Abstract
The noninvasive assessment of anticancer treatment efficacy is very important for the improvement of therapeutic window. The purpose of the present study was to evaluate the antitumoral effects of the vascular targeting agent, combretastatin A-4 phosphate (CA-4-P), at selected time points after repeated intraperitoneal drug administrations (25 mg/kg), using diffusion-weighted magnetic resonance imaging (DW-MRI). The experiments were performed during an overall follow-up period of 3 weeks on WAG/Rij rats with subcutaneously growing rhabdomyosarcomas. Each animal served as its own baseline. The DW-MRI studies were quantified by calculating the apparent diffusion coefficient (ADC) for different low and high b-values to separate the effects on tumor vasculature and cellular integrity. The changes in ADC as well as the extent of necrosis development (proportional to the tumor volume), measured on the MR images, were of comparable magnitude after each treatment. All ADC values showed a significant decrease at 6 hours, followed by a significant increase at 2 days for various CA-4-P administrations. DW-MRI allowed us to monitor both reduction in perfusion and changes in the extent of tumor necrosis after CA-4-P injection. Repeated CA-4-P administration retains efficacy in rat rhabdomyosarcomas, with similar findings after each drug administration.
Keywords: vascular targeting, anticancer therapy, DW-MRI, necrosis, CA-4-P
Introduction
To overcome the limits of classic tumor treatments such as chemotherapy and radiation therapy, new approaches must be developed. One possibility is to selectively target vascularity to inhibit the development of new blood vessels (antiangiogenesis), or to destroy postangiogenic vessels (vascular targeting) in tumors [1,2].
One family of vascular targeting agents (VTAs) is comprised of low-molecular-weight compounds, which inhibit tubulin polymerization with inherent microtubule destabilization, causing acute vascular shutdown in solid tumors [3,4]. One of the lead compounds in this family is combretastatin A-4 phosphate (CA-4-P). Acute events on tumor vessel structure and function, with subsequent extensive tumor necrosis, have been described for this compound in various rodent models and some explanations on the temporal mechanism of action have been discussed [5–10]. Although the antitumor effects demonstrated in these preclinical studies were severe, a thin rim of viable tumor tissue consistently remained, from which expansion rapidly occurred. These studies were based on single treatments with doses inducing little or no systemic toxicity.
In experimental settings using CA-4-P, the response evaluation has been performed with various noninvasive methods such as 31P magnetic resonance spectroscopy and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). This follow-up was studied up to, at most, 24 hours after administration [11,12]. The dynamic imaging results were consistent with vascular volume and perfusion changes. With the exception of growth delay evaluation [7,13,14], no information is available on the efficacy of repeated drug administration in preclinical studies.
In phase I clinical trials, escalating doses of CA-4-P were applied at regular intervals, and the effects were evaluated with DCE-MRI and positron emission tomography (PET), in addition to clinical and pharmacokinetic parameters [15–18]. Yet, the effects on the tumors were not studied specifically after each drug administration.
We have shown earlier, using the rat rhabdomyosarcoma tumor model, that the antitumor effect of a single treatment with CA-4-P can be monitored noninvasively using diffusion-weighted magnetic resonance imaging (DW-MRI) [19].
The aim of the present study was to noninvasively evaluate the effects of CA-4-P on rat rhabdomyosarcomas after three equally spaced intraperitoneal injections over a period of 3 weeks using DW-MRI, and to relate the time course of changes in necrotic versus viable tumor tissue.
Materials and Methods
Tumor Model
The experiments were performed on 13 male adult WAG/Rij rats, weighing 280 to 300 g. The animals were wrapped in a warm towel to keep the body temperature constant during the average imaging period of 10 minutes. Rhabdomyosarcoma tumors (1-mm3 pieces) were implanted subcutaneously in the flank region at the level of the kidneys on opposite sides. The baseline tumor volume before treatment was 3.6 ± 1.3 cm3, reached at 2 weeks postimplantation.
The study protocol was approved by the local ethical committee for animal care and use. Rats were kept in a conventional housing facility and given access to food and water ad libitum.
MRI
The rats were examined in a 1.5-T whole-body MR system (SonataVision; Siemens, Erlangen, Germany) with a 40-mT/m maximum gradient capability. A four-channel phased-array wrist coil was used to obtain all MR images, allowing parallel imaging (parallel imaging reduction factor [generalized autocalibrating partially parallel acquisition, or GRAPPA] of two in all series). To avoid movement, rats were placed supine into a plastic holder with tube connection to a gas anesthesia system (2% isoflurane in a 20% oxygen and 80% room air mixture). For intravenous access, the penile vein was cannulated.
A coronal T1-weighted spin-echo sequence was used as localizer. A transverse T1-weighted spin-echo (553 msec/15 msec [repetition time/echo time], matrix size of 120 x 256) and a T2-weighted turbo spin-echo sequence (5860 msec/99 msec, matrix size of 160 x 256, turbo factor 19) were performed with a section thickness of 2 mm and an intersection gap of 0.2 mm. The field of view was 81.3 x 130 mm2, covering both tumors entirely (20 slices). The time of acquisition was 1:32 minutes for the T1-weighted sequence with two averages and 1:34 minutes for the T2-weighted sequence with three averages.
