Abstract
To better understand the mechanisms of transformation by the oncogene HER-2, we transduced the human mammary epithelial (HME) cell line MCF-10A with HER-2 and developed a cell line that appeared to moderately overexpress HER-2. These MCF-10HER-2 cells were unable to grow in the absence of epidermal growth factor (EGF). However, coexpression of HER-2 with the HPV-16 oncoproteins E6 and E7 resulted in EGF-independent cells that expressed very high levels of constitutively activated HER-2. Interestingly, coexpression of E7 with HER-2 resulted in cells that were EGF-independent for growth but did not express HER-2 to high levels, and coexpression of E6 with HER-2 resulted in cells expressing higher levels of HER-2, which were still dependent on EGF for growth and survival. The MCF-10HER-2E7 and HER-2/E6E7 cells exhibited constitutive activation of a form of epidermal growth factor receptor (EGFR) that had a faster electrophoretic mobility than EGFR activated by exogenous growth factors. Exposure of cells with EGFR activation to ZD1839 (Iressa), at concentrations specific for EGFR, had little or no influence on proliferation of cells with amplified HER-2 but little or no EGFR. These results indicate that HER-2, E6, and E7 cooperate with endogenous EGFR to yield fully transformed cells.
Keywords: Her-2, HPV E6, HPV E7, EGFR, human breast cancer
Introduction
The HER-2 oncogene has been implicated as a causal factor in breast cancer development for many years. The clinical data linking the amplification and overexpression of HER-2 to prognosis [1–3] are supported by laboratory studies demonstrating a role for HER-2 overexpression in malignant transformation. In addition, many studies have examined the HER-2 oncoprotein as a signaling receptor and have studied its interaction with other members of the HER family of growth factor receptors [4–14]. Together, these studies have laid the groundwork for a detailed mechanistic understanding of how HER-2 overexpression drives the malignant behavior of human breast cancer cells.
In previous work, we have attempted to understand the role of HER-2 overexpression and signaling in the progressive acquisition of growth factor independence exhibited by human breast cancer cells with a HER-2 gene amplification. Normal human mammary epithelial (HME) cells, be they primary cultures derived from reduction mammoplasties, spontaneously immortalized cell lines, or cells artificially immortalized by viral oncoproteins or telomerase, require multiple growth factors for continuous survival and proliferation under serum-free conditions in vitro [15–21]. More specifically, these HME cells require stimulation by an insulin-like growth factor (IGF) and an epidermal growth factor (EGF)-like factor in the presence of other nutrients and hormones to proliferate continuously. Interestingly, heregulins are unique in their ability to act as either an insulin-like or EGF-like growth factor toward mammary epithelial cells [19].
Our previous work in rat models of mammary carcinogenesis demonstrated a direct relationship between the progressive acquisition of growth factor independence and malignant growth potential in vivo [22]. In this rat model, growth factor independence was mediated by constitutive activation of the rat homologue of HER-2, c-neu [23,24]. More recently, we demonstrated a direct correlation between progressive growth factor independence in human breast cancer cells with a HER-2 amplification and the level of expression of HER-2 [25,26]. In both rat and human models, the acquisition of EGF and IGF independence was associated with other transformed phenotypes and with malignant potential in vivo, and multiple growth factor independence correlated with the overall level of HER-2 expression at the message and protein levels. We were able to recapitulate this phenotypic progression by transducing HME cell lines with a HER-2 retroviral expression vector, and once again found a direct correlation between the level of HER-2 expression and the degree of growth factor independence exhibited by the cells [26]. Interestingly, when the HER-2 vector was used to infect the spontaneously immortalized MCF-10A cell line, only moderate levels of HER-2 expression were obtained, and the cells were independent of IGF but not EGF. When the HPV-16-immortalized cell line H16N2 was infected with the same vector, cells independent of both insulin and EGF that expressed very high levels of HER-2 emerged, and these HER-2-transformed cells exhibited a spectrum of transformed phenotypes [27,28]. Whereas these findings upheld the correlation between HER-2 expression levels and multiple growth factor independence, they also suggested that HER-2 can interact with human papilloma virus (HPV) oncoproteins in important ways. Thus, the present study was aimed at examining the interaction between HER-2 and the HPV-16 E6 and E7 viral oncoproteins in mediating EGF independence. The results reveal important interactions that provide insights into how HER-2 may require and interact with other genetic alterations during breast cancer progