Abstract
Effective pathogenesis by the fungus Sclerotinia sclerotiorum requires the secretion of oxalic acid. Studies were conducted to determine whether oxalate aids pathogen compatibility by modulating the oxidative burst of the host plant. Inoculation of tobacco leaves with an oxalate-deficient nonpathogenic mutant of S. sclerotiorum induced measurable oxidant biosynthesis, but inoculation with an oxalate-secreting strain did not. Oxalate inhibited production of H2O2 in tobacco and soybean cultured cell lines with a median inhibitory concentration of ∼4 to 5 mM, a concentration less than that measured in preparations of the virulent fungus. Several observations also indicate that the inhibitory effects of oxalate are largely independent of both its acidity and its affinity for Ca2+. These and other data demonstrate that oxalate may inhibit a signaling step positioned upstream of oxidase assembly/activation but downstream of Ca2+ fluxes into the plant cell cytosol.
INTRODUCTION
Sclerotinia is a ubiquitous phytopathogenic Ascomycete fungus capable of infecting a wide range of plants (Boland and Hall, 1994). The general inability of economically important crops to develop germ plasm resistant to this pathogen has focused attention on the need for a more detailed understanding of the pathogenic factors involved in disease development. Oxalic acid secretion by Sclerotinia appears to be an essential determinant of its pathogenicity (Maxwell and Lumsden, 1970; Noyes and Hancock, 1981; Marciano et al., 1983; Godoy et al., 1990; Dutton and Evans, 1996; Zhou and Boland, 1999). Evidence for such involvement is based on the recovery of millimolar concentrations of oxalate from infected tissues (Bateman and Beer, 1965; Maxwell and Lumsden, 1970; Marciano et al., 1983; Godoy et al., 1990) and from the manual injection of oxalate, or of culture filtrate containing oxalate, into plants and observation of the development of Sclerotinia diseaselike symptoms independent of the pathogen (Bateman and Beer, 1965; Noyes and Hancock, 1981). Such correlative evidence has been strengthened by the observation that mutants of Sclerotinia that are deficient in the ability to synthesize oxalate are nonpathogenic, whereas revertant strains that regain their oxalate biosynthetic capacity exhibit normal virulence (Godoy et al., 1990). Importantly, the main, if not the only, difference between these strains is their ability to synthesize and secrete oxalic acid (Godoy et al., 1990).
Speculation regarding the mechanism or mechanisms by which oxalate secretion might enhance Sclerotinia virulence currently centers on three modes of action (reviewed in Dutton and Evans, 1996). First, because several of the fungal enzymes secreted during invasion of plant tissues (e.g., polygalacturonase) have maximal activities at low pH, various researchers have postulated that oxalate might aid Sclerotinia virulence by shifting the apoplastic pH to a value better suited for enzymatic degradation of plant cell walls (Bateman and Beer, 1965). Second, because oxalate may be directly toxic to host plants, presumably because of its acidity, the secretion of oxalate has been suggested to weaken the plant, thereby facilitating invasion (Noyes and Hancock, 1981). Finally, chelation of cell wall Ca2+ by the oxalate anion has been proposed both to compromise the function of Ca2+-dependent defense responses and to weaken the plant cell wall (Bateman and Beer, 1965). Although each of these hypotheses has its logical appeal, evidence supporting them is incomplete, and arguments against their validity have also been made (Dutton and Evans, 1996).
One of the earliest resistance responses mounted by infected plant tissues against an invading microbe is the oxidative burst, that is, the controlled release of O2.− and H2O2 at the site of pathogen ingress (recent reviews include those by Wojtaszek, 1997; Blumwald et al., 1998; Ebel and Mithofer, 1998; Bolwell, 1999). The oxidative burst is thought to be required for several subsequent defense responses and is expressed in most if not all plant species (Wojtaszek, 1997). For oxalate to suppress this response, it would have to either inhibit the activated free radical–generating oxidase directly or block a signaling step leading to activation of the oxidase. Recent biochemical data indicate that oxidase activation involves tightly regulated signal transduction pathways, including the recognition of pathogen- or plant-derived elicitor molecules by plasma membrane or cytosolic receptors (Wendehenne et al., 1995; Nennstiel et al., 1998) and the activation of several signaling enzymes, including GTP binding proteins (Legendre et al., 1992; Kawasaki et al., 1999), phospholipases (Legendre et al., 1993a; Chandra et al., 1996), and protein kinases (Schwacke and Hager, 1992; Chandra and Low, 1995; Cazale et al., 1999). Of relevance to the present study, the oxidative burst is also known to be suppressed at low pH (Legendre et al., 1993b) and to require an increase in cytosolic Ca2+ (Schwacke and Hager, 1992; Tavernier et al., 1995; Chandra et al., 1997; Jabs et al., 1997). Because release of oxalate could conceivably both lower the pH and chelate the Ca2+ ions, we have hypothesized that oxalic acid might enhance fungal pathogenicity by inhibiting the oxidative burst of the host plant.
In this report we demonstrate that the oxidative burst is indeed suppressed in the presence of oxalate generated by virulent Sclerotinia. In contrast, an oxalate-deficient nonpathogenic strain was unable to inhibit the oxidative burst. On the basis of above considerations, we also have explored mechanisms by which oxalate secretion by Sclerotinia might reduce H2O2 production. Although a decrease in apoplastic pH cannot be dismissed as a contributing factor, we show that chelation of Ca2+ ions is largely unimportant. Most importantly, however, we demonstrate that oxalate blocks a signaling event in the oxidative burst pathway even at the optimal pH of the pathway. Together, these data reveal a previously undescribed function of oxalate secretion by Sclerotinia, namely, to suppress active oxygen generation and thereby to compromise the defense responses of the host plant.
