SB at 33 μg/mL) for 90 min, and then washed three times with PBS containing 0.05% Tween-20 (v/v). The samples were mounted with Citifluor (Ted Pella, Inc., Redding, CA) and photographed with an Axioskop microscope (Zeiss) equipped with a Nikon UFX camera.
Acknowledgments
We thank Natasha Raikhel for providing us with the CTPP+ and CTPP− lectin plant lines and Andrew Staehelin for sending us antibody to PGA/RG-I. We are grateful to Michael Hahn and Keith Roberts for the CRCC and JIM antibodies, respectively. We also thank Helen Shio and Chun-Hai Dong for expert technical assistance with the microscopy experiments. We especially thank Earl Zablackis for performing the cell wall analysis experiments and for sharing his data. We are grateful to Michael Hahn, Particia Moore, Natasha Raikhel, and Andrew Staehelin for useful discussions and to Ulrich Klare, Benedikt Kost, and Robert McGrath for helpful discussions and careful reading of the manuscript. We also thank an anonymous reviewer for helpful suggestions. This work was supported by National Science Foundation Grant No. 9420038 to D.E.S., and N.-H.C. T.K. was supported by a Human Frontiers in Science Program organization grant.
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baus, A.D., Franzmann, L., and Meinke, D.W. (1986). Growth in vitro of arrested embryos from lethal mutants of Arabidopsis thaliana. Theor. Appl. Genet. 72, 577–586. [DOI] [PubMed] [Google Scholar]
- Belanger, K.D., and Quatrano, R.S. (2000). Polarity: The role of localized secretion. Curr. Opin. Plant Biol. 3, 67–72. [DOI] [PubMed] [Google Scholar]
- Bouget, F., Berger, F., and Brownlee, C. (1998). Position dependent control of cell fate in the Fucus embryo: Role of intercellular communication. Development 125, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Busch, M., Mayer, U., and Jürgens, G. (1996). Molecular analysis of the Arabidopsis pattern formation gene GNOM: Gene structure and intragenic complementation. Mol. Gen. Genet. 250, 681–691. [DOI] [PubMed] [Google Scholar]
- Campbell, J.L., and Schekman, R. (1997). Selective packaging of cargo molecules into endoplasmic reticulum–derived COPII vesicles. Proc. Natl. Acad. Sci. USA 94, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle, L.A., and Meinke, D.W. (1994). A FUSCA gene of Arabidopsis encodes a novel protein essential for plant development. Plant Cell 6, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin, P., Paris, S., Antonny, B., Robineau, S., Béraud-Dufour, S., Jackson, C.L., and Chabre, M. (1996). A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature 384, 481–484. [DOI] [PubMed] [Google Scholar]
- Driouich, A., Faye, L., and Staehelin, L.A. (1993). The plant Golgi apparatus: A factory for complex polysaccharides and glycoproteins. Trends Biochem. Sci. 18, 210–214. [DOI] [PubMed] [Google Scholar]
- Freshour, G., Clay, R.P., Fuller, M.S., Albersheim, P., Darvill, A.G., and Hahn, M.G. (1996). Developmental and tissue-specific structural alterations of the cell wall polysaccharides of Arabidopsis thaliana roots. Plant Physiol. 110, 1413–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, R.C., and Emr, S.D. (1997). COPI-independent anterograde transport: Cargo-selective ER to Golgi protein transport in yeast COPI mutants. J. Cell Biol. 136, 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau, E., Traas, J., Desnos, T., Grandjean, O., Caboche, M., and Hofte, H. (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 114, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke, C.S., and Berleth, T. (1998). The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 17, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, W.A. (1962). Pectic substances. In Botanical Histochemistry: Principles and Practice (San Francisco: W.H. Freeman), pp. 201–202.