A DW-MRI echo-planar sequence was performed with a large range of b-values (b = 0, 50, 100, 150, 200, 250, 300, 500, 750, and 1000 sec/mm2). The following parameters were used for this sequence: TR = 3300 milliseconds, TE = 124 milliseconds, matrix size of 96 x 192, four averages, and a total time of acquisition of 2:35 minutes. The duration of both diffusion-sensitizing gradient pulses was 25 milliseconds each, without gap between the positive and negative lobes. Gradient strength was adjusted for each b-value. Apparent diffusion coefficient (ADC) maps were calculated from the native diffusion images with the built-in software tools of the MRI scanner.
All sequences were acquired with the same geometry to maintain comparability between the different imaging sequences.
Study Design
The abovedescribed MR protocol was performed on 13 rats with bilateral tumors (n = 26).
Six rats (12 tumors) were examined before and after each of the three intraperitoneal CA-4-P (OXiGENE, Watertown, MA) injections at a dose of 25 mg/kg body weight (based on earlier experiments) [7] administered with an interval period of 9 days. Treatment monitoring was performed by applying the entire MRI protocol according to the following schedule: 1) baseline study prior to first CA-4-P administration; 2) imaging at 6 hours, 2 days, and 9 days (baseline for second treatment) after the first CA-4-P administration; 3) 6 hours, 2 days, and 9 days (baseline for third treatment) after the second CA-4-P administration; and 4) 6 hours and 2 days (three of six animals) after the third drug administration. After the third CA-4-P administration, three of these animals were sacrificed at 6 hours (n = 6 tumors) and the remaining three at 2 days (n = 6 tumors).
For correlation between MRI and histopathology after a single CA-4-P administration, four rats were used: one animal (n = 2 tumors) was sacrificed before treatment, and one animal at 6 hours, 2 days, and 9 days, respectively.
As controls, the remaining three rats (n = 6 tumors) underwent the same study protocol without CA-4-P treatment to compare tumor growth and to assess potential influences of MR examinations on animals and tumor analysis.
Image Analysis
Image analysis was performed off-line on a LINUX work-station using a dedicated software (BioMAP; Novartis, Basel, Switzerland). In the transverse T1-weighted sequence, the entire tumors were manually delineated on each slice by two observers in consensus. For each tumor, the delineations on all slices were merged to produce a three-dimensional region of interest (ROI) per tumor from which the system automatically calculated the volume.
Analysis of the DW-MRI images was performed in a first step by copying the ROIs of the T1-weighted images onto the ADC maps at each time point. In a second step, several circular ROIs were placed in the periphery (the outer 5 mm) as well as in the center (more than 10 mm from the edge) of each tumor. The respective ROIs were merged to obtain one center ROI and one peripheral ROI for each tumor. Afterward, the ROIs of the ADC maps were copied to the corresponding original diffusion images, from which the average intensities for each different b-value could be obtained. The signal intensities of the ADC maps from the DW-MR images were graded as hypointense, isointense, and hyperintense by visual comparison.
The ADC is a quantitative parameter calculated from the diffusion-weighted MR images. Le Bihan et al. [20] hypothesized that the ADC reflects not only diffusion but also microcirculation of blood (perfusion). Using lower b-values in the ADC calculations shows a greater influence of perfusion, whereas by using only higher b-values, the true diffusion coefficient of the tissue is approximated. The hypothesis has been validated for this rat tumor model in a previous study; a strong correlation was observed between the perfusion influence in the DW-MR images and the perfusion parameters from a dynamic contrast-enhanced MR sequence after treatment with CA-4-P [21]. In the present study, the ADCs of the tumors were therefore calculated separately for a range of low b-values (b = 0, 50, and 100 sec/mm2; ADClow) and high b-values (b = 500, 750, and 1000 sec/mm2; ADChigh) to better differentiate the relative contribution of perfusion and true diffusion. The ADC values were calculated using a least squares solution of the following system of equations:
S(i) = S0exp(-biADC)
where S(i) is the signal intensity measured on the ith b-value image and bi is the corresponding b-value. S0 is a variable that estimates the intrinsic signal intensity (for b = 0 sec/mm2). To limit the influence of noise (from the signal measurements) on ADC calculations, diffusion images with at least three different b-values were used for calculations of ADChigh and ADClow.
The amount of necrosis in the tumors was estimated on the ADC maps (calculated from all used b-value images) by measuring the in-plane ratio of necrotic diameter versus total diameter of the tumor in the two perpendicular main directions of a central tumor slice. To reduce intraobserver variability and minimize the influence of the chosen tumor slice, the measurement was repeated on three of the center slices through the tumor and averaged afterward.
Histologic Analysis and Correlation with Imaging Results
Histopathologic correlation was obtained 6 hours, 2 days, and 9 days after a single CA-4-P administration, and 6 hours and 2 days after the third treatment. After surgical excision of the tumors, sections were made in the transverse plane corresponding to the MR sections. The tumors were fixed in a 10% formaldehyde solution. Following paraffin embedding, 5-µm sections were stained with hematoxylin and eosin. Tissue sections were assessed for cell viability (discernible heterochromatin and euchromatin in the nucleus, occurrence of mitoses) and vasculature (vessel constriction, dilatation, or congestion). All sections were screened at different magnifications up to x400 by an experienced histopathologist (E.K.V.) who was blinded to all treatment and MRI-related information. Thereafter, the histologic sections were compared with the corresponding transverse ADC maps.
Statistical Analysis
Statistical analysis was carried out with the software packages Microsoft Excel 9.0 and Analyse-It 1.68 (Analyse-It Ltd., Leeds, England, UK). Absolute numeric data are reported as mean ± standard deviation (SD), whereas percentage values are given compared to the baseline value. For statistical analysis, comparison between the treated rats at the subsequent time points was performed using the paired two-tailed Student's t test, whereas comparison of the treated rats to the controls was performed using unpaired two-tailed Student's t tests. A P-value smaller than .05 was considered significant.