RESULTS
Oxalate Suppresses the Plant Cell Oxidative Burst
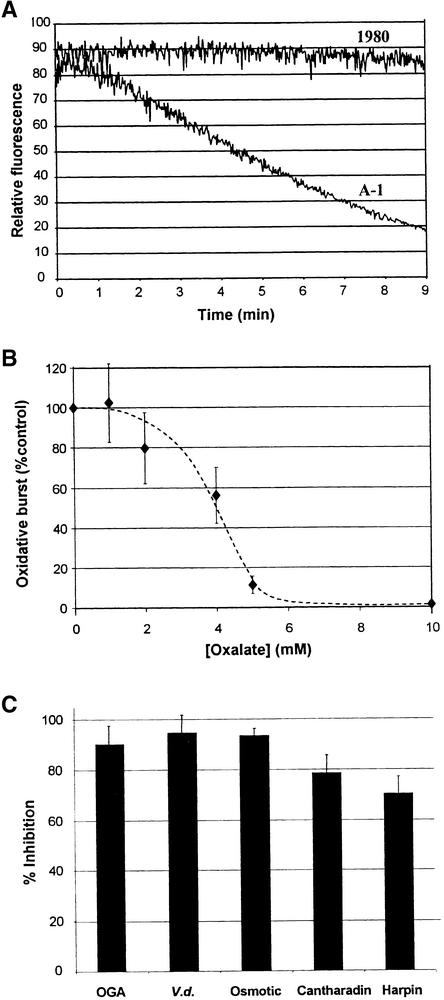
To evaluate whether oxalate secretion by Sclerotinia might result in inhibition of the plant cell oxidative burst, we inoculated tobacco leaves with both a wild-type (1980) and an oxalate-deficient (A-1) strain of the fungus. Although only the oxalate-secreting 1980 strain successfully colonized the leaf tissue (Figure 1A), only the oxalate-deficient A-1 mutant induced a measurable oxidative burst in the tobacco leaves, as indicated by the accumulation of the oxidized blue form of nitroblue tetrazolium (NBT) dye (Figures 1A and 1B). Consistent with previous reports (Godoy et al., 1990), filtrates from the A-1 strain contained ∼120-fold less oxalate than filtrates from the 1980 strain (0.11 ± 0.03 versus 12.4 ± 5.4 mM oxalic acid, respectively). To determine whether this large difference in oxalate concentration might differentially affect the capacity of plant cells to mount an oxidative burst, we adjusted the pH of the two fungal filtrates to that of our plant cell suspension cultures (pH 5.7) and reexamined their impact on the oligogalacturonic acid (OGA)–induced oxidative burst in both tobacco and soybean cells. As shown in Figure 2A, the oxalate-containing filtrate from the 1980 strain almost quantitatively suppressed oxidant production in tobacco cells, whereas the oxalate-deficient medium from the A-1 strain allowed unrestricted H2O2 production. Furthermore, addition of 11 mM oxalate to the A-1 filtrate at pH 5.7 converted this filtrate to an equally potent inhibitor of the elicitor-induced oxidative burst in suspension cultures (data not shown). Similar results were also obtained in suspension-cultured soybean cells (data not shown). We conclude that oxalate either directly or in combination with other fungal components strongly suppresses the oxidative burst activity of the host plant.
Figure 1.
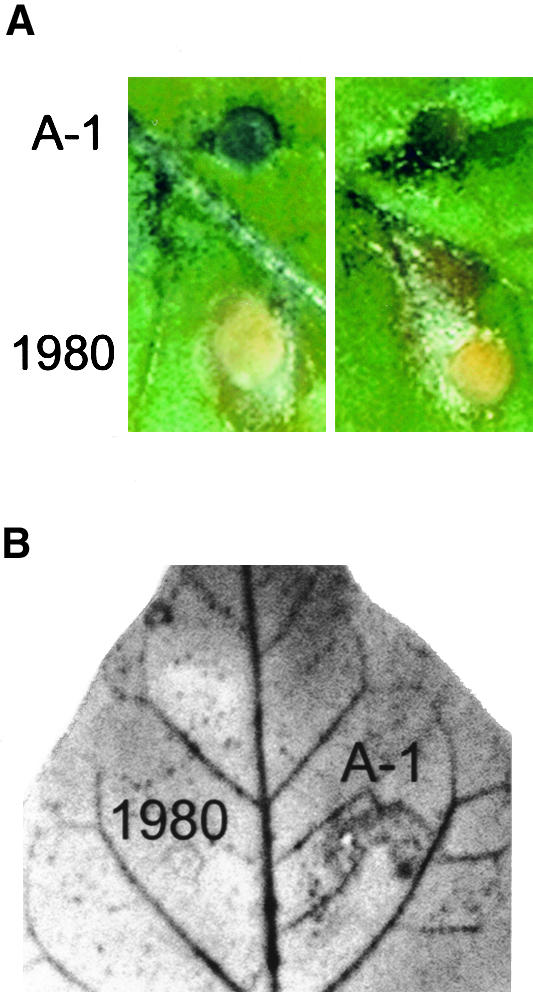
Differential Oxidant Accumulation in Leaves Infected with an Oxalate-Producing (1980) and an Oxalate-Deficient (A-1) Strain of the Fungal Pathogen Sclerotinia.
