Abstract
Phytochromes are primary photoreceptors mediating diverse responses ranging from induction of germination to floral induction in higher plants. We have isolated novel recessive rep1 (reduced phytochrome signaling 1) mutants, which exhibit a long-hypocotyl phenotype only under far-red light but not under red light. Physiological characterization showed that rep1 mutations greatly reduced a subset of phytochrome A–regulated responses, including the inhibition of hypocotyl elongation, cotyledon expansion, modulation of gravitropic growth of hypocotyl, and induction of the CAB (encoding chlorophyll a/b binding protein) gene, without affecting the accumulation of anthocyanin, far-red-preconditioned blocking of greening, induction of germination, and induction of CHS (encoding chalcone synthase) and FNR (encoding ferredoxin-NADP+ oxidoreductase) genes. These results suggest that REP1 is a positive signaling component, functioning in a branch of the phytochrome A signaling pathway. Molecular cloning and characterization of the REP1 gene revealed that it encodes a light-inducible, putative transcription factor containing the basic helix-loop-helix motif.
INTRODUCTION
Plants are equipped with versatile photoperception and signaling systems to incorporate the constant changes of quantity, quality, duration, or direction of the ambient light into their developmental programs (Kendrick and Kronenberg, 1994). Perception of the broad spectrum of light, ranging from UV-B to far red (FR), is mediated by several distinct photoreceptors, including red (R)/FR-absorbing phytochromes, blue/UV-A light–absorbing cryptochromes, phototropins, and UV-B light photoreceptors (Furuya, 1993; Huala et al., 1997; Cashmore et al., 1999). Among these photoreceptors, phytochromes have been best characterized at biochemical, molecular, and physiological levels. Phytochromes, which primarily mediate diverse responses to R light and FR light (Smith, 1995; Fankhauser and Chory, 1997; Deng and Quail, 1999), are dimeric proteins, existing in two photointerconvertible forms, Pr (R light–absorbing phytochrome) and Pfr (FR light–absorbing phytochrome). When irradiated with R light, the Pr form is transformed into the Pfr form; conversely, the Pfr form can be converted back to the Pr form by irradiation with FR light (Butler et al., 1959). This photoreversibility enables phytochromes to function as a molecular light switch. Arabidopsis has five members of the phytochrome gene family, PHYA to PHYE (Sharrock and Quail, 1989; Clack et al., 1994). Whereas PHYA encodes the type I photolabile phytochrome, PHYB to PHYE have been suggested to encode type II photostable phytochromes (Furuya, 1993). Physiological analysis of phytochrome-deficient mutants and transgenic plants overexpressing these phytochromes has demonstrated not only distinct but also overlapping functions for each phytochrome throughout the plant development (Reed et al., 1994; Quail et al., 1995; Furuya and Schäfer, 1996; Whitelam and Devlin, 1997).
Phytochrome A (PhyA) is the primary photoreceptor for the high irradiance response (HIR) to FR light including deetoliation such as inhibition of hypocotyl elongation and cotyledon expansion (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993; Johnson et al., 1994). In addition to its role in deetiolation, PhyA mediates accumulation of anthocyanin (Kunkel et al., 1996) and FR-preconditioned blocking of greening (van Tuinen et al., 1995; Barnes et al., 1996b; Runge et al., 1996). FR light also modulates the gravitropic response of hypocotyl growth and germination (Poppe et al., 1996; Robson and Smith, 1996; Shinomura et al., 1996; Hangarter, 1997). These physiological and developmental changes are often accompanied by the changes in gene expression (Terzaghi and Cashmore, 1995). For example, CAB (encoding chlorophyll a/b binding protein) and CHS (encoding chalcone synthase) are induced (Bowler et al., 1994; Barnes et al., 1996a; Hamazato et al., 1997), whereas others, such as PHYA (encoding phytochrome A) and PORA (encoding protochlorophyllide oxidoreductase A) are repressed by FR light through the action of PhyA (Runge et al., 1996; Canton and Quail, 1999).
Although the structure and function of phytochromes have been extensively characterized (Cherry et al., 1993; Boylan et al., 1994; Xu et al., 1995; Lapko et al., 1997; Yeh and Lagarias, 1998; Hennig et al., 1999; Kircher et al., 1999; Yamaguchi et al., 1999), the downstream signaling mechanisms are poorly understood. Several approaches have been undertaken to identify the components involved in phytochrome signaling. Pharmacological studies have identified several positive components in the PhyA signaling pathway (Romero et al., 1991; Neuhaus et al., 1993; Bowler et al., 1994). The proposed pathway involves heterotrimeric G proteins, cGMP, and calcium/calmodulin, which mediate PhyA-dependent induction of the CAB, CHS, and FNR genes. The yeast two-hybrid screening revealed that several components, including PIF3 (Ni et al., 1998), PKS1 (Fankhauser et al., 1999), and NDPK2 (Choi et al., 1999), interact with both PhyA and PhyB. The different subcellular localization and differential effects of these interacting proteins on phytochrome-dependent responses imply that phytochrome may utilize multiple interacting partners to regulate various photoresponses (Furuya and Kim, 2000; Neff et al., 2000).
Genetic approaches using mutant screening have been fruitful in identifying various components in the phytochrome signaling pathway (Koornneef et al., 1980; Chory et al., 1989; Ahmad and Cashmore, 1996; Wei and Deng, 1996; Lin and Cheng, 1997; Wagner et al., 1997; Genoud et al., 1998). Several genetic components of PhyA signaling have been isolated. The eid1 and spa1 mutants show enhanced FR-HIR (Hoecker et al., 1998, 1999; Büche et al., 2000), whereas far1, fhy1, fhy3, fin2, fin219, pat1, and vlf mutants show defective PhyA-dependent responses (Whitelam et al., 1993; Yanovsky et al., 1997; Soh et al., 1998; Hudson et al., 1999; Bolle et al., 2000; Hsieh et al., 2000). So far, FAR1, FIN219, PAT1, and SPA1 genes have been characterized at the molecular level, but their biochemical functions are not defined (Hoecker et al., 1999; Hudson et al., 1999; Bolle et al., 2000; Hsieh et al., 2000).
To identify downstream components in the PhyA signaling pathway, we screened T-DNA insertional lines and isolated two novel allelic mutants, rep1-1 (reduced phytochrome signaling 1-1) and rep1-2, that showed a long hypocotyl phenotype under FR light but not under R light. Phenotypic analysis revealed that the rep1 mutation abrogated a subset of PhyA-mediated responses, including deetiolation, gravitropic modulation of hypocotyl growth, and induction of the CAB gene, but it did not affect the accumulation of anthocyanin, FR-preconditioned blocking of greening, induction of germination, and induction of CHS and FNR genes by FR light. Our results showed that the REP1 gene encodes a transcription factor containing the basic helix-loop-helix (bHLH) motif and that the corresponding transcript is induced by light. These results suggest that REP1 may function as a positive component in a branch pathway of PhyA signaling in Arabidopsis.
RESULTS
Isolation of rep1 Mutants
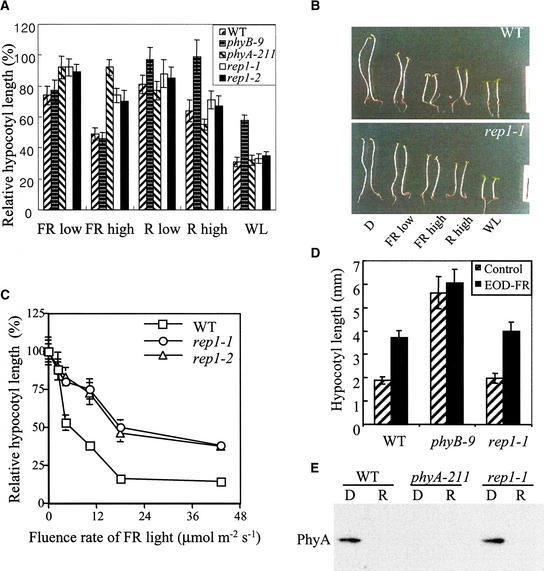
To isolate signaling components in the PhyA signaling pathway, we conducted genetic screening for mutants defective in FR light–induced seedling development. Based on phenotypic criteria of hypocotyl elongation and cotyledon expansion, three mutants insensitive to both R and FR light and five mutants with reduced sensitivity to FR but not to R light were recovered. We focused on two allelic mutants, designated rep1-1 and rep1-2, that had a long-hypocotyl phenotype under FR light but not under R light. First, the hypocotyl elongation phenotypes under various light conditions were examined to test whether the rep1 mutant is defective in PhyA-specific signaling. Wild-type seedlings exhibited a short hypocotyl phenotype when grown under R, FR, or white (W) light. As shown in Figure 1A, the rep1 mutants exhibited a long-hypocotyl phenotype only under FR light, showing a short hypocotyl under R and W light, similar to the phyA-211 mutant. In addition to inhibited hypocotyl elongation, cotyledon expansion was also impaired in the rep1-1 mutant under FR light (Figure 1B). These results suggested that the rep1 mutation affects PhyA signaling, leading to impaired HIR, such as the inhibition of hypocotyl elongation and cotyledon expansion. To test the defects of rep1 mutations in FR-HIR in detail, we examined the fluence rate response for inhibition of hypocotyl elongation. As shown in Figure 1C, compared with the wild type, rep1 mutants showed reduced inhibition of hypocotyl elongation under various fluence rates of FR light. However, the rep1 mutants exhibited partial sensitivity to FR light. To examine whether the rep1 mutation affects the low fluence response, which is primarily regulated by PhyB (Robson et al., 1993), we examined the end-of-day (EOD) FR response. The rep1-1 mutant exhibited normal response to EOD-FR treatment (Figure 1D), suggesting that rep1-1 mutation does not affect the PhyB-mediated low fluence response.