Results
Tumor Volume
The tumor volume at the respective time points is given in Figure 1 for the treated rats as well as for the control animals. At 9 days after the first CA-4-P administration, a significant difference was found between the tumor volumes of the treated rats (n = 12 tumors) as compared to the control rats (n = 6 tumors) (P = .007). This difference was more significant at later time points: P = .004 after 11 days (2 days after the second CA-4-P administration), P = .002 after 18 days, and P = .0002 after 20 days (2 days after the third CA-4-P administration).
Figure 1.
Bar charts representing the tumor volume of the CA-4-P-treated rats (■) compared to the controls (□). A significant difference in volumes (P = .007) can already be observed between treated and untreated tumors after 9 days. The decrease in tumor volume observed between 18 and 20 days and the related increased SD in the treated tumors are due to the randomly selected sacrifice of three (of six) of the treated rats for histology on day 18. Error bars, ± SD.
Before CA-4-P Administration
The rat rhabdomyosarcomas evaluated at the start of the present study showed a variable proportion of necrosis on DW-MRI, mostly located in the central part of the tumors.
The ADCaverage of the entire tumor volume before treatment was 1.24 ± 0.15 (x 10-3 mm2/sec), whereas ADClow was 1.62 ± 0.18 (x 10-3 mm2/sec) and ADChigh was 1.14 ± 0.15 (x 10-3 mm2/sec). When looking at the values of center and periphery separately, higher ADC values were found in the center of the tumor (in 10-3 mm2/sec, ADCaverage of 1.69, ADClow of 1.88, and ADChigh of 1.64) and lower values in the periphery (in 10-3 mm2/sec, ADCaverage of 1.11, ADClow of 1.43, and ADChigh of 1.05).
After First CA-4-P Administration Functional changes (n = 12 tumors)
ADC of the whole tumor volume. Six hours after the first CA-4-P treatment (Figure 2B), no overall visual changes in hypointense and hyperintense regions compared to the baseline (Figure 2A) could be appreciated on the ADC maps, but all calculated ADC values decreased significantly (ADCaverage, P < .001; ADClow, P < .001; ADChigh, P = .002) (Table 1). At 2 days after the first treatment, almost the entire tumor volume was uniformly hyperintense on the ADC maps (Figure 2C) and all calculated ADC values were significantly increased (P < .001). At 9 days postinjection, a broad hypointense rim was seen surrounding a large hyperintense center (Figure 2D). At the same time, both ADCaverage and ADChigh decreased significantly (P < .001), whereas the ADClow showed no significant changes compared to the previous time point (P = .08) (Figure 3).
Figure 2.
ADC maps of transverse DW-MRI images of a rat rhabdomyosarcoma, calculated using all b-values (ADCaverage). The images are given before (A) and at different time points in between and after repeat CA-4-P administration: 6 hours (B), 2 days (C), 9 days (D), 9 days and 6 hours (E), 11 days (F), 18 days (G), 18 days and 6 hours (H), and 20 days (I). Doses of CA-4-P were administered every 9 days after day 0 (A), at day 9 (D), and at day 18 (G). The 9-day time points (D and G) after each administration served as baseline for the following evaluations. Each row represents the time points before (A, D, and G), at 6 hours (B, E, and H), and at 2 days (C, F, and I) after each CA-4-P administration. Changes on the ADC map were similar after each CA-4-P administration. Before the injection (A, D, and G), a central hyperintensity was visible with a broad hypointense periphery, corresponding to a necrotic center surrounded by solid tumor tissue. No visible changes were found 6 hours later (B, E, and H), indicating still viable tumor tissue in the periphery. After 2 days, the entire tumor volume became hyperintense (C, F, and I), showing extension of the necrotic area. This was slightly less pronounced 2 days after the third CA-4-P administration in this example tumor (I).
Table 1.
Absolute (x 10-3 mm2/sec) and Relative (%) ADC Values during Repetitive CA-4-P Administration.