(A) NBT-treated tobacco leaves were inoculated on either side of their primary leaf veins with agar plugs embedded with the fungal hyphae of either the A-1 oxalate-deficient mutant of Sclerotinia or the wild-type 1980 strain, as previously described (Godoy et al., 1990). After 2 days of growth in high humidity, samples were removed and photographed. The agar plugs embedded with the A-1 fungus accumulated the insoluble oxidized blue form of NBT, reflecting the accumulation of oxidant in the infected area, but the embedded plugs of 1980 did not. Sections of two representative leaves are shown.
(B) A tobacco leaf inoculated as shown in (A) with the 1980 and A-1 strains of Sclerotinia was washed in ethanol to remove the chlorophyll and then photographed. All fungal material washed away in the ethanol. Similar results were obtained in four independent experiments.
Figure 2.
Inhibition of Plant Cell Oxidative Burst by Oxalate.
(A) Suspension-cultured tobacco cells were filtered and resuspended in preparations from either a wild-type oxalate-secreting strain (1980) or a mutant oxalate-deficient strain (A-1) of Sclerotinia and were monitored for OGA-induced H2O2 production, as described in Methods. OGA (5 μg/mL), an elicitor of the oxidative burst, and pyranine (1 μg/mL) were added immediately before beginning to record fluorescence. The data shown are representative of four separate experiments.
(B) Soybean cells were monitored for OGA-stimulated oxidative burst activity in the presence of increasing concentrations of pH-buffered oxalate (pH 5.7, added to the cell growth media), as described in Methods. Data are presented as the percentage of the control ±sd, where 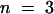 or more for each data point.
or more for each data point.
(C) The relative rates of H2O2 production in soybean cells were compared in the presence and absence of 10 mM oxalate after stimulation with elicitors of the oxidative burst: OGA (5 μg/mL), Verticilium elicitor (V.d.; 2% v/v), hypoosmotic shock (Osmotic; 1:1 dilution of sample with distilled H2O), cantharadin (2 μM), or harpin (6 μg/mL). Data are presented as the average percentage (±sd) of inhibition of the rate of pyranine quenching relative to that of controls measured on the same day. 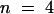 or more in every case.
or more in every case.
To explore whether additional components in the fungal filtrates might be required for expression of the inhibitory potency of oxalate, we also examined the effect of adding oxalate directly to suspension-cultured cells in the absence of any other fungal material. As shown in Figure 2B, oxalic acid directly inhibited the OGA-stimulated production of H2O2 by soybean cells, even in the absence of other fungal components. Thus, oxalate was found to suppress OGA-induced oxidant biosynthesis with a median inhibitory concentration (IC50) of ∼4 mM and maximal inhibition at ∼6 mM. Importantly, this inhibitory effect was independent of the nature of the elicitor, displaying roughly equivalent potency in blocking the oxidative burst initiated by five distinct stimuli, including the plant cell wall–derived OGA elicitor, an elicitor from Verticilium wilt fungus, hypoosmotic shock, the protein phosphatase inhibitor cantharadin, and the Erwinia protein elicitor harpin (Figure 2C). Thus, the measured IC50 values were ∼4 to 5 mM for all modes of elicitation of both soybean and tobacco cells alike (data not shown). Interestingly, however, although maximal inhibition was reached by ∼6 to 7 mM oxalate for all five stimuli, the maximal percentage of inhibition varied slightly. Thus, as shown in Figure 2C, the harpin-induced burst was maximally inhibited to only ∼30% of the control value, perhaps indicating that this elicitor induces H2O2 production by both oxalate-sensitive and oxalate-insensitive mechanisms. Regardless, the IC50 values for oxalate inhibition of the oxidative bursts stimulated by all of theses elicitors fall within the physiological range of oxalate concentrations seen in planta during Sclerotinia infections (Bateman and Beer, 1965; Maxwell and Lumsden, 1970; Marciano et al., 1983; Godoy et al., 1990), indicating that the general ability of oxalate to inhibit the burst could explain its role as a virulence factor.
Investigation of the Mechanism of Oxalate Inhibition
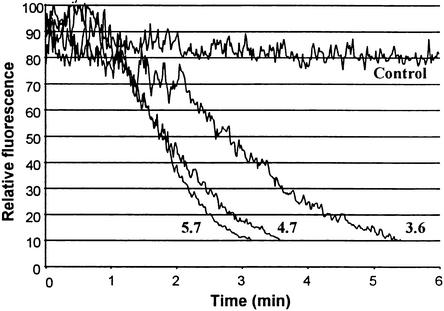
Although mechanistic studies of oxalate's potentiation of fungal pathogenicity have not been conclusive, most hypotheses have centered on the possible effect of oxalate on plant cell Ca2+ and apoplast pH. Indeed, the fungal filtrates of the A-1 and 1980 strains of Sclerotinia were found to have pH 4.7 and 3.6, respectively, suggesting that accumulation of oxalate had the anticipated effect on filtrate acidity (Godoy et al., 1990). To evaluate whether the magnitude of this pH change was sufficient to impact oxidative burst activity, we adjusted tobacco cell suspensions to these lower pHs and compared their rates of OGA-stimulated H2O2 biosynthesis with control suspensions at their normal pH of 5.7. As shown in Figure 3, lowering the pH of the medium to 3.6 indeed led to greater inhibition of oxidative burst activity than that seen in pH 4.7 medium. However, neither pH was as inhibitory as oxalate administration, and whether such lower pHs might actually be imposed on infected tissue in planta is also uncertain. Because oxalate remains a potent suppressor of the oxidative burst even at high pHs (Figure 2), clearly other inhibitory mechanisms than apoplast acidification must be operative.