- Knox, J.P., Linstead, P.J., King, J., Cooper, C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. [DOI] [PubMed] [Google Scholar]
- Liners, F., Gaspar, T., and Van Cutsem, P. (1994). Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: Consequences for intercellular adhesion. Planta 192, 545–556. [Google Scholar]
- Liu, C.-M., Xu, Z.-H., and Chua, N.-H. (1993). Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69. [DOI] [PubMed] [Google Scholar]
- Lukowitz, W., Mayer, U., and Jürgens, G. (1996). Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84, 61–71. [DOI] [PubMed] [Google Scholar]
- Lupashin, V.V., Hamamoto, S., and Schekman, R.W. (1996). Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J. Cell Biol. 132, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M.A., and Staehelin, L.A. (1992). Domain-specific and cell type–specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J. Cell Biol. 118, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndon, R.F. (1990). Plant Development: The Cellular Basis. (London: Unwin Hyman).
- Matsouka, K., Bassham, D.C., Raikhel, N.V., and Nakamura, K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, R., Raventos, D., and Chua, N.-H. (1996). det1, cop1, and cop9 mutations cause inappropriate expression of several gene sets. Plant Cell 8, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, U., Buttner, G., and Jürgens, G. (1993). Apical–basal pattern formation in the Arabidopsis embryo: Studies on the role of the GNOM gene. Development 117, 149–162. [Google Scholar]
- McCann, M.C., and Roberts, K. (1991). Architecture of the cell wall. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (London: Academic Press), pp. 109–129.
- Meinke, D.W. (1985). Embryo-lethal mutants of Arabidopsis thaliana: Analysis of mutants with a wide range of lethal phenotypes. Theor. Appl. Genet. 69, 543–552. [DOI] [PubMed] [Google Scholar]
- Moore, P.J., Darvill, A.G., Albersheim, P., and Staehelin, L.A. (1986). Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 82, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Patton, D.A., and Meinke, D.W. (1990). Ultrastructure of arrested embryos from lethal mutants of Arabidopsis thaliana. Am. J. Bot. 77, 653–661. [DOI] [PubMed] [Google Scholar]
- Peyroche, A., Paris, S., and Jackson, C.L. (1996). Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature 384, 479–481. [DOI] [PubMed] [Google Scholar]
- Puhlmann, J., Bucheli, E., Swain, M.J., Dunning, N., Albersheim, P., Darvill, A.G., and Hahn, M.G. (1994). Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1-2)-linked fucosyl-containing epitope. Plant Physiol. 104, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve, R.M. (1959). A specific hydroxylamine–ferric chloride reaction for histochemical localization of pectin. Stain Technol. 34, 209–211. [DOI] [PubMed] [Google Scholar]
- Sata, M., Donaldson, J.G., Moss, J., and Vaughan, M. (1998). Brefeldin A–inhibited guanine nucleotide-exchange activity of Sec7 domain from yeast Sec7 with yeast and mammalian ADP ribosylation factors. Proc. Natl. Acad. Sci. USA 95, 4204–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell, D.E., Leu, W.-M., Gillmor, C.S., Xia, G., Feldmann, K.A., and Chua, N.-H. (1994). EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77, 1051–1062. [DOI] [PubMed] [Google Scholar]
- Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C.L., Paris, S., Galweiler, L., Palme, K., and Jurgens, G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286, 316–318. [DOI] [PubMed] [Google Scholar]
- Stephenson, M.B., and Hawes, M.C. (1994). Correlation of pectin methyltransferase activity in root caps of pea with root border cell separation. Plant Physiol. 106, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroemen, C.W., Langeveld, S., Mayer, U., Ripper, R., Jurgens, G., Van Kammen, A., and De Vries, S.C. (1996). Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 8, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadegari, R., de Paiva, G.R., Laux, T., Koltunow, A.M., Apuya, N., Zimmerman, J.L., Fischer, R.L., Harada, J.J., and Goldberg, R.B. (1994). Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell 6, 1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis, E., Huang, J., Müller, B., Darvill, A.G., and Albersheim, P. (1995). Characterization of the cell wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]