Figure 1.
Morphological and Biochemical Analysis of rep1 Mutant under Various Light Conditions.
(A) Measurement of hypocotyl lengths. The seedlings were grown under various light conditions for 4 days. The hypocotyl lengths of the wild type (WT) and mutants were normalized to their respective hypocotyl lengths in darkness. The average length of the dark-grown seedlings was 13.61, 12.45, 14.20, 12.52, and 13.72 mm for the wild-type, phyB-9, phyA-211, rep1-1, and rep1-2 seedlings, respectively. The fluence rates used were 2.5, 7, 2, 10, and 15 μmol m−2 sec−1 for FR low, FR high, R low, R high, and white light (WL), respectively. Each measurement was performed with at least 25 seedlings. The error bars indicate standard deviations.
(B) Comparison of seedling morphology of the wild type (WT; top) and rep1-1 mutant (bottom) that were grown for 4 days under the light conditions indicated in (A). Note that the cotyledon expansion of the rep1-1 mutant under FR low conditions is partially defective compared with that of the wild type.  .
.
(C) Fluence rate responses of inhibition of hypocotyl elongation under FR light. The seedlings were grown for 4 days under various fluence rates of FR light (0, 2.5, 4.4, 8.8, 19, and 45 μmol m−2 sec−1). The data shown are averages of relative hypocotyl lengths from at least 20 seedlings, normalized to their respective hypocotyl length in darkness. The error bars indicate standard deviations. s, sec; WT, wild type.
(D) End-of-day (EOD) FR responses of the wild type and mutants. The mean hypocotyl lengths from at least 20 seedlings are shown. Hatched bars, no EOD-FR treatments; black bars, EOD-FR treatments. The error bars indicate standard deviations. WT, wild type.
(E) Immunoblot analysis of PhyA. The seedlings were grown in darkness for 4 days (D) and then irradiated with R light, 10 μmol m−2 sec−1, for 6 hr (R). The specificity of the antibody to PhyA was verified by the absence of signal from the phyA-211 mutant.
Genetic Analysis
As shown in Table 1, the phenotypic analysis of F1 heterozygotes and subsequent F2 progeny derived from crossing wild-type plants and rep1-1 mutants indicated that rep1-1 is a single recessive mutation. Complementation tests showed that the rep1-1 mutant is not allelic to phyA, fhy1, or fhy3 mutants. We then performed chromosomal mapping of the rep1 mutation by using cleaved amplified polymorphic sequences (CAPS) markers. In the 30 F2 progenies, the REP1 locus exhibited three recombinant chromosomes with the NCC1 marker but no recombinant chromosomes with the PVV4 marker. This result suggested that the REP1 locus might reside on the upper arm of chromosome 1, closely linked with the PVV4 marker. Because there are no known PhyA signaling mutations around the chromosomal position of REP1, these results indicate that rep1 is a novel mutation involved in PhyA signaling. Given that defects in the photoresponses of the rep1-1 mutant were slightly more severe than those of the rep1-2 mutant, most of the phenotypic analysis presented below was performed with the rep1-1 mutant after two backcrosses with the wild type.
Table 1.
Genetic Analysis of rep1 Mutation
| Cross | Generation | Long | Short | χ2a |
|---|---|---|---|---|
| WT × rep1-1 | F1 | 7 | ||
| rep1-1 × WT | F1 | 9 | ||
| F2 | 49 | 125 | 0.843 (P > 0.1) | |
| rep1-1 × rep1-2 | F1 | 10 | ||
| F2 | 120 | |||
| rep1-1 × phyA-211 | F1 | 8 | ||
| rep1-1 × fhy1 | F1 | 5 | ||
| rep1-1 × fhy3 | F1 | 10 |
χ2 test was based on the expected segregation ratio of 3:1. The phenotypic criterion used was hypocotyl length of seedlings grown under FR light for 4 days. Hypocotyls >8 mm in length were categorized as long hypocotyl.
Immunoblot Analysis
The amount of PhyA expressed is critical for the photosensitivity to FR light (Boylan and Quail, 1991; Whitelam et al., 1993). To examine whether the reduced photosensitivity of the rep1 mutant was due to the presence of less PhyA, we performed immunoblot analysis with antibody against PhyA. In the dark-grown seedlings, the amount of PhyA in the rep1-1 mutant was similar to that in the wild type (Figure 1E). Furthermore, the degradation of PhyA in continuous R light was also normal in the rep1-1 mutant. Thus, the defective photosensitivity of rep1 is not the result of a reduced amount of photoactive PhyA, which suggests that REP1 controls the downstream signaling of PhyA.
Phenotypic Analysis of the rep1 Mutant
Because PhyA regulates diverse responses to FR light in addition to deetiolation (Smith, 1995; Whitelam and Devlin, 1997), we examined various PhyA-dependent responses of the rep1-1 mutant, such as gravitropic hypocotyl growth, anthocyanin accumulation, FR-preconditioned blocking of greening, and germination, to further investigate the roles of REP1 in the PhyA signaling pathway.
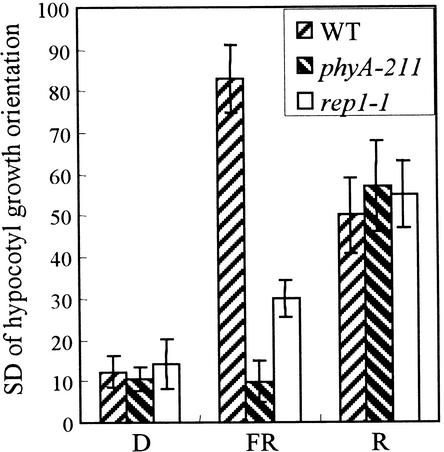
PhyA is shown to reduce negative gravitropism of the hypocotyl (Poppe et al., 1996; Robson and Smith, 1996). Thus, the hypocotyl of wild-type plants grows in a randomized orientation, whereas that of a phyA-211 mutant exhibits growth oriented opposite to the direction of gravity under FR light. In the rep1-1 mutant, the modulation of gravitropism of hypocotyl growth by FR light was partially affected (Figure 2). In contrast, the rep1-1 mutant showed randomized hypocotyl growth under R light, similar to the wild type and phyA-211 mutant. This indicates that REP1 is involved in the randomization of hypocotyl growth only under FR light.
Figure 2.
Gravitropic Response of Hypocotyl Growth.
The seedlings were grown vertically for 4 days in darkness, FR light (7 μmol m−2 sec−1), or R light (10 μmol m−2 sec−1). The bars show the standard deviations of the hypocotyl growth orientations from at least 50 seedlings. The higher scores indicate the greater decrease in the gravitropism of hypocotyl growth. The error bars are standard errors from three independent experiments. WT, wild type.
Accumulation of anthocyanin is known to be induced by FR light in Arabidopsis seedlings (Kunkel et al., 1996). Wild-type seedlings grown under FR light show accumulation of anthocyanin in the cotyledon and at the junction between hypocotyl and cotyledon, whereas the phyA-211 mutant does not accumulate any detectable amounts. The rep1-1 mutant accumulates about as much anthocyanin as does the wild type under the same conditions (Figure 3A). Furthermore, the rep1 mutants were normal in both kinetics and fluence rate responses of accumulation of anthocyanin compared with the wild type (Figures 3B and 3C), indicating that the rep1 mutation does not affect the PhyA signaling that leads to accumulation of anthocyanin.