Figure 3.
Effect of Lowering the Media pH on Elicitor-Induced Oxidative Burst.
Suspension-cultured tobacco cells were filtered and resuspended in fresh Murashige and Skoog (1962) media adjusted to the indicated pH (4.7 and 3.6) with hydrochloric acid and then monitored for OGA-stimulated H2O2 production as described in Figure 1. Unelicited control cells were suspended in fresh media at pH 5.7. These data are representative of three different experiments.
Investigations into the Role of Ca2+ Chelation in the Inhibition by Oxalate
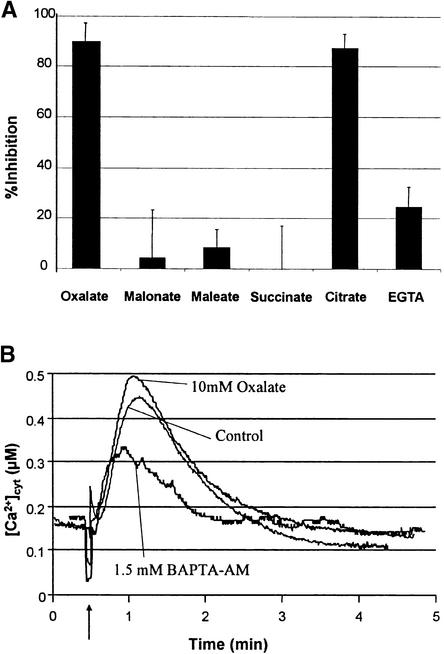
Because high affinity for Ca2+ is a well-recognized property of oxalate, a second mechanism investigated was related to the chelation by oxalate of extracellular Ca2+. To explore whether Ca2+ sequestration might explain oxalate's potency, we compared the inhibitory activity of oxalate with that of other chelators of similar and greater affinity for Ca2+. As shown in Figure 4A, whereas both oxalate and citrate inhibited H2O2 production by >90%, EGTA, a Ca2+ chelator with at least 104-fold greater affinity for Ca2+ (Martel and Smith, 1977), inhibited the OGA-stimulated oxidative burst by only ∼25%. Furthermore, although oxalate and malonate bind Ca2+ with similar binding constants (Martel and Smith, 1977), only oxalate displays a measurable ability to inhibit the oxidative burst (Figure 4A). Apparently, therefore, the binding of extracellular Ca2+ is not the primary mechanism by which oxalate suppresses the oxidative burst.
Figure 4.
Role of Ca2+ Chelation in Oxalate Inhibition of the Oxidative Burst.
(A) The inhibitory potencies of oxalate, malonate, maleate, succinate, citrate, and EGTA were measured by adding their pH-buffered (pH 5.7) sodium salts to soybean cells at 10 mM. The relative rates of pyranine quenching as a result of OGA stimulation were then compared in treated and untreated cells and reported as in Figure 2C. Similar results were also obtained for OGA-activated tobacco cells (data not shown). Data shown are the average of three independent experiments ±sd.
(B) Cytosolic Ca2+ concentrations were monitored in aequorin-transformed tobacco cells in the presence and absence of 10 mM oxalate or 1.5 mM BAPTA-AM, as described in Methods. At the time indicated by the arrow, oxalate and 5 μg/mL OGA were delivered simultaneously into the 1-mL cell suspension in the luminometer cuvette. BAPTA-AM in DMSO was delivered into the cuvette 5 min before OGA stimulation. Control and oxalate-treated cells were treated with DMSO at 5 min before OGA stimulation as a solvent control. Data are representative of four independent experiments.
We also considered that oxalate, by virtue of its smaller size or some other unique property, might be more permeable than other chelators to plant cells and thereby more capable of chelating intracellular Ca2+. In this regard, we previously had shown that several elicitors stimulate cytosolic Ca2+ fluxes and that the expression of these Ca2+ pulses is required for the ensuing oxidative bursts (Chandra et al., 1997). Accordingly, we measured elicitor-stimulated cytosolic Ca2+ transients in aequorin-transformed tobacco cells in the presence and absence of inhibitory concentrations of oxalate. As shown in Figure 4B, 10 mM oxalate is clearly not inhibitory but instead invariably increases the OGA-stimulated Ca2+ pulse. Oxalate was also ineffective at inhibiting the Verticilium elicitor–stimulated Ca2+ transient (data not shown). In contrast, 1,2-bis-(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-tetra(acetoxymethyl) ester (BAPTA-AM), a membrane-permeable Ca2+ chelator, measurably inhibited the OGA-activated oxidative burst (Figure 4B). Thus, unlike BAPTA-AM, perhaps oxalate does not enter the cell and disrupt cytosolic Ca2+ fluxes. We concluded, therefore, that oxalate does not inhibit H2O2 production by reducing free Ca2+ concentrations in the cell cytosol but rather inhibits H2O2 at some point downstream of cytosolic Ca2+ entry.
Oxalate Does Not Inhibit the Assembled/Activated Oxidant-Generating Complex
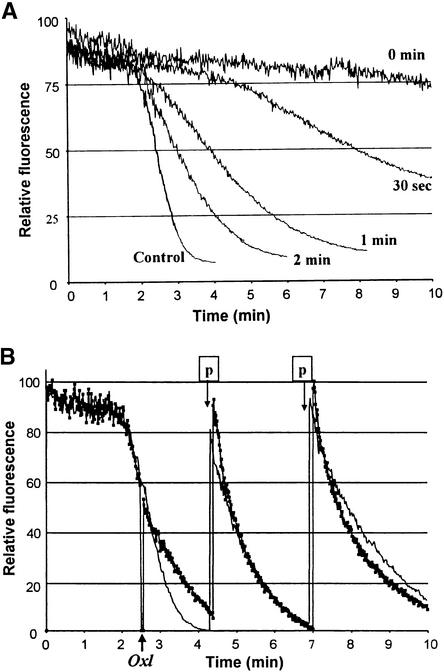
Although little is known regarding the signaling/assembly processes that occur downstream of Ca2+ entry, one event that must necessarily transpire at a downstream locus is the synthesis of O2.−/H2O2 by an activated oxidase (Groom et al., 1996; Blumwald et al., 1998; Keller et al., 1998; Bolwell, 1999). To learn whether oxalate might directly inhibit the activity of this enzyme complex, we examined the impact of adding oxalate at progressively later times after OGA stimulation. As seen in Figure 5A, addition of oxalate at 30 sec after OGA stimulation measurably reduced the burst activity. However, treatment with oxalate after increasingly extended delays resulted in progressively weaker inhibition of the oxidative burst. This trend continued until oxalate became totally innocuous when added after H2O2 production had reached its maximal rate (Figure 5B). Similar results were also found with Verticilium elicitor–stimulated soybean cells and with both OGA- and Verticilium elicitor–stimulated tobacco cells (data not shown). These data suggest that oxalate inhibits the oxidative burst at a stage preceding the catalysis of O2 reduction by an assembled/activated oxidase.
Figure 5.
Lack of Inhibition of the Oxidative Burst by Oxalate Once the Burst Had Been Initiated.
(A) Soybean cells (1.5 mL) were placed in a fluorescence spectrophotometer with 1 μg/mL pyranine. OGA (5 μg/mL) was added immediately before the beginning of each recording, and 10 mM oxalate was then added at the indicated times after OGA addition. (B) As in (A), except that 10 mM oxalate was added at the time indicated by the arrow. Note the decrease in fluorescence as the shutter closed while the oxalate solution was being added. The trace marked by small black squares represents the data collected from oxalate-treated cells. At the time points marked p, additional reduced pyranine was added to the cuvettes to allow for continuous recording of the oxidative burst.
The data shown in (A) and (B) are representative of three independent experiments each. After the oxalate was added, inner-filter effects caused by the formation of a white precipitate were corrected by increasing the lamp energy.
DISCUSSION
The plant pathogen Sclerotinia can infect >400 species of plants with diverse phylogenetic backgrounds, thus making it one of the most successful and economically important of the fungal pathogens (Boland and Hall, 1994). Identifying determinants of Sclerotinia pathogenicity is obviously important for developing strategies to combat its spread among agronomically important crops, particularly because traditional control measures have proven to be inadequate. Sclerotinia is also a member of a group of at least 12 fungal plant pathogens that generate and secrete millimolar concentrations of oxalate into their surroundings. In several cases, oxalate secretion has been shown to be required for pathogenesis (Dutton and Evans, 1996). Therefore, considerable research interest has been focused on identifying mechanisms by which oxalate aids in the colonization of plant tissues by these fungi. Our data demonstrate a probable site of action for fungally secreted oxalate in facilitating fungal infections: suppression of the oxidative burst of the host plant. Indeed, inhibition of H2O2 production by oxalate was shown to be complete at concentrations measured in leaf material infected with pathogenic strains of Sclerotinia (Godoy et al., 1990), and its potency was equally expressed in two diverse plant species (tobacco and soybean) stimulated by five unrelated elicitors.
Two highly plausible hypotheses that have previously been offered to explain the essential role of oxalate in Sclerotinia pathogenicity ascribe its potency either to its acidity, which is believed to aid fungal invasion by direct cellular toxicity or by development of a more suitable apoplastic pH for cell wall–degrading enzymes (or both), or to its affinity for Ca2+, which may weaken plant barriers by leaching the stabilizing cation from the host plant cell wall (reviewed in Dutton and Evans, 1996). We explored both mechanisms, anticipating that one or both might account for potency of oxalate in inhibiting H2O2 production. Although oxalate secretion was accompanied by a lowered pH of the growth medium sufficient to partially suppress the oxidative burst, oxalate nevertheless remained inhibitory even when the pH decrease was prevented, suggesting that pH reduction alone was insufficient to explain complete suppression of oxidant production by oxalate. Furthermore, over the course of each experiment performed, the suspension cells remained viable, indicating that the inhibition was not attributable to any acidic toxicity of oxalate. Additional lines of evidence further suggested that Ca2+ chelation by oxalate did not facilitate its suppression of H2O2 production. Nevertheless, we cannot completely discount calcium chelation by oxalate as a mechanism of suppressing the oxidative burst in planta, for as we have noted previously (Apostol et al., 1987), inhibition of the oxidative burst by Ca2+ chelators such as EGTA is strongly time and dose dependent. Thus, if suspension-cultured cells are incubated for extended periods (>10 min) with chelator concentrations greatly exceeding the total Ca2+ concentration of the media (∼3 mM), internal Ca2+ stores may become depleted, resulting in a loss of oxidative burst activity. Thus, when plant tissues are invaded by an oxalate-secreting fungus and the oxalate is present in high concentrations for an extended period (Maxwell and Lumsden, 1970), suppression of the oxidative burst by Ca2+ chelation could ensue. However, because depletion of internal Ca2+ pools was specifically prevented in our studies by exposing the cells to oxalate for only short periods (<1 min), an additional site of oxalate action must be operative, presumably downstream of the observed Ca2+ pulse required for signaling the burst (Figure 3B) (Chandra et al., 1997).
Oxalate chelation of some cation other than Ca2+ (e.g., Fe2+, Fe3+, Cu2+, and Mn2+) might also be responsible for its inhibitory effects. However, EGTA chelates these and most other di- and trivalent cations with 10- to 104-fold greater affinity than oxalate (Martel and Smith, 1977) but without substantially inhibiting the oxidative burst (Figure 2C), thus indicating that inhibition by oxalate may be completely independent of its cation binding capacity.
Because stimulation of the oxidative burst is also known to require activation of protein kinases (Schwacke and Hager, 1992; Chandra and Low, 1995; Lebrun-Garcia et al., 1998; Cazale et al., 1999; Romeis et al., 1999), oxalate conceivably could interrupt the initiation of the oxidative burst–signaling program by altering the function of one or more relevant kinases. However, because the oxidative burst induced by the protein phosphatase inhibitor cantharadin is potently inhibited by prior exposure to oxalate (Figure 2C), the blockade by oxalate most probably occurs at a point downstream of protein phosphorylation. Another possible site of action of the inhibition by oxalate is superoxide dismutase. However, because oxalate does not inhibit the burst after it has already begun (Figure 5), the oxalate-sensitive event must occur before both the reduction of O2 by an activated oxidase and the dismutation of O2.− to H2O2. Further experimentation is obviously required to identify the exact molecular target or targets of oxalate inhibition.
Given that many plant species can generate an oxidative burst to resist pathogen attack, oxalate suppression of the oxidative burst could help explain the broad host range exhibited by Sclerotinia and related oxalate-secreting fungi. Our data have shown that oxalate is effective in blocking the burst in two unrelated plant species (soybean and tobacco) as stimulated by a variety of elicitors. If sufficient quantities of oxalate can be discharged from the fungus before the plant has detected the intruder, then a primary site of disease resistance (i.e., the oxidative burst) will be compromised. Based on the data in Figure 4, the relative rates of pathogen detection by the plant cell and oxalate secretion by the invading fungus are clearly the most critical variables in determining the success of this strategy (see also Zhou and Boland, 1999).
Because of the potentially devastating effects of oxalate on disease susceptibility, one might think that the plant kingdom would have evolved countermeasures to offset the advantage of an oxalate-secreting pathogen. Indeed, oxalate triggers a marked increase in the production of phytoalexins in cotton (Davis et al., 1992), and other plant species have evolved mechanisms of removing apoplastic oxalate and producing H2O2 in the process by expression of the enzyme oxalate oxidase (Lane et al., 1993; Wojtaszek, 1997; Zhou et al., 1998). These latter species include wheat and barley, which perhaps not surprisingly are not suitable hosts for Sclerotinia (Boland and Hall, 1994). In light of the findings in this and previous papers, we predict that expression of oxalate oxidase in susceptible plant species may render them more resistant to otherwise highly virulent oxalate-secreting pathogens.
METHODS
Plant Transformation and Cell Cultures
The aequorin-encoding DNA plasmid pMAQ2, developed by Knight et al. (1991), was purchased from Molecular Probes and used for Agrobacterium-mediated transformation of tobacco leaf disks (Nicotiana tabacum var Wisconsin 38) by the method of Liu et al. (1994). Transformants were selected on media containing 200 mg/L kanamycin. Leaf material from the original transformants was then used to establish callus and suspension cultures, as described previously (Chandra et al., 1997). Cell cultures prepared from wild-type tobacco cells of the same lineage responded similarly to stimulants of the oxidative burst. This similarity in behavior of wild-type and aequorin transformants indicates that incorporation and expression of the aequorin gene do not interfere with the pathways under study.
Suspension cultures of soybean cells (Glycine max var Kent) were established and maintained as previously described (Legendre et al., 1993b). All experiments were performed within 18 to 36 hr of transfer to fresh media, when the suspension cultures were most responsive to elicitors.
Elicitors
Oligogalacturonic acid (OGA) (Legendre et al., 1993b) and an elicitor derived from the fungus Verticilium dahlia (Davis et al., 1992) were prepared as previously described. Harpin, a protein elicitor of the oxidative burst and the hypersensitive response isolated from the plant pathogen Erwinia amylovora (Wei et al., 1992; Baker et al., 1993), was generously provided by Dr. Steven Beer (Cornell University, Ithaca, NY). Cantharadin, a phosphatase inhibitor and potent stimulator of H2O2 production in several plant species (Shirasu et al., 1997; Van Gestelen et al., 1998), was purchased from Calbiochem (La Jolla, CA).
Fungal Cultures and Preparations
A wild-type isolate (1980) and an oxalate-deficient, near-isogenic phototropic mutant (A-1) of Sclerotinia sclerotiorum were grown on potato dextrose agar plates as previously described (Godoy et al., 1990). After 10 days of growth, the soluble material was extracted from the cultures by adding warm (37°C) 200 mM aqueous sucrose (1 mL/mL of original culture media) to the fungal mat. The agar with the embedded fungus was then blended with a spatula, and the resulting material was filtered sequentially through Whatman paper and a 10-kD cutoff membrane (Bio-Rad, Hercules, CA). These filtrates were first analyzed for their initial pH and then adjusted to the pH of the plant cell media (pH 5.7) with NaOH. Analysis of the protein content and osmolarity showed negligible differences between the filtrates from the two fungi. Oxalate concentrations of the filtrates were determined with an enzyme-based colorimetric assay, as described elsewhere (Yriberri and Possen, 1980).
Fungal Inoculations and Measurements of Reactive Oxygen Species in Whole Tobacco Leaves
Tobacco plants were grown at 22°C, with a 14-hr-light/10-hr-dark cycle. The upper surfaces of fully expanded mature leaves were painted with a solution containing 1 mM nitroblue tetrazolium (NBT) and 5 mM Mes adjusted to pH 5.5 and then were inoculated with fungus as previously described (Godoy et al., 1990). We had to take care not to wound the leaves, because that led to an abundant accumulation of the oxidized blue form of the dye throughout the leaf, indicating that wounding may induce an oxidative burst (see also Orozco-Cardenas and Ryan, 1999). In some cases, leaves were washed in 95% ethanol for 48 hr to remove endogenous chlorophyll and thereby increase the contrast of the dark blue ethanol-insoluble NBT stain.
Fluorometric Determination of H2O2 Production
The production of H2O2 was monitored in cell cultures by measuring the oxidative quenching of pyranine (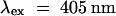 ,
, 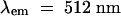 ; Molecular Probes), as previously described (Low and Heinstein, 1986). Briefly, 1.5 mL of suspension-cultured cells was added to a fluorometer cuvette and treated with 1 μg/mL pyranine. After addition of an elicitor, the rate of H2O2 biosynthesis was approximated by measuring the maximum rate of quenching of the fluorescent dye. Because this rate varied in control cells on different days, all data on modified cells are presented as a percentage of the rate for the control cells tested on the same day. Fluorescence tracings of oxalate-treated cells were corrected for inner-filter effects of white calcium oxalate precipitation. When desired, chelating agents were added at the time of elicitor stimulation, except in the case of 1,2-bis-(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-tetra(acetoxymethyl) ester (BAPTA-AM; Molecular Probes, Eugene, OR), which was added 5 min prior to elicitation.
; Molecular Probes), as previously described (Low and Heinstein, 1986). Briefly, 1.5 mL of suspension-cultured cells was added to a fluorometer cuvette and treated with 1 μg/mL pyranine. After addition of an elicitor, the rate of H2O2 biosynthesis was approximated by measuring the maximum rate of quenching of the fluorescent dye. Because this rate varied in control cells on different days, all data on modified cells are presented as a percentage of the rate for the control cells tested on the same day. Fluorescence tracings of oxalate-treated cells were corrected for inner-filter effects of white calcium oxalate precipitation. When desired, chelating agents were added at the time of elicitor stimulation, except in the case of 1,2-bis-(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-tetra(acetoxymethyl) ester (BAPTA-AM; Molecular Probes, Eugene, OR), which was added 5 min prior to elicitation.
Aequorin-Dependent Ca2+ Measurement
Luminescence measurements were conducted on aequorin-transformed tobacco cells as a method of monitoring changes in the concentration of cytosolic Ca2+ (Knight et al., 1991; Chandra et al., 1997). When desired, 10 mM oxalate or 1.5 mM BAPTA-AM was incubated with the cells 5 min prior to stimulation with elicitor. After each luminescence recording on stimulated cells, residual functional aequorin was quantified by injecting 0.2 mL of a solution containing 0.5 M CaCl2 and 10% (v/v) Nonidet P-40 into the luminometer cuvette and recording the resulting increase in luminescence. Luminescence data were then transformed into the corresponding cytosolic Ca2+ concentrations by a computer program as previously described (Chandra et al., 1997).
Acknowledgments
This work was supported by National Science Foundation Grant No. MCB-9725934 and BARD Grant No. US-3094-99CR.
References
- Apostol, I., Low, P.S., Heinstein, P., Stipanovic, R.D., and Altman, D.W. (1987). Inhibition of elicitor-induced phytoalexin formation in cotton and soybean cells by citrate. Plant Physiol. 84, 1276–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.J., Orlandi, E.W., and Mock, N.M. (1993). Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 102, 1341–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman, D.F., and Beer, S.V. (1965). Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotiorum rolfsii. Phytopathology 55, 204–211. [PubMed] [Google Scholar]
- Blumwald, E., Aharon, G.S., and Lam, B.C.H. (1998). Early signal transduction pathways in plant–pathogen interactions. Trends Plant Sci. 3, 342–346. [Google Scholar]
- Boland, G.J., and Hall, R. (1994). Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 16, 93–100. [Google Scholar]
- Bolwell, G.P. (1999). Role of active oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 2, 287–294. [DOI] [PubMed] [Google Scholar]
- Cazale, A.C., Droillard, M.J., Wilson, C., Heberle-Bors, E., Barbier-Brygoo, H., and Lauriere, C. (1999). MAP kinase activation by hypo-osmotic stress of tobacco cell suspensions: Towards the oxidative burst response? Plant J. 19, 297–307. [DOI] [PubMed] [Google Scholar]
- Chandra, S., and Low, P.S. (1995). Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc. Natl. Acad. Sci. USA 92, 4120–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, S., Heinstein, P.F., and Low, P.S. (1996). Activation of phospholipase A by plant defense elicitors. Plant Physiol. 110, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, S., Stennis, M., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272, 28274–28280. [DOI] [PubMed] [Google Scholar]
- Davis, D.A., Tsao, D., Seo, J.H., Emery, A., Low, P.S., and Heinstein, P. (1992). Enhancement of phytoalexin accumulation in cultured plant cell by oxalate. Phytochemistry 31, 1603–1607. [Google Scholar]
- Dutton, M.V., and Evans, C.S. (1996). Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 42, 881–895. [Google Scholar]
- Ebel, J., and Mithofer, A. (1998). Early events in the elicitation of plant defense. Planta 206, 335–348. [Google Scholar]
- Godoy, G., Steadman, J.R., Dickman, M.B., and Dam, R. (1990). Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol. Mol. Plant Pathol. 37, 179–191. [Google Scholar]
- Groom, Q.J., Torres, M.A., Fordham-Skelton, A.P., Hammond-Kosack, K.E., Robinson, N.J., and Jones, J.D. (1996). rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 10, 515–522. [DOI] [PubMed] [Google Scholar]
- Jabs, T., Tschope, M., Colling, C., Hahlbrock, K., and Scheel, D. (1997). Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA 94, 4800–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, T., Henmi, K., Ono, E., Iwano, M., Satoh, H., and Shimamoto, K. (1999). The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 96, 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, T., Damude, H.G., Werner, D., Doerner, P., Dixon, R.A., and Lamb, C. (1998). A plant homologue of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Lane, B.G., Dunwell, J.M., Ray, J.A., Schmitt, M.R., and Cuming, A.C. (1993). Germin, a protein marker of early plant development, is an oxalate oxidase. J. Biol. Chem. 268, 12239–12242. [PubMed] [Google Scholar]
- Lebrun-Garcia, A., Ouaked, F., Chiltz, A., and Pugin, A. (1998). Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 15, 773–781. [DOI] [PubMed] [Google Scholar]
- Legendre, L., Heinstein, P.F., and Low, P.S. (1992). Evidence for participation of GTP binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J. Biol. Chem. 267, 20140–20147. [PubMed] [Google Scholar]
- Legendre, L., Heinstein, P.F., Crain, R.C., and Low, P.S. (1993. a). Phospholipase C activation during the elicitation of the oxidative burst in cultured plant cells. J. Biol. Chem. 268, 24559–24563. [PubMed] [Google Scholar]
- Legendre, L., Rueter, S., Heinstein, P.F., and Low, P.S. (1993. b). Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 102, 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D., Raghothama, K.G., Hasegawa, P.M., and Bressan, R.A. (1994). Osmotin over-expression in potato delays development of disease symptoms. Proc. Natl. Acad. Sci. USA 91, 1888–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, P.S., and Heinstein, P.F. (1986). Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch. Biochem. Biophys. 249, 472–479. [DOI] [PubMed] [Google Scholar]
- Marciano, P., DiLenna, P., and Magro, P. (1983). Oxalic acid, cell wall degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol. Plant Pathol. 22, 339–345. [Google Scholar]
- Martel, A.E., and Smith, R.M. (1977). Critical Stability Constants. Vol. 3. (New York and London: Plenum Press).
- Maxwell, D.P., and Lumsden, R.D. (1970). Oxalic acid production by Sclerotinia sclerotiorum in infected bean and in culture. Phytopathology 60, 1395–1398. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nennstiel, D., Scheel, D., and Nurnberger, T. (1998). Characterization and partial purification of an oligopeptide elicitor receptor from parsley (Petroselinium crispum). FEBS Lett. 43, 405–410. [DOI] [PubMed] [Google Scholar]
- Noyes, R.D., and Hancock, J.G. (1981). Role of oxalic acid in the sclerotinia wilt of sunflower. Physiol. Plant Pathol. 18, 123–132. [Google Scholar]
- Orozco-Cardenas, M., and Ryan, C.A. (1999). Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 96, 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J.D. (1999). Rapid Avr9- and Cf-9–dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke, R., and Hager, A. (1992). Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta 187, 136–141. [DOI] [PubMed] [Google Scholar]
- Shirasu, K., Nakajima, H., Rajesekhar, V.K., Dixon, R.A., and Lamb, C.J. (1997). Salicylic acid potentiates an agonist gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier, E., Wendehenne, D., Blein, J.P., and Pugin, A. (1995). Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 109, 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestelen, P., Ledeganck, P., Wynant, I., Caubergs, R.J., and Asard, H. (1998). The cantharidin-induced oxidative burst in tobacco BY-2 cell suspension cultures. Protoplasma 205, 83–92. [Google Scholar]
- Wei, Z.M., Laby, R.J., Zumoff, C.H., Bauer, D.W., He, S.Y., Collmer, A., and Beer, S.V. (1992). Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Wendehenne, D., Binet, M.N., Blein, J.P., Ricci, P., and Pugin, A. (1995). Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 374, 203–207. [DOI] [PubMed] [Google Scholar]
- Wojtaszek, P. (1997). Oxidative burst: An early plant response to pathogen infection. Biochem. J. 322, 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yriberri, J., and Possen, S. (1980). A semi-automatic enzymic method for estimating urinary oxalate. Clin. Chem. 26, 881–884. [PubMed] [Google Scholar]
- Zhou, F., Zhang, Z., Gregersen, P.L., Mikkelsen, J.D., de Neergaard, E., Collinge, D.B., and Thordal-Christensen, H. (1998). Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol. 117, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., and Boland, G.J. (1999). Mycelial growth and production of oxalic acid by virulent and hypovirulent isolates of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 21, 93–99. [Google Scholar]