| Time after the First CA-4-P Administration | |||||||||
| Before | 6 hours | 2 days | 9 days | 9 days, 6 hours | 11 days | 18 days | 18 days, 6 hours | 20 days | |
| Whole volume | |||||||||
| ADCaverage | 1.24 ± 0.15 | 1.08 ± 0.19 | 1.78 ± 0.17 | 1.43 ± 0.13 | 1.30 ± 0.14 | 1.70 ± 0.25 | 1.34 ± 0.23 | 1.19 ± 0.26 | 1.69 ± 0.19 |
| * (%) | 100 | 87 | 145 | 116 | 106 | 139 | 108 | 96 | 138 |
| † (%) | 100 | 91 | 120 | 93 | 83 | 120 | |||
| ‡ (%) | 100 | 89 | 122 | ||||||
| ADClow | 1.62 ± 0.18 | 1.13 ± 0.19 | 1.94 ± 0.20 | 1.88 ± 0.20 | 1.56 ± 0.24 | 2.05 ± 0.26 | 1.72 ± 0.23 | 1.32 ± 0.30 | 2.00 ± 0.14 |
| * (%) | 100 | 70 | 121 | 117 | 97 | 127 | 107 | 82 | 130 |
| † (%) | 100 | 83 | 109 | 92 | 70 | 111 | |||
| ‡ (%) | 100 | 76 | 114 | ||||||
| ADChigh | 1.14 ± 0.15 | 1.00 ± 0.13 | 1.74 ± 0.23 | 1.24 ± 0.15 | 1.14 ± 0.13 | 1.58 ± 0.30 | 1.16 ± 0.20 | 1.06 ± 0.26 | 1.55 ± 0.19 |
| * (%) | 100 | 89 | 154 | 109 | 101 | 138 | 102 | 93 | 137 |
| † (%) | 100 | 92 | 127 | 93 | 85 | 127 | |||
| ‡ (%) | 100 | 90 | 130 | ||||||
| Center | |||||||||
| ADCaverage | 1.69 ± 0.36 | 1.55 ± 0.38 | 1.96 ± 0.30 | 2.20 ± 0.28 | 2.15 ± 0.24 | 2.16 ± 0.23 | 2.12 ± 0.27 | 2.06 ± 0.27 | 2.06 ± 0.14 |
| * (%) | 100 | 91 | 119 | 135 | 132 | 133 | 131 | 126 | 132 |
| † (%) | 100 | 98 | 99 | 97 | 94 | 93 | |||
| ‡ (%) | 100 | 97 | 97 | ||||||
| ADClow | 1.88 ± 0.30 | 1.65 ± 0.49 | 2.00 ± 0.21 | 2.41 ± 0.23 | 2.29 ± 0.24 | 2.39 ± 0.23 | 2.43 ± 0.19 | 2.25 ± 0.21 | 2.32 ± 0.08 |
| * (%) | 100 | 87 | 108 | 131 | 124 | 129 | 132 | 122 | 129 |
| † (%) | 100 | 95 | 99 | 101 | 94 | 96 | |||
| ‡ (%) | 100 | 93 | 94 | ||||||
| ADChigh | 1.64 ± 0.46 | 1.51 ± 0.44 | 1.90 ± 0.36 | 1.99 ± 0.32 | 1.99 ± 0.29 | 1.95 ± 0.27 | 1.87 ± 0.32 | 1.93 ± 0.28 | 1.81 ± 0.17 |
| * (%) | 100 | 92 | 120 | 128 | 129 | 127 | 122 | 124 | 116 |
| † (%) | 100 | 100 | 99 | 94 | 97 | 91 | |||
| ‡ (%) | 100 | 104 | 96 | ||||||
| Periphery | |||||||||
| ADCaverage | 1.11 ± 0.14 | 0.97 ± 0.21 | 1.80 ± 0.27 | 1.04 ± 0.14 | 0.94 ± 0.13 | 1.59 ± 0.32 | 1.210.30 | 0.89 ± 0.11 | 1.66 ± 0.18 |
| * (%) | 100 | 88 | 164 | 94 | 86 | 144 | 111 | 81 | 156 |
| † (%) | 100 | 91 | 155 | 119 | 87 | 168 | |||
| ‡ (%) | 100 | 77 | 118 | ||||||
| ADClow | 1.43 ± 0.21 | 1.02 ± 0.26 | 1.89 ± 0.27 | 1.46 ± 0.33 | 1.04 ± 0.22 | 1.83 ± 0.33 | 1.48 ± 0.35 | 0.93 ± 0.20 | 2.00 ± 0.28 |
| * (%) | 100 | 71 | 133 | 103 | 74 | 129 | 104 | 66 | 142 |
| † (%) | 100 | 74 | 131 | 107 | 66 | 148 | |||
| ‡ (%) | 100 | 66 | 120 | ||||||
| ADChigh | 1.05 ± 0.16 | 0.99 ± 0.27 | 1.73 ± 0.28 | 0.98 ± 0.13 | 0.88 ± 0.14 | 1.47 ± 0.29 | 1.15 ± 0.26 | 0.88 ± 0.12 | 1.55 ± 0.19 |
| * (%) | 100 | 95 | 168 | 94 | 86 | 141 | 112 | 85 | 156 |
| † (%) | 100 | 91 | 151 | 119 | 90 | 163 | |||
| ‡ (%) | 100 | 79 | 115 | ||||||
Relative changes compared to the baseline before the first CA-4-P administration.
Relative changes compared to the baseline before the second CA-4-P administration.
Relative changes compared to the baseline before the third CA-4-P administration.
Figure 3.
Bar charts showing the changes in ADC values (calculated from the transverse DW-MRI images) over the entire tumor volume at the different time points before and after CA-4-P administrations as indicated on the graph (arrows). The ADCaverage values are calculated from the DW-MRI images with all employed b-values (ADClow values from b-values: 0, 50, and 100 sec/mm2; ADChigh values from b-values: 500, 750, and 1000 sec/mm2). All values are given in percentages compared to baseline. A significant decrease was found 6 hours after each CA-4-P administration, indicating the vascular shutdown as confirmed by histology. Thereafter, a significant increase occurred after 2 days (days 2, 11, and 20), corresponding to expansion of necrosis.
ADC of ROIs at the center and periphery of the tumor. At 6 hours after the first CA-4-P administration, all ADC values in the center and periphery showed a significant decrease compared to baseline, except for the ADChigh in the peripheral ROIs (ADCaverage, P = .01 and P = .04; ADClow, P = .02 and P < .001; and ADChigh, P = .01 and P = .49, for the center and periphery, respectively; Figure 4). On day 2 after treatment, all ADC values increased significantly (P = .007 for ADClow in the center of the tumor, P < .001 for all other ADC values). At 9 days, again a significant decrease was found in all ADC values (P < .005), except for the ADChigh in the center (P = .24).
Figure 4.
Bar charts showing the changes in ADC values (calculated from the transverse DW-MRI images) of ROIs placed at the periphery of the tumors at different time points before and after CA-4-P administrations. The time points for injections are indicated by arrows on the graph. The ADCaverage values are calculated from the DW-MRI images with all employed b-values (ADClow values from b-values: 0, 50, and 100 sec/mm2; ADChigh values from b-values: 500, 750, and 1000 sec/mm2). All values are given in percentages compared to baseline. During the first 6 hours after each CA-4-P administration, the ADClow decreased significantly due to vascular shutdown. Two days after each treatment, a significant increase was found for all ADC values, corresponding to an increase of the necrotic fraction.
Correlation between histopathology and ADC maps Six hours after drug administration, histopathology showed some interstitial edema at the transition between the tumor periphery and the necrotic center. The tumor cells in the periphery had still a viable profile (several mitoses seen), whereas the vessels were congested or constricted. At this time point after CA-4-P administration, the ADC maps clearly showed a broad remaining hypointense rim with a hyperintense center (Figure 2B), similar to the pretreatment images (Figure 2A). These findings suggested a remaining viable tumor tissue in the periphery, which was confirmed with histologic examination.
The histology at 2 days showed a strongly reduced size of the periphery containing a high rate of mitoses. In parallel, the necrotic portion of the tumor was substantially larger with only pyknotic cells present. At this time point, the ADC maps showed extensive hyperintensity (Figure 2C).
Seven days later, a broad rim of viable tumor cells with a very high rate of mitoses was seen histologically. This corresponded with the hypointense viable periphery on the ADC maps surrounding the hyperintense necrotic center (Figure 2D).
After the Second CA-4-P Administration Functional changes (n = 12 tumors)
ADC of the whole tumor volume. The changes of DW-MRI found after the second treatment were similar to the findings after the first CA-4-P injection (Figure 3, Table 1).
Six hours after the second treatment, the ADC maps showed no visible changes from the previous time point (Figure 2E), but all ADC values decreased significantly (ADCaverage and ADClow, P < .001; and ADChigh, P = .003). Two days later, the entire tumor was hyperintense on the ADC maps (Figure 2F) and a significant increase was observed for all ADC values (P < .001). At 9 days after the second CA-4-P administration, all ADC values decreased significantly (P < .001), and a new hypointense rim could be appreciated on the ADC maps (Figure 2G).
ADC of the ROIs at the center and periphery of the tumor. Six hours after the second CA-4-P administration, only the ADClow values at both the center and periphery decreased significantly (P = .02 and P = .003, respectively) (Figure 4). In the periphery, the ADCaverage and ADChigh values also decreased significantly (P = .04 and P = .02), whereas at the center, these two values remained largely unchanged (P = .14 and P = .97). Two days later, no significant changes were observed in all ADC values at the center (ADCaverage, P = .61; ADClow, P = .14; ADChigh, P = .22), whereas all ADC values in the periphery increased significantly (P < .001). At the 9-day time point, no significant changes were found in the center for ADCaverage (P = .12) and ADClow (P = .35) and a slightly significant change for ADChigh (P = .04), whereas all ADC values in the periphery decreased significantly (ADCaverage, P < .001; ADClow, P = .006; and ADChigh, P = .001).
After the Third CA-4-P Administration Functional changes
ADC of the whole tumor volume. The changes of DW-MRI found after the third treatment were similar to the findings following the first and second CA-4-P injections (Figure 3, Table 1).
Six hours after the third CA-4-P administration (n = 12 tumors), no visible changes were found on the ADC maps compared to the previous time point (Figure 2H), but again a significant decrease was observed for all calculated ADC values (ADCaverage and ADClow, P < .001; and ADChigh, P = .006). At this time point, three rats were sacrificed for histopathologic analysis of the tumors (n = 6).
At 2 days after the third injection (n = 6 tumors), the hyperintense area of the tumors strongly increased on the ADC maps (Figure 2I) and the corresponding calculated ADC values increased significantly (ADCaverage, P = .002; ADClow, P < .001; and ADChigh, P = .004).
ADC of the ROIs at the center and periphery of the tumor. In the tumor center, 6 hours after the third CA-4-P administration, only the ADClow decreased significantly (P < .001), whereas the ADCaverage and ADChigh remained mainly unchanged (P = .19 and .22, respectively) (Figure 4). The periphery showed a significant decrease in all ADC values at this time point (ADCaverage, P = .002; ADClow, P < .001; and ADChigh, P = .002). At 2 days after the third treatment, all ADC values in the periphery were significantly increased (ADCaverage, P < .001; ADClow, P = .001; and ADChigh, P = .001). At the center, all ADC values remained unchanged (ADCaverage, P = .86; ADClow, P = .44; and ADChigh, P = .26).
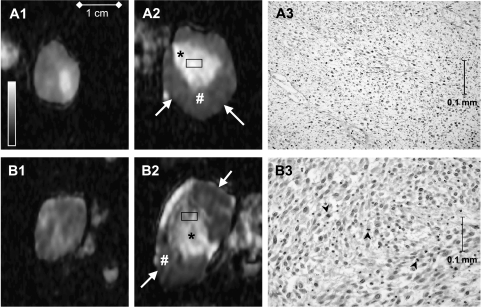
Correlation between histopathology and ADC maps At 6 hours after the third CA-4-P administration, four of the six excised tumors showed complete necrosis of the center (Figure 5, A3), whereas the other two revealed some clusters of remaining viable-looking tumor cells with some mitoses seen in the center (Figure 5, B3). In comparison to the findings after the first treatment, the demarcation between the central necrosis and the actively proliferating tumor in the periphery was more pronounced. The ADC maps (Figure 2H) showed still viable tumor tissue in the periphery, confirmed by histologic findings. The ADC maps of the tumors with complete central necrosis on histology had a higher ADC value compared to both tumors with clusters of viable cells in the center.
Figure 5.
Transverse ADC maps before the first (A1 and B1) and 6 hours after the third CA-4-P administration (A2 and B2) of two different rat rhabdomyosarcomas (A and B). Rectangles in the ADC maps (A2 and B2) approximate the location of the shown histologic (x400) panels (A3 and B3). On the ADC maps after the third treatment (A2 and B2), three regions can be depicted in each tumor: rim (arrows), viable tissue (#), and necrosis (*). Tumors (A) and (B) have different intensities in the necrotic center of the ADC maps (A2 and B2) (*). The higher intensity in tumor (A) corresponded histologically to complete necrosis (A3). The lower intensity in tumor (B) corresponded to the presence of still viable cells in tumor (B) (B3). Note the well recognizable euchromatin and heterochromatin in most nuclei and some mitotic figures (B3, arrowheads). Retrospectively, a difference between these two tumors could already be appreciated visually before the first treatment (A1 and B1).
At 2 days (n = 6 tumors), only a small rim of viable tumor cells was visible on histology accompanied by extension of the necrotic fraction. Five of the six tumors showed complete necrosis of the center, corresponding to the homogeneous bright center in the ADC maps (Figure 2I). In the remaining tumor, some clusters of viable cells were visible on histologic examination, which appeared heterogeneous and hypointense in the center of the respective ADC map.
Necrotic Tumor Area
The necrotic tumor area at the respective time points is given in Figure 6 for the treated rats as well as for the control animals. In the untreated rats, a variable but persistent increase in necrotic tumor area occurs. This is not the case in the treated animals, as the tumors experience massive necrosis induction after each CA-4-P administration. There are no significant differences between the subsequent time points of maximal necrosis proportionate to the respective tumor volumes (2-day time point after each CA-4-P administration) (between 2 and 9 days, P = .08; between 9 and 18 days, P = .92).
Figure 6.
Bar charts representing the percentage of necrotic tumor area of the treated rats (■) and the controls (□) during the follow-up sequence. In the untreated rats, a variable but persistent increase in necrotic tumor area occurs. This is not the case in the treated animals, as the tumors experience massive necrosis induction 2 days after each CA-4-P administration. Error bars, ± SD.
Retrospective ADC Assessment
During histopathologic examinations of the repeatedly treated tumors, three tumors (of 12) showed some remaining viable-looking cells in the necrotic center. These tumors had also the lowest ADC values in the central necrosis just before excision. Retrospectively, these same three tumors showed the lowest ADC values during the entire examination sequence. Even before the first treatment, these three tumors had outlying lower values compared to the nine other tumors in both ADCaverage and ADChigh (in 10-3 mm2/sec): ADCaverage values were 1.15, 0.91, and 1.05 (compared to the average of 1.24) and ADChigh values were 1.00, 0.83, and 0.95 (compared to the average of 1.14).
Discussion
From a clinical point of view, the evaluation of effects developing within hours to a few days in the tumor microenvironment after the use of anticancer agents is very important. This information can be used to predict the response in tumors, and allows the modulation of the ongoing treatment. The potential of using individual tumor-adapted treatments and the introduction of novel anticancer therapies further support the need for noninvasive imaging approaches. One such novel treatment strategy, vascular targeting using small molecules such as CA-4-P, has been shown to induce severe intratumoral damage at doses below the maximum tolerated dose (Refs. [3,22] and references therein). Based on the broad information on drug activity obtained with preclinical rodent tumors, clinical phase I trials have been performed using escalating doses of CA-4-P administered at regular time intervals [17,23].
The use of gadolinium-based DCE-MRI already provided useful information on the rapidly induced and severe changes in tumor blood flow and vessel permeability after treatment with VTAs [16,23–25]. DW-MRI has been initiated recently as a potential noninvasive technique to monitor the antitumoral effects of radiotherapy and chemotherapy with the aim to predict therapy outcome [26–36]. In contrast to conventional MRI, viable tumor can be differentiated from tumor necrosis with DW-MRI [34,35]. High cellular density is related to a low ADC value, reflecting the lack of mobility of water protons, whereas high ADC values relate to necrotic tissue with inherent diffusion of water protons due to loss of cell membrane integrity [34,36]. We have already shown earlier, using a rat rhabdomyosarcoma R1 tumor model, that DW-MRI allows assessment of intratumoral cell integrity changes after a single CA-4-P administration [19,21].
The present study involves the same rat tumor model, but the set-up of the study was extended toward repeated injections of CA-4-P and the potential of DW-MRI to evaluate the reproducibility of the induced intratumoral damage after each administration. The results illustrate that CA-4-P retained a similar efficacy after the treatment with a second and a third equal-sized dosage. The changes in ADC values after each treatment were of the same order of magnitude, with an initial decrease 6 hours after treatment followed by a strong increase at 2 days posttreatment. In a previous study using the same tumor model, the initial ADC decrease was found to be a result of both diffusion restriction due to ischemia (reflected in a decrease in ADChigh) and a decrease in perfusion (reflected by a stronger decrease in ADClow) [21]. The subsequent increase in all ADC values coincided with necrosis formation.