Figure 3.
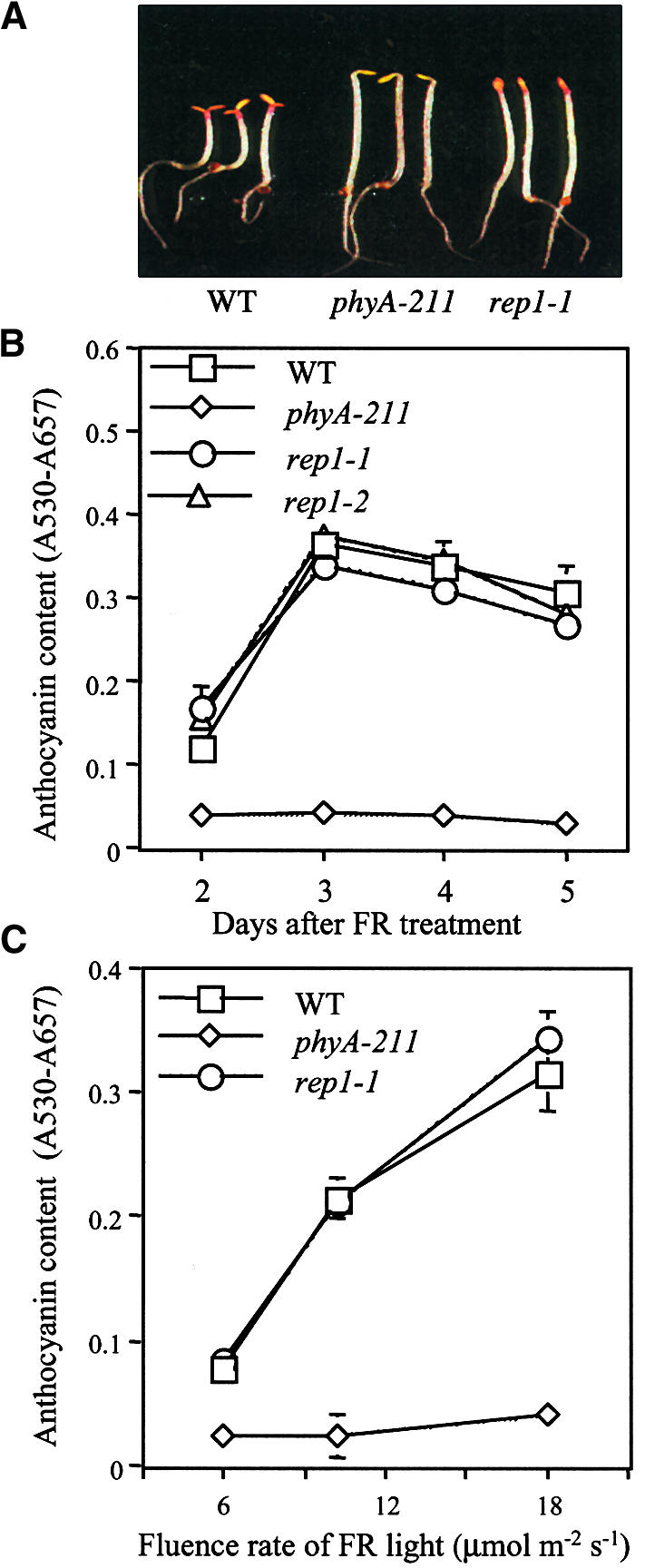
FR-Induced Accumulation of Anthocyanin of rep1 Mutant.
(A) The seedling phenotypes of the wild type (WT) and phyA-211 and rep1-1 mutants grown under FR light (19 μmol m−2 sec−1) for 3 days.
(B) Kinetics of accumulation of anthocyanin. The seedlings were grown under FR light (19 μmol m−2 sec−1) and harvested at various days after FR treatment. Each measurement was performed with 100 seedlings. The bars indicate standard deviations from three independent measurements.
(C) Fluence rate responses of the accumulation of anthocyanin. Anthocyanin measurement was performed with seedlings grown for 3 days under indicated fluence rates of FR light. The bars indicate standard deviations from three independent measurements. s, sec.
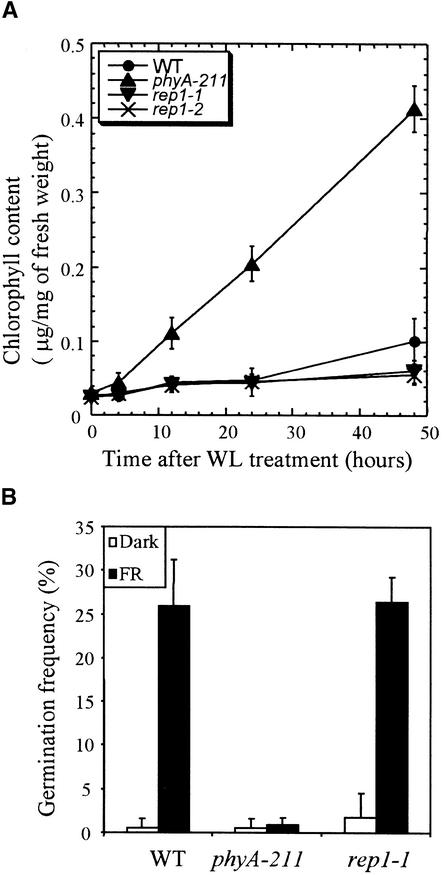
To test whether the rep1 mutation is involved in regulating the PhyA-mediated FR-preconditioned blocking of greening (van Tuinen et al., 1995; Barnes et al., 1996b), we grew the seedlings for 5 days under FR light before transfer to W light. Wild-type seedlings showed delayed greening of cotyledons when transferred to W light, leading to reduced viability. Although the phyA-211 mutant grown under FR light exhibited fast and substantial greening of cotyledons when exposed to W light, the rep1 mutant seedlings showed delayed greening of cotyledons, similar to the wild type (Figure 4A). Therefore, the rep1 mutation does not affect FR-preconditioned blocking of greening.
Figure 4.
FR-Preconditioned Blocking of Greening and Germination Responses in the rep1-1 Mutant.
(A) FR-preconditioned blocking of greening. The seedlings were grown on Murashige and Skoog (1962) medium for 5 days in FR light (7 μmol m−2 sec−1) and then irradiated with white light (WL; 15 μmol m−2 sec−1) for the times indicated. Each measurement was from 50 seedlings. The error bars indicate standard deviations of three independent measurements.
(B) PhyA-dependent germination responses. Germination frequencies of wild type (WT), phyA-211, and rep1-1 mutant seeds were measured. The seeds were treated with FR light for 15 min just after imbibition and returned to darkness for 2 days. Seeds were then incubated in darkness for an additional 5 days, without (Dark) or with treatment with FR light (7 μmol m−2 sec−1) for 15 min. Each measurement was performed with at least 150 seeds. The error bars indicate the standard deviations of three independent measurements.
We examined PhyA-dependent induction of germination, given demonstrations that seed germination can be induced by a pulse of FR light (Botto et al., 1996; Shinomura et al., 1996). Wild-type seeds showed a marked increase in germination frequency when exposed to FR light, whereas the phyA-211 mutant showed no response to FR light. The induction of germination in the rep1-1 mutant exposed to FR light was comparable to that in the wild type (Figure 4B). This suggests that PhyA signaling for the induction of germination is normal in the rep1-1 mutant.
Light-Dependent Gene Expression
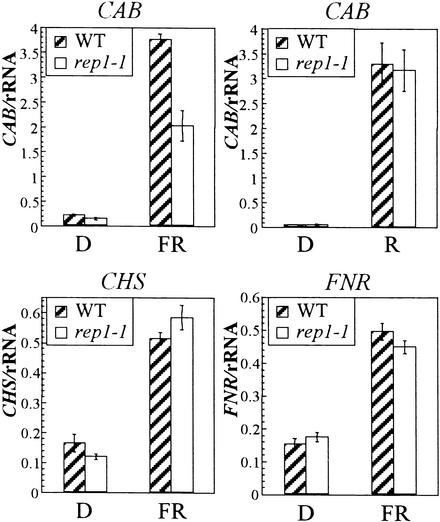
PhyA regulates the expression of several genes in response to FR light (Kuno et al., 2000). Previous pharmacological studies proposed that distinctive signaling pathways regulate the PhyA-dependent induction of CAB, CHS, and FNR genes (Neuhaus et al., 1993; Bowler et al., 1994). To assess the roles of REP1 in PhyA-dependent gene expression, we examined the transcript levels of three light-inducible genes: CAB, CHS, and FNR. When irradiated with FR light for 24 hr, wild-type plants showed a marked increase in the amounts of transcripts of these three genes (Figure 5). Interestingly, the rep1-1 mutant exhibited a differential defect; its CHS and FNR genes were induced as usual by FR light, whereas induction of the CAB gene was greatly reduced. The normal induction of the CHS gene in the rep1-1 mutant is consistent with the normal response of this mutant in the accumulation of anthocyanin (Figure 3). To test whether the defect of the rep1-1 mutant for induction of the CAB gene is FR light specific, we examined R light–mediated induction of the gene. The R light–mediated induction of CAB in the rep1-1 mutant was comparable to that in the wild type (Figure 5), suggesting that REP1 may regulate the induction of a subset of light-inducible genes, including CAB, under FR light.
Figure 5.
PhyA-Dependent Gene Expression of Three Light-Inducible Genes: CAB, CHS, and FNR.
The seedlings were grown on Murashige and Skoog (1962) medium containing 2% sucrose for 4 days in darkness and then transferred to FR light or R light or kept in darkness (D) for an additional 24 hr before extraction of total RNA. Ten micrograms of total RNA was loaded and normalized by 18S rRNA probe. Signals were quantified with a PhosphoImager (Fuji, FLA2000). The values were from two independent experimental sets. A similar trend was repeated in two other independent experiments. Error bars indicate se.
Molecular Cloning of the REP1 Gene
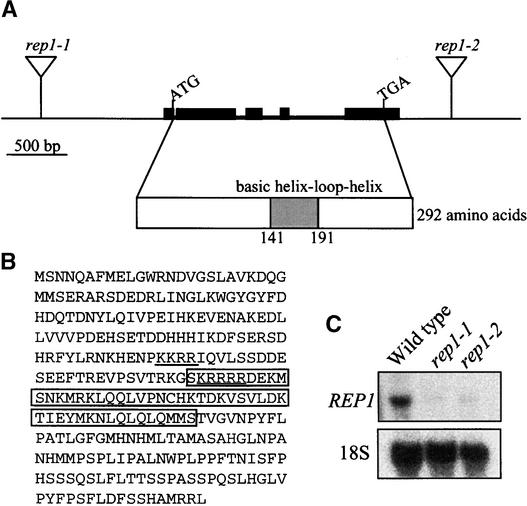
The rep1-1 and rep1-2 mutants were isolated from T-DNA insertional lines generated by T. Jack (Campisi et al., 1999). First, we performed genetic cosegregation analysis. All of the 165 seedlings with a long-hypocotyl phenotype among the segregating F2 population derived from the cross between the wild type and the rep1-1 mutant were resistant to kanamycin, implying a tight linkage between T-DNA and rep1-1 mutation. The rep1-2 mutant also showed a tight linkage with T-DNA. Using the T-DNA border sequence, we isolated the flanking genomic sequences of the right border of T-DNA of rep1-1 and rep1-2 mutants through thermal asymmetric interlaced polymerase chain reaction (PCR). The physical linkage between T-DNA and the rep1-1 mutant was again confirmed by PCR by using the flanking genomic sequence and the T-DNA border (data not shown). Using the flanking genomic sequences obtained, we isolated the full-length cDNA (Figure 6). Sequence analysis indicated that the T-DNA is inserted in the promoter region, 1099 bp upstream of the start codon in the rep1-1 mutant, and is inserted in the 3′ region of the open reading frame, 570 bp downstream of the stop codon in the rep1-2 mutant (Figure 6A). To test whether the T-DNA insertion affected the expression of the REP1 gene, we performed RNA gel blot analysis with the REP1 gene as a probe. A single band of ∼1.2 kb was detected in wild-type seedlings, whereas both rep1-1 and rep1-2 mutants showed drastically lower amounts of the transcript (Figure 6C). Together, the tight linkage between T-DNA and mutant phenotypes, the sequence analysis, and the RNA gel blot analysis of the two independent alleles of rep1 mutation allow us to conclude that the reduced photosensitivity of the rep1-1 and rep1-2 mutants is due to a decrease in expression of the REP1 gene.
Figure 6.
Molecular Cloning of the REP1 Gene.
(A) Genomic structure of the REP1 gene. The locations of start codon (ATG) and stop codon (TGA) are shown. The triangles indicate the positions of T-DNA insertions in the rep1-1 and rep1-2 mutants, respectively. The structure of cDNA is shown along with the regions for the bHLH motif. Filled boxes indicate exons, and lines between boxes indicate introns.
(B) Deduced amino acid sequence of the REP1 gene (GenBank accession number AF288287). The bHLH motif is boxed, and the putative bipartite nuclear localization signal is underlined.
(C) RNA gel blot analysis of rep1 mutants. Total RNA was extracted from seedlings of the wild type and rep1-1 and rep1-2 mutants that had been grown in darkness for 4 days and then subjected to RNA gel blot analysis with a REP1 cDNA probe. Ten micrograms of total RNA was loaded onto each lane. The 18S rRNA probe was used as a loading control.
Comparison with genomic sequences revealed that the REP1 gene consists of five exons separated by four introns. BLAST search with the deduced amino acids of the REP1 gene indicated that it belongs to a large gene family of bHLH transcription factors (Figure 6). However, except for the bHLH motif, REP1 has no homologous domain to any other known proteins, including members of the bHLH family, which suggests specific roles for REP1 among bHLH family members in Arabidopsis.
Nuclear Localization of REP1
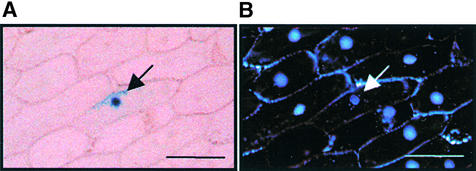
The deduced amino acid sequence of the REP1 gene contains a bipartite nuclear localization signal (Figure 6B). To determine the subcellular localization of REP1, we constructed a fusion protein of REP1 and β-glucuronidase (GUS) and introduced it into onion epidermal cells by using particle bombardment. Microscopic analysis revealed that the REP1–GUS fusion protein was localized in the nucleus (Figure 7), whereas control GUS protein was observed throughout the cell (data not shown), confirming that REP1 is localized in the nucleus.
Figure 7.
Subcellular Localization of the REP1–GUS Protein.
A construct encoding REP1 cDNA fused to GUS driven by the cauliflower mosaic virus 35S promoter was introduced into onion epidermal cells by particle bombardment.
(A) After 24 hr of incubation under W light, the cells were stained for GUS activity. The arrow indicates the nucleus.
(B) Fluorescence staining with 4′,6-diamidino-2-phenylindole identifies the nucleus (arrow).
 .
.
Expression of the REP1 Gene
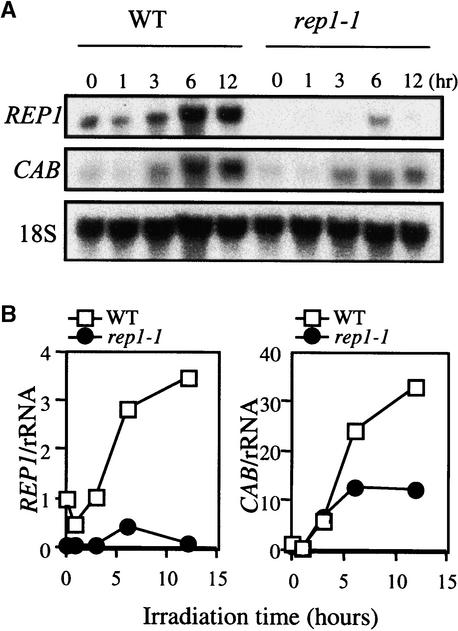
To determine whether the expression of the REP1 gene is light dependent, we examined the amount of REP1 transcript. In the wild type, the REP1 transcript increased after 3 hr of irradiation with FR light. The rep1-1 mutant exhibited much less REP1 transcript, irrespective of light conditions (Figure 8). This observation prompted us to examine whether the accumulation of REP1 transcript is related to induction of the CAB gene. Interestingly, the early induction of CAB by 3 hr of irradiation was similar for both the wild type and the rep1-1 mutant, whereas in the late phase of the induction after 6 hr, the CAB transcript was greatly reduced in rep1-1 (Figure 8). This temporal defect in induction of the CAB gene in the rep1-1 mutant appears to correlate with the kinetics of accumulation of the REP1 transcript. These results suggest that light induction of the REP1 gene may be responsible for the late induction of the CAB gene under FR light.
Figure 8.
Expression Analysis of the REP1 Gene.
(A) RNA gel blot analysis of CAB and REP1 transcripts in the wild type (WT) and rep1-1 mutants. The seedlings were grown on Murashige and Skoog (1962) medium for 4 days in darkness and then irradiated with FR light (7 μmol m−2 sec−1) for the indicated times before harvest. The 18S rRNA was used as a loading control.
(B) Quantitative measurement of the relative amounts of CAB and REP1 transcripts shown in (A). The values denotes relative expressions and were calculated by first normalizing each signal against that for 18S rRNA and then against the amount of expression in the wild type for each particular gene. The signals were quantified with a PhosphoImager (Fuji, FLA 2000).
DISCUSSION
Here, we report the genetic identification of the REP1 gene that encodes a nuclear protein, potentially acting as a transcription factor. From the molecular and physiological analyses of rep1 mutants, the possible functions of REP1 can be inferred. First, REP1 is a positive regulator of PhyA signaling because the recessive rep1-1 mutant exhibited defective photoresponses under FR light but not under R or W light. Second, REP1 might be involved in a branch pathway of PhyA signaling. The rep1-1 mutant showed defects in a subset of PhyA-dependent responses, including deetiolation, gravitropic modulation of hypocotyl growth, and induction of the CAB gene, while exhibiting normal responses in the accumulation of anthocyanin, FR-preconditioned blocking of greening, induction of germination, and induction of the CHS and FNR genes. Moreover, the amount of the PhyA photoreceptor in rep1-1 mutant is comparable with that in the wild type. Third, being a bHLH protein, REP1 may be directly involved in light-dependent gene expression. The bHLH proteins can directly bind to DNA containing the E-box motif CANNTG (Patikoglou and Burley, 1997). Moreover, several bHLH proteins in plants have been shown to bind to DNA, preferentially to the G-box motif CACGTG, located in the promoter region of numerous photoresponsive genes (Kawagoe and Murai, 1996; de Pater et al., 1997; Martinez-Garcia et al., 2000).
Several reports have dealt with the genetic components in PhyA signaling (Whitelam et al., 1993; Yanovsky et al., 1997; Hoecker et al., 1998, 1999; Soh et al., 1998; Hudson et al., 1999; Bolle et al., 2000; Büche et al., 2000; Hsieh et al., 2000). The rep1 mutation is distinct from the spa1 and eid1 mutations that enhance sensitivity to FR light. The far1, fhy1, fhy3, fin2, fin219, and pat1 mutations show less sensitivity to continuous FR light in a way similar to the rep1 mutation. Our complementation test and the chromosomal location of the REP1 gene indicated that REP1 is a novel genetic component. Furthermore, rep1 mutants exhibited phenotypes distinct from these other known PhyA signaling mutants. All the mutants examined are pleiotropic, affecting various PhyA-dependent responses, including deetiolation, accumulation of anthocyanin, and induction of CAB and CHS genes (Barnes et al., 1996a; Hoecker et al., 1998, 1999; Soh et al., 1998; Hudson et al., 1999; Bolle et al., 2000; Büche et al., 2000; Hsieh et al., 2000). In contrast, the rep1 mutants responded normally to FR light with regard to the accumulation of anthocyanin and the induction of CHS under that condition (Figures 3 and 5). In addition, the rep1 mutant is normal in FR-preconditioned blocking of greening and induction of germination under FR light, implying that REP1 may define a novel branch of PhyA signaling in Arabidopsis.
Previously, pharmacological studies proposed a biochemical model in which PhyA signaling involves distinct signaling components to induce the expression of CAB, CHS, and FNR genes (Neuhaus et al., 1993; Bowler et al., 1994). The proposed pathway involves heterotrimeric G proteins that mediate expression of both CHS and CAB. Further downstream signaling is divided into cGMP-dependent and calcium/calmodulin-dependent pathways, which are responsible for CHS and CAB gene expression, respectively. Both cGMP- and calcium/calmodulin-dependent pathways are required for induction of the FNR gene. Our finding that REP1 is necessary for the induction of CAB, but not CHS and FNR, under FR light correlates with the biochemical model and suggests that REP1 may be under the control of the calcium/calmodulin-dependent signaling pathway.
Recently, PhyA and PhyB have been shown to be translocated into the nucleus when exposed to light (Sakamoto and Nagatani, 1996; Kircher et al., 1999; Yamaguchi et al., 1999), suggesting that one of the primary actions of phytochromes is to regulate nuclear processes such as transcription. So far, a few transcription factors have been demonstrated to function in phytochrome signaling in Arabidopsis. PIF3 (PHYTOCHROME INTERACTING FACTOR3) contains the bHLH motif (Ni et al., 1998, 1999); CCA1 (CIRCADIAN CLOCK ASSOCIATED1), which has a single MYB domain (Wang et al., 1997); and a basic leucine zipper (bZIP) protein, HY5 (LONG HYPOCOTYL5) (Koornneef et al., 1980; Oyama et al., 1997). PIF3 was identified as a phytochrome-interacting protein and binds with both PhyA and PhyB. It can also bind to the G-box element in the promoter of the CCA1 gene to induce the transcription of the CCA1 gene (Martinez-Garcia et al., 2000). In turn, the induced CCA1 increases the amount of CAB gene transcript by binding to the promoter of the CAB gene (Wang et al., 1997). However, the rapid decrease of CCA1 after acute induction under continuous light suggests that one or more additional factor(s) might be required for the sustained increase of CAB gene expression under continuous light (Wang et al., 1997; Wang and Tobin, 1998). We propose that REP1 is responsible for the sustained induction of the CAB gene under FR light on the basis of the following observations: (1) The late phase of the induction of CAB after 6 hr under FR light was greatly affected in the rep1-1 mutant, whereas the early phase of induction after 3 hr in the rep1-1 mutant was more or less similar to that in the wild type. (2) The REP1 gene transcript in the wild type can be induced after at least 3 hr under FR light (Figure 8). (3) The acute induction and rapid decline of the CCA1 gene under FR light was not affected by rep1-1 mutation (data not shown). Interestingly, the increased CAB transcript at 6 hr was maintained over longer periods in the rep1-1 mutant, without further induction (Figure 8), suggesting that additional factors, besides REP1, may be responsible for the late induction of CAB.
HY5, a bZIP protein, is involved in downstream signaling of multiple photoreceptors, including PhyA and PhyB (Koornneef et al., 1980; Oyama et al., 1997). Interestingly, the hy5 mutation abrogates a subset of PhyA-dependent responses under FR light, including inhibition of hypocotyl elongation, induction of the CHS gene, and accumulation of anthocyanin, while minimally affecting FR-preconditioned blocking of greening and phytochrome-dependent induction of the CAB gene (Koornneef et al., 1980; Barnes et al., 1996a; Anderson et al., 1997; Pepper and Chory, 1997; Ang et al., 1998). Our observations that the rep1-1 mutation impaired inhibition of hypocotyl elongation and induction of CAB suggest that HY5 and REP1 may play not only independent but also redundant roles in PhyA signaling. Further double mutant analysis should not only help define the respective roles of REP1 and HY5 in PhyA signaling but also reveal the genetic interaction in the signaling network.
A question of particular interest is why REP1 is necessary for PhyA signaling but dispensable for PhyB signaling. One possibility is that expression of the REP1 gene might be induced by FR light, not R light. When we examined the transcript quantities of the REP1 gene, we found that the gene was induced not only by FR light (Figure 8) but also by R or W light (data not shown). Thus, expression of the REP1 gene is not fully responsible for the FR-specific defects of rep1 mutants. Instead, the function of REP1 may be regulated by one or more additional mechanisms, including post-translational modifications by PhyA-specific signaling components. Recent studies have shown that besides transcriptional regulation, post-translational modifications, such as phosphorylation or destabilization, play critical roles in the regulation of transcription factors in plants (Gu et al., 2000; Osterlund et al., 2000; Worley et al., 2000). Alternatively, additional components redundantly acting for PhyB signaling under R light may compensate for the decreased REP1 gene expression in the rep1 mutants. Finally, the residual amount of REP1 transcripts in the rep1 mutants may be sufficient for PhyB signaling, leading to normal PhyB-dependent responses.
In summary, we present molecular genetic evidence that REP1, a nuclear protein containing the bHLH motif, is necessary for a branch pathway of PhyA signaling that regulates various photoresponses, including inhibition of hypocotyl elongation, gravitropic modulation of hypocotyl growth, and late, sustained induction of the CAB gene. Further molecular, biochemical, and genetic analyses of the REP1 gene should provide important clues for deciphering phytochrome signaling in Arabidopsis.
METHODS
Plant Material and Light Conditions
The T-DNA mutagenized seeds of ∼28,000 lines (CS31087 and CS21995, generated by Dr. Tom Jack [Dartmouth College, Hanover, NH] and Dr. Detlef Weigel [The Salk Institute, La Jolla, CA], respectively) were obtained from the Arabidopsis Biological Resource Center (ABRC; Columbus, OH) and used for mutant screening. The seeds of the wild-type and of the phyA-211 and phyB-9 mutant lines were also obtained from ABRC. The fhy1 and fhy3 seeds were kindly provided by Dr. Garry Whitelam (Leicester University, UK).
Various fluxes of continuous white (W) light were obtained by using fluorescent tubes (FLR40D/A; Osram, Seoul, Republic of Korea). For far-red (FR) and red (R) light irradiation, we used growth chambers (model E-30LED1; Percival Scientific, Inc., Boone, IA) equipped with FR and R light–emitting diodes. For all light treatments, the temperature was maintained at 22 to 24°C.
Screening of Mutants with Reduced Phytochrome Signaling
For screening FR-insensitive mutants, seeds were sterilized as previously described (Soh et al., 1998), sown on Murashige and Skoog (1962) (MS) plates with 0.8% agar, and grown in FR light (7 μmol m−2 sec−1). After 4 days, seedlings showing long hypocotyl or unexpanded cotyledons were transferred to fresh plates of MS medium containing 2% sucrose. After growing for 2 weeks more under fluorescent light in a growth room (Vision Scientific Co., Seoul, Republic of Korea), the seedlings were transferred to soil for seed-setting. The self-pollinated seeds were collected from individual plants. Two separate portions of the individual seeds from the next generation were grown on MS plates in FR (7 μmol m−2 sec−1) or R (10 μmol m−2 sec−1) light for 4 days. Hypocotyl length was the phenotype scored. The homozygous rep mutants were backcrossed twice to the wild type for further genetic and physiological analyses.
Physiological Analysis
For measuring hypocotyl length, seeds were surface-sterilized for 5 min in commercial bleach, rinsed at least five times with sterile distilled water, and then sown onto MS medium containing 0.8% agar. After cold treatment at 4°C for 3 days, the plates were placed in W light (20 μmol m−2 sec−1) for 12 hr at 23 °C to improve germination and then transferred to the appropriate light conditions. Data were collected from 40% of the longest seedlings, to minimize variation in hypocotyl lengths among the seedlings, as previously described (Soh et al., 1998).
For end-of-day treatment with FR light experiments, seedlings were first treated with W light for 12 hr to promote germination and then transferred to a short-day growth chamber, where they were grown for another 5 days in cycles of 8 hr of W light (15 μmol m−2 sec−1) and 16 hr of darkness. FR light treatment (7 μmol m−2 sec−1) was given at the end of each day for 15 min.
For the examination of the gravitropic response, the seeds were sown in a row onto MS medium containing 1.2% agar. The seedlings were grown vertically and photographed to measure the angles of hypocotyl growth, as described by Kim et al. (1998).
For measurement of anthocyanin content, seedlings were grown on MS medium containing 2% sucrose in darkness or in FR light for 3 days and then harvested after W light irradiation for 12 hr to induce germination. Samples of 100 seedlings were harvested and ground into particles in liquid nitrogen. Anthocyanin was extracted as previously described (Soh et al., 1998), and its content was calculated as described by Mancinelli (1990).
For the FR-preconditioned blocking of the greening experiment, seedlings were grown on MS medium for 5 days in darkness or in FR light (7 μmol m−2 sec−1) and then transferred to W light (20 μmol m−2 sec−1). Samples of 50 seedlings were harvested and homogenized. Chlorophyll was extracted in 95% ethanol at 4°C. Chlorophyll content was estimated spectrophotometrically (Kim et al., 1996).
The germination test was performed as described by Shinomura et al. (1996). Seeds were surface sterilized and sown on aqueous medium containing 0.7% agar. The seeds were then irradiated with FR light (7 μmol m−2 sec−1) for 15 min and kept in darkness. After 48 hr, the seeds were kept in darkness or irradiated with FR light (7 μmol m−2 sec−1) for 15 min. The seeds were then incubated in darkness for another 5 days before germination frequency was measured.
Genetic Mapping
The mutation was mapped by using cleaved amplified polymorphic sequences (CAPS) markers, as described by Konieczny and Ausubel (1993). F2 seeds were obtained from the cross between Landsberg erecta and rep1-1 mutant (Columbia-6 background) plants and then scored for seedling phenotypes in FR light (7 μmol m−2 sec−1). DNA was prepared from 30 individual mutant progeny and used for CAPS mapping. The map distance was estimated as described by Koornneef and Stam (1991).
Immunoblot Analysis
For immunoblot analysis, the seedlings were grown on MS medium for 4 days in darkness and then kept dark or transferred to R light (10 μmol m−2 sec−1) for 6 hr. Approximately 100 seedlings were harvested and ground to extract proteins (Martinez-Garcia et al., 1999). Proteins were separated by SDS-PAGE (8%) and then transferred to polyvinylidenedifluoride membrane. Protein gel blotting was performed as described before (Soh et al., 1998) with a polyclonal antibody against pea phytochrome A as primary antibody and anti–rabbit antibody (Promega) as secondary antibody. The immunoblot was developed with the ECL-Plus (Amersham-Pharmacia, Biotech, UK) electrochemiluminescence system according to the manufacturer's instructions.
RNA Gel Blot Analysis
Total cellular RNA was extracted from whole seedlings by using the RNeasy Miniprep kit (Qiagen). RNA gel blot analysis was performed as described (Soh et al., 1998). The CAB2 gene probe was obtained from Dr. J. Chory (The Salk Institute, La Jolla, CA); the CHS gene probe and FNR gene probe were from the ABRC. The Brassica 18S rRNA probe was described before (Park et al., 1993). For detection of REP1 transcript, the full-length cDNA of REP1 was used as probe.
Cloning of REP1 Gene and Sequence Analysis
To obtain the genomic DNA flanking the T-DNA border in rep1-1 and rep1-2 mutants, we performed thermal asymmetric interlaced polymerase chain reaction (PCR) analysis, as described by Liu et al. (1995), using primer sets within the T-DNA designed to amplify the adjacent flanking DNA (Campisi et al., 1999). Fragments 1250 and 1020 bp long adjacent to the right border of the T-DNA in rep1-1 and rep1-2 mutants, respectively, were amplified and cloned into pGEM-T Easy vector (Promega). Sequence analysis and database search were performed with the NCBI BLAST2.0 program at The Arabidopsis Information Resource (www.tair.org). The same bacterial artificial chromosome (BAC) clone, T6A9, was found to match the flanking genomic sequence of both rep1-1 and rep1-2 mutants, and an expressed sequence tag clone, AI99564, corresponding to the 3′ end of cDNA, was identified to match the flanking genomic sequence of rep1-2. A full-length 1100-bp cDNA was isolated by PCR using proof-reading PWO polymerase (Boehringer Mannheim) as described (Choi et al., 2000) with Arabidopsis (Columbia) cDNA library plasmid DNA and cloned into a TOPO TA cloning vector after the addition of 3′A overhangs with Taq polymerase after amplification, according to the manufacturer's instructions (Invitrogen, The Netherlands). The primers were 5′-GTATATGACACAAATGGTTC-3′ for the 3′ end of the REP1 gene and MATCHMAKER 5′ AD LD-Insert Screening Amplifier (Clontech Laboratories, Inc., Palo Alto, CA) for the vector. Subsequent sequence analysis and comparison with the genomic sequence of BAC clone T6A9 revealed that the REP1 gene consists of five exons separated by four introns. The sequencing analysis was done with a genetic analyzer (model ABI 310; Perkin-Elmer), according to the manufacturer's instructions.
Subcellular Localization of REP1–β-Glucuronidase Fusion Protein
To construct a REP1–β-glucuronidase (GUS) fusion protein, we amplified REP1 cDNA with proof-reading PWO polymerase (Boehringer Mannheim) and cloned this into the BamHI site of a pBI221 vector (Clontech). The primer combinations used were 5′-CCAAACTTTCGGATCCGATATCTC-3′ for upstream of the start codon and 5′-GCTACTTACGGATCCTAGTCTTCTC-3′ for the 3′ region of REP1, replacing the stop codon. The BamHI sites introduced are underlined. The pBI221 and REP1–GUS plasmids were introduced into onion epidermal cells by using a helium biolistic particle delivery system (Bio-Rad), as described by Shieh et al. (1993). After incubation for 24 hr at 23°C, GUS activity was determined by X-Gluc (Sigma) and use of a light microscope (Olympus Optical, Inc., Tokyo). For identification of nuclei, the same cells were stained with 1 mg/mL 4′,6-diamidino-2-phenylindole (Sigma) and visualized with a fluorescence microscope (Olympus).
Acknowledgments
We thank Dr. Chung-Mo Park for providing polyclonal PhyA antibody; Drs. Soo Young Kim and Giltsu Choi for providing the Arabidopsis cDNA library; Youn-Sik Kwak for help with immunoblot analysis; Drs. Mamatha Hanumappa, Soo Young Kim, Jungmook Kim, Chung-Mo Park, and Giltsu Choi for critical reading of the manuscript; and the members of the light-signaling group of the Kumho Life and Environmental Science Laboratory for helpful discussions. These studies were in part supported by the U.S. Public Health Service National Institutes of Health (Grant No. GM36956), the National Research Laboratory (Grant No. PYUNG1-7), from the Korea Institute of Science and Technology Evaluation and Planning, and Brain Korea 21 funding from the Korea Science and Engineering Foundation to P.-S.S.
References
- Ahmad, M., and Cashmore, A.R. (1996). The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 10, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Anderson, S.L., Somers, D.E., Miller, A.J., Hanson, K., Chory, J., and Kay, S.A. (1997). Attenuation of phytochrome A and B signaling pathways by the Arabidopsis circadian clock. Plant Cell 9, 1727–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, L.-H., Chattopadhyay, S., Wei, N., Oyama, T., Okada, K., Batschauer, A., and Deng, X.-W. (1998). Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1, 213–222. [DOI] [PubMed] [Google Scholar]
- Barnes, S.A., Quaggio, R.B., Whitelam, G.C., and Chua, N.-H. (1996. a). fhy1 defines a branch point in phytochrome A signal transduction pathways for gene expression. Plant J. 10, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Barnes, S.A., Nishizawa, N.K., Quaggio, R.B., Whitelam, G.C., and Chua, N.-H. (1996. b). Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A–mediated change in plastid development. Plant Cell 8, 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle, C., Koncz, C., and Chua, N.-H. (2000). PAT1, a member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 14, 1269–1278. [PMC free article] [PubMed] [Google Scholar]
- Botto, J.F., Sanchez, R.A., Whitelam, G.C., and Casal, J.J. (1996). Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 110, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler, C., Neuhaus, G., Yamagata, H., and Chua, N.-H. (1994). Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 77, 73–81. [DOI] [PubMed] [Google Scholar]
- Boylan, M.T., and Quail, P.H. (1991). Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc. Natl. Acad. Sci. USA 88, 10806–10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan, M., Douglas, N., and Quail, P.H. (1994). Dominant negative suppression of Arabidopsis photoresponses by mutant phytochrome A sequences identifies spatially discrete regulatory domains in the photoreceptor. Plant Cell 6, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büche, C., Poppe, C., Schäfer, E., and Kretsch, T. (2000). eid1: A new Arabidopsis mutant hypersensitive in phytochrome A–dependent high-irradiance responses. Plant Cell 12, 547–558. [PMC free article] [PubMed] [Google Scholar]
- Butler, W.L., Norris, K.H., Siegelman, H.W., and Hendricks, S.B. (1959). Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc. Natl. Acad. Sci. USA 45, 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi, L., Yang, Y., Yi, Y., Heilig, E., Herman, B., Cassista, A.J., Allen, D.W., Xiang, H., and Jack, T. (1999). Generation of enhancer trap lines in Arabidopsis and characterization of expression patterns in the inflorescence. Plant J. 17, 699–707. [DOI] [PubMed] [Google Scholar]
- Canton, F.R., and Quail, P.H. (1999). Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 121, 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284, 760–765. [DOI] [PubMed] [Google Scholar]
- Cherry, J.R., Hondred, D., Walker, J.M., Keller, J.M., Hershey, H.P., and Vierstra, R.D. (1993). Carboxy-terminal deletion analysis of oat phytochrome A reveals the presence of separate domains required for structure and biological activity. Plant Cell 5, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, G., Yi, H., Lee, J., Kwon, Y.K., Soh, M.S., Shin, B., Luka, S., Hahn, T.R., and Song, P.S. (1999). Phytochrome signaling is mediated through nucleoside diphosphate kinase 2. Nature 401, 610–613. [DOI] [PubMed] [Google Scholar]
- Choi, H., Hong, J., Ha, J., Kang, J., and Kim, S.Y. (2000). ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 275, 1723–1730. [DOI] [PubMed] [Google Scholar]
- Chory, J., Peto, C., Feinbaum, R., Pratt, L., and Ausubel, F. (1989). Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58, 991–999. [DOI] [PubMed] [Google Scholar]
- Clack, T., Mathews, S., and Sharrock, R.A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes—The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 25, 413–427. [DOI] [PubMed] [Google Scholar]
- Deng, X.-W., and Quail, P.H. (1999). Signaling in light-controlled development. Semin. Cell Dev. Biol. 10, 121–129. [DOI] [PubMed] [Google Scholar]
- de Pater, S., Pham, K., Memelin, J., and Kijne, J. (1997). RAP-1 is an Arabidopsis MYC-like R protein homologue, that binds to G-box sequence motifs. Plant Mol. Biol. 34, 169–174. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., and Chory, J. (1997). Light control of plant development. Annu. Rev. Cell Dev. Biol. 13, 203–229. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., Yeh, K.C., Lagarias, J.C., Zhang, H., Elich, T.D., and Chory, J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284, 1539–1541. [DOI] [PubMed] [Google Scholar]
- Furuya, M. (1993). Phytochromes—Their molecular species, gene families, and functions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 617–645. [Google Scholar]
- Furuya, M., and Kim, B.C. (2000). Do phytochromes interact with diverse partners? Trends Plant Sci. 5, 87–89. [DOI] [PubMed] [Google Scholar]
- Furuya, M., and Schäfer, E. (1996). Photoperception and signaling of induction reactions by different phytochromes. Trends Plant Sci. 1, 301–307. [Google Scholar]
- Genoud, T., Millar, A.J., Nishizawa, N., Kay, S.A., Schäfer, E., Nagatani, A., and Chua, N.H. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y.-Q., Yang, C., Thara, V.K., Zhou, J., and Martin, G.B. (2000). Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. Plant Cell 12, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazato, F., Shinomura, T., Hanzawa, H., Chory, J., and Furuya, M. (1997). Fluence and wavelength requirements for Arabidopsis CAB gene induction by different phytochromes. Plant Physiol. 115, 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter, R.P. (1997). Gravity, light and plant form. Plant Cell Environ. 20, 796–800. [DOI] [PubMed] [Google Scholar]
- Hennig, L., Büche, C., Eichenberg, K., and Schäfer, E. (1999). Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings. Plant Physiol. 121, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker, U., Xu, Y., and Quail, P.H. (1998). SPA1: A new genetic locus involved in phytochrome A–specific signal transduction. Plant Cell 10, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker, U., Tepperman, J.M., and Quail, P.H. (1999). SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 284, 496–499. [DOI] [PubMed] [Google Scholar]
- Hsieh, H.-L., Okamoto, H., Wang, M., Ang, L.-H., Matsui, M., Goodman, H., and Deng, X.-W. (2000). FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 14, 1958–1970. [PMC free article] [PubMed] [Google Scholar]
- Huala, E., Oeller, P.W., Liscum, E., Han, I.S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Hudson, M., Ringli, C., Boylan, M.T., and Quail, P.H. (1999). The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev. 13, 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, E., Bradley, M., Harberd, N.P., and Whitelam, G.C. (1994). Photoresponses of light-grown phyA mutants of Arabidopsis. Plant Physiol. 105, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe, Y., and Murai, N. (1996). A novel basic region/helix-loop-helix protein binds to a G-box motif CACGTG of the bean seed storage protein b-phaseolin gene. Plant Sci. 116, 47–57. [Google Scholar]
- Kendrick, R.E., and Kronenberg, G.H.M. (1994). Photomorphogenesis in Plants, 2nd ed. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Kim, B.C., Soh, M.S., Kang, B.J., Furuya, M., and Nam, H.G. (1996). Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J. 9, 441–456. [DOI] [PubMed] [Google Scholar]
- Kim, B.C., Soh, M.S., Hong, S.H., Furuya, M., and Nam, H.G. (1998). Photomorphogenic development of the Arabidopsis shy2–1D mutation and its interaction with phytochromes in darkness. Plant J. 15, 61–68. [DOI] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bogner, L., Kim, L., Adam, E., Harter, K., Schäfer, E., and Nagy, F. (1999). Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11, 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., and Stam, P. (1991). Genetic analysis. In Methods in Arabidopsis Research, C. Koncz, N.-H. Chua, and J. Schell, eds (Singapore: World Scientific), pp. 83–99.
- Koornneef, M., Rolff, E., and Spruit, C.J.P. (1980). Genetic control of light inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z. Pflanzenphysiol. 100, 147–160. [Google Scholar]
- Kunkel, T., Neuhaus, G., Batschauer, A., Chua, N.-H., and Schäfer, E. (1996). Functional analysis of yeast-derived phytochrome A and B phycocyanobilin adducts. Plant J. 10, 625–636. [DOI] [PubMed] [Google Scholar]
- Kuno, N., Muramatsu, T., Hamazato, F., and Furuya, M. (2000). Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol. 122, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapko, V.N., Jiang, X.Y., Smith, D.L., and Song, P.S. (1997). Posttranslational modification of oat phytochrome A: Phosphorylation of a specific serine in a multiple serine cluster. Biochemistry 36, 10595–10599. [DOI] [PubMed] [Google Scholar]
- Lin, Y., and Cheng, C.L. (1997). A chlorate-resistant mutant defective in the regulation of nitrate reductase gene expression in Arabidopsis defines a new HY locus. Plant Cell 9, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8, 457–463. [DOI] [PubMed] [Google Scholar]
- Mancinelli, A.L. (1990). Interaction between light quality and light quantity in the photoregulation of anthocyanin production. Plant Physiol. 92, 1191–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia, J.F., Monte, E., and Quail, P.H. (1999). A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J. 20, 251–257. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia, J.F., Huq, E., and Quail, P.H. (2000). Direct targeting of light signals to a promoter element–bound transcription factor. Science 288, 859–863. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nagatani, A., Reed, J.W., and Chory, J. (1993). Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 102, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., Fankhauser, C., and Chory, J. (2000). Light: An indicator of time and place. Genes Dev. 14, 257–271. [PubMed] [Google Scholar]
- Neuhaus, G., Bowler, C., Kern, R., and Chua, N.-H. (1993). Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell 73, 937–952. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for the normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 1–20. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- Osterlund, M.T., Hardtke, C.S., Wei, N., and Deng, X.-W. (2000). Targetted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. [DOI] [PubMed] [Google Scholar]
- Oyama, T., Shimura, Y., and Okada, K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11, 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y.S., Kwak, J.M., Kwon, O.-Y., Kim, Y.S., Lee, D.S., Cho, M.J., Lee, H.H., and Nam, H.G. (1993). Generation of expressed sequence tags of random root cDNA clones of Brassica napus by single-run partial sequencing. Plant Physiol. 103, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, B.M., and Quail, P.H. (1993). hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell 5, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patikoglou, G., and Burley, S.K. (1997). Eukaryotic transcription factor–DNA complexes. Annu. Rev. Biophys. Biomol. Struct. 26, 289–325. [DOI] [PubMed] [Google Scholar]
- Pepper, A.E., and Chory, J. (1997). Extragenic suppressors of Arabidopsis det1 mutant identify elements of flowering-time and light-response regulatory pathways. Genetics 145, 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe, C., Hangarter, R.P., Sharrock, R.A., Nagy, F., and Schäfer, E. (1996). The light-induced reduction of the gravitropic growth orientation of seedlings of Arabidopsis thaliana (L.) Heynh. is a photomorphogenic response mediated synergistically by the far-red–absorbing forms of phytochrome A and B. Planta 199, 511–514. [DOI] [PubMed] [Google Scholar]
- Quail, P.H., Boylan, M.T., Parks, B.M., Short, T.W., Xu, Y., and Wagner, D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268, 675–680. [DOI] [PubMed] [Google Scholar]
- Reed, J.W., Nagatani, A., Elich, T.D., Fagan, M., and Chory, J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104, 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson, P.R.H., and Smith, H. (1996). Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol. 110, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson, P.R.H., Whitelam, G.C., and Smith, H. (1993). Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 102, 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, L.C., Sommer, D., Gotor, C., and Song, P.S. (1991). G-proteins in etiolated Avena seedlings: Possible phytochrome regulation. FEBS Lett. 282, 341–346. [DOI] [PubMed] [Google Scholar]
- Runge, S., Sperling, U., Frick, G., Apel, K., and Armstrong, G.A. (1996). Distinct roles for light-dependent NADPH: protochlorophyllide oxidoreductase (POR) A and B during greening in higher plants. Plant J. 9, 513–523. [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., and Nagatani, A. (1996). Nuclear localization activity of phytochrome B. Plant J. 10, 859–868. [DOI] [PubMed] [Google Scholar]
- Sharrock, R.A., and Quail, P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3, 1745–1757. [DOI] [PubMed] [Google Scholar]
- Shieh, M.W., Wessler, S.R., and Raikhel, N.V. (1993). Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol. 101, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura, T., Nagatani, A., Hanzawa, H., Kubota, M., Watanabe, M., and Furuya, M. (1996). Action spectra for phytochrome A– and B–specific photoinhibition of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93, 8129–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, H. (1995). Physiological and ecological function within the phytochrome family. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 289–315. [Google Scholar]
- Soh, M.S., Hong, S.H., Hanzawa, H., Furuya, M., and Nam, H.G. (1998). Genetic identification of FIN2, a far red light–specific signaling component of Arabidopsis thaliana. Plant J. 16, 411–419. [DOI] [PubMed] [Google Scholar]
- Terzaghi, W.B., and Cashmore, A.R. (1995). Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 445–474. [Google Scholar]
- van Tuinen, A., Kerckhoffs, L.H.J., Nagatani, A., Kendrick, R.E., and Koornneef, M. (1995). Far-red light–insensitive, phytochrome A–deficient mutants of tomato. Mol. Gen. Genet. 246, 133–141. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Hoecker, U., and Quail, P.H. (1997). RED1 is necessary for phytochrome B–mediated red light–specific signal transduction in Arabidopsis. Plant Cell 9, 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.-Y., and Tobin, E.M. (1998). Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217. [DOI] [PubMed] [Google Scholar]
- Wang, Z.-Y., Kenigbuch, D., Sun, L., Harel, E., Ong, M.S., and Tobin, E.M. (1997). A myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9, 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, N., and Deng, X.W. (1996). The role of the COP/DET/FUS genes in light control of Arabidopsis seedling development. Plant Physiol. 112, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam, G.C., and Devlin, P.F. (1997). Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 20, 752–758. [Google Scholar]
- Whitelam, G.C., Johnson, E., Peng, J., Carol, P., Anderson, M.L., Cowl, J.S., and Harberd, N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley, C.K., Zenser, N., Ramos, J., Rouse, D., Leyser, O., Theologies, A., and Callis, J. (2000). Degradation of Aux/IAA proteins is essential for normal auxin signaling. Plant J. 21, 553–562. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Parks, B.M., Short, T.W., and Quail, P.H. (1995). Missense mutations define a restricted segment in the C-terminal domain of phytochrome A critical to its regulatory activity. Plant Cell 7, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagtani, A. (1999). Light-dependent translocation of a phytochrome B–GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 3, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky, M.J., Casal, J.J., and Luppi, J.P. (1997). The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J. 12, 659–667. [DOI] [PubMed] [Google Scholar]
- Yeh, K.C., and Lagarias, J.C. (1998). Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 95, 13976–13981. [DOI] [PMC free article] [PubMed] [Google Scholar]