With the exception of the ADClow calculated at 6 hours after the second and third injections, no significant change in values was observed in the center of the tumor. The center was identified on histology as being necrotic, both after the third CA-4-P administration as well as in a separate series of tumors after a single CA-4-P administration. Hence, the ADC changes calculated in the periphery of the tumors should determine the ADC changes of the whole tumor. The translation was indeed proven by the measurements in the periphery of the tumors, which showed similar significant changes in ADCaverage, as well as in ADClow and ADChigh. Importantly, the extent of necrosis development in relation to the evolution in size of the tumors, as measured on the MR images, was very similar after each drug treatment. The parallel increase in ADC measured at 2 days after each CA-4-P administration represents this positive response to treatment noninvasively. The hypointense rim appreciated on the ADC maps at 9 days after each treatment represents viable cell-rich tissue from which tumor growth has occurred prior to the next CA-4-P treatment. It is in this peripheral area that the accelerated development of new pathologic vessels takes place and provides nutrients for tumor expansion as seen after each treatment. These newly formed microvessels may provide an improved tumor perfusion, which, together with the major influence of necrosis formation (reflected in ADChigh), is translated in an increase in ADClow 2 days after each CA-4-P administration. Changes in ADClow are, as indicated by us and others, related to a change in perfusion as well as true diffusion [19,20]. Very recently, preclinical in vivo data have been published on the impact of cell density on the potential of microvessels to provide adequate intratumoral perfusion [37]. In that study, the reduction of cell density on treatment paralleled an increase in the fraction of tumor vessels with open lumen. Such a mechanism may play a role in the increased ADClow at 2 days after CA-4-P treatment when extensive necrosis is prominently present, as measured in our study. Finally, lower values of ADC are related to high cellular density in the tumors. A study by Taouli et al. [38] analyzing focal liver lesions by DW-MRI showed that metastatic lesions and hepatocellular carcinomas had the lowest ADC. These authors attributed this finding to the higher tumoral content of these lesions, which restricts water diffusion. Retrospectively assessed, the three tumors with the lowest ADC before sacrifice were those that still showed viable tumor cells in the necrotic center after the third CA-4-P treatment (histologically proven). These three tumors had also a smaller ADC value before the first CA-4-P treatment, compared to the others.
Within the present tumor model and treatment strategy, our results show that DW-MRI allows to differentiate between viable and nonviable tumor tissues. However, the question as to the time point of the change from nonperfused (including transient vascular function deficit) but viable tissue to necrotic tumor tissue remains. Therefore, follow-up at additional time intervals between 6 and 48 hours after drug administration may provide more insights into the complex nature of tumor damage from the use of VTAs.
Conclusion
The present data demonstrate that repeated CA-4-P administration, at the time intervals used, induces reproducible effects on subcutaneously growing rhabdomyosarcomas in rats. Similar results were obtained up to 2 days after each drug administration, supporting the absence of drug-induced resistance during this treatment course. DW-MRI is therefore a promising noninvasive tool for in vivo monitoring of such treatment-induced changes and provides information on both vascular changes as well as cellular integrity. Noninvasively obtained early knowledge of response to therapy is clinically useful. It helps in making decisions as to treatment changes of individual patients, thereby preventing unnecessary toxicity of prolonged ineffective treatment of nonresponding tumors.
Abbreviations
- ADC
apparent diffusion coefficient
- CA-4-P
combretastatin A-4 phosphate
- DCE-MRI
dynamic contrast-enhanced magnetic resonance imaging
- DW-MRI
diffusion-weighted magnetic resonance imaging
- GRAPPA
generalized autocalibrating partially parallel acquisition
- PET
positron emission tomography
- ROI
region of interest
- SD
standard deviation
- VTA
vascular targeting agent
Footnotes
Harriet C. Thoeny was supported by a grant from the Bernese Cancer League and by the Senta and Kurt Hermann Foundation.
References
- 1.Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- 2.Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 1990;9:267–282. doi: 10.1007/BF00046365. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–427. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- 4.Thorpe PE, Chaplin DJ, Blakey DC. The first international conference on vascular targeting: meeting overview. Cancer Res. 2003;63:1144–1147. [PubMed] [Google Scholar]
- 5.Tozer GM, Kanthou C, Parkins CS, Hill SA. The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, Stratford MR, Dennis MF, Chaplin DJ. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 1999;59:1626–1634. [PubMed] [Google Scholar]
- 7.Landuyt W, Verdoes O, Darius DO, Drijkoningen M, Nuyts S, Theys J, Stockx L, Wynendaele W, Fowler JF, Maleux G, et al. Vascular targeting of solid tumours: a major “inverse” volume-response relationship following combretastatin A-4 phosphate treatment of rat rhabdomyosarcomas. Eur J Cancer. 2000;36:1833–1843. doi: 10.1016/s0959-8049(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 8.Prise VE, Honess DJ, Stratford MR, Wilson J, Tozer GM. The vascular response of tumor and normal tissues in the rat to the vascular targeting agent, combretastatin A-4-phosphate, at clinically relevant doses. Int J Oncol. 2002;21:717–726. [PubMed] [Google Scholar]
- 9.Kanthou C, Tozer GM. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood. 2002;99:2060–2069. doi: 10.1182/blood.v99.6.2060. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed B, Van Eijk LI, Bouma-Ter Steege JC, Van Der Schaft DW, Van Esch AM, Joosten-Achjanie SR, Lambin P, Landuyt W, Griffioen AW. Vascular targeting effect of combretastatin A-4 phosphate dominates the inherent angiogenesis inhibitory activity. Int J Cancer. 2003;105:20–25. doi: 10.1002/ijc.11010. [DOI] [PubMed] [Google Scholar]
- 11.Beauregard DA, Thelwall PE, Chaplin DJ, Hill SA, Adams GE, Brindle KM. Magnetic resonance imaging and spectroscopy of combretastatin A4 prodrug-induced disruption of tumour perfusion and energetic status. Br J Cancer. 1998;77:1761–1767. doi: 10.1038/bjc.1998.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell RJ, Wilson J, Prise VE, Vojnovic B, Rustin GJ, Lodge MA, Tozer GM. Evaluation of the anti-vascular effects of combretastatin in rodent tumours by dynamic contrast-enhanced MRI. NMR Biomed. 2002;15:89–98. doi: 10.1002/nbm.754. [DOI] [PubMed] [Google Scholar]
- 13.Chaplin DJ, Pettit GR, Hill SA. Anti-vascular approaches to solid tumour therapy: evaluation of combretastatin A4 phosphate. Anticancer Res. 1999;19:189–195. [PubMed] [Google Scholar]
- 14.Hill SA, Chaplin DJ, Lewis G, Tozer GM. Schedule dependence of combretastatin A4 phosphate in transplanted and spontaneous tumour models. Int J Cancer. 2002;102:70–74. doi: 10.1002/ijc.10655. [DOI] [PubMed] [Google Scholar]
- 15.Anderson HL, Yap JT, Miller MP, Robbins A, Jones T, Price PM. Assessment of pharmacodynamic vascular response in a phase l trial of combretastatin A4 phosphate. J Clin Oncol. 2003;21:2823–2830. doi: 10.1200/JCO.2003.05.186. [DOI] [PubMed] [Google Scholar]
- 16.Galbraith SM, Maxwell RJ, Lodge MA, Tozer GM, Wilson J, Taylor NJ, Stirling JJ, Sena L, Padhani AR, Rustin GJ. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J Clin Oncol. 2003;21:2831–2842. doi: 10.1200/JCO.2003.05.187. [DOI] [PubMed] [Google Scholar]
- 17.Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, Gumbrell L, Price PM. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- 18.Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–3416. [PubMed] [Google Scholar]
- 19.Thoeny HC, De Keyzer F, Chen F, Ni Y, Landuyt W, Verbeken EK, Bosmans H, Marchal G, Hermans R. Diffusion-weighted magnetic resonance imaging for monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756–764. doi: 10.1148/radiol.2343031721. [DOI] [PubMed] [Google Scholar]
- 20.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol. 1992;159:591–599. doi: 10.2214/ajr.159.3.1503032. [DOI] [PubMed] [Google Scholar]
- 21.Thoeny HC, De Keyzer F, Vandecaveye V, Chen F, Sun X, Bosmans H, Hermans R, Verbeken EK, Boesch C, Marchal G, et al. Comparison of dynamic contrast-enhanced MRI and diffusion-weighted MRI for monitoring the effect of a vascular targeting agent on tumors: a study in rodents. Radiology. 2005 doi: 10.1148/radiol.2372041638. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, Giantonio B, Zimmer R, Petros WP, Stratford M, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21:4428–4438. doi: 10.1200/JCO.2003.12.986. [DOI] [PubMed] [Google Scholar]
- 24.Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–3416. [PubMed] [Google Scholar]
- 25.McIntyre DJ, Robinson SP, Howe FA, Griffiths JR, Ryan AJ, Blakey DC, Peers IS, Waterton JC. Single dose of the antivascular agent, ZD6126 (N-acetylcolchinol-O-phosphate), reduces perfusion for at least 96 hours in the GH3 prolactinoma rat tumor model. Neoplasia. 2004;6:150–157. doi: 10.1593/neo.03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M, Pipe JG, Bonnett J, Evelhoch JL. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br J Cancer. 1996;73:61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chenevert TL, Stegman LD, Taylor JMG, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 28.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 29.DeVries AF, Kremser C, Hein PA, Griebel J, Kreczy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958–965. doi: 10.1016/s0360-3016(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 30.Jennings D, Hatton BN, Guo J, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MR. Neoplasia. 2002;4:255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chenevert TL, Meyer CR, Moffat BA, Rehemtulla A, Mukherji SK, Gebarsi SS, Quint DJ, Robertson PL, Lawrence TS, Junck L, et al. Diffusion MRI: a new strategy for assessment of cancer therapeutic efficacy. Mol Imaging. 2002;1:336–343. doi: 10.1162/15353500200221482. [DOI] [PubMed] [Google Scholar]
- 32.Mardor Y, Pfeffer R, Spiegelmann R, Roth Y, Maier SE, Nissim O, Berger R, Glicksman A, Baram J, Orenstein A, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094–1100. doi: 10.1200/JCO.2003.05.069. [DOI] [PubMed] [Google Scholar]
- 33.Kauppinen RA. Monitoring cytotoxic tumour treatment response by diffusion magnetic resonance imaging and proton spectroscopy. NMR Biomed. 2002;15:6–17. doi: 10.1002/nbm.742. [DOI] [PubMed] [Google Scholar]
- 34.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion-weighted magnetic resonance imaging. Magn Reson Med. 2000;43:823–836. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W, Mathur A, Gooding CA, Genant HK. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998;206:227–235. doi: 10.1148/radiology.206.1.9423677. [DOI] [PubMed] [Google Scholar]
- 36.Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45:208–213. doi: 10.1016/s0720-048x(02)00310-8. [DOI] [PubMed] [Google Scholar]
- 37.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 38.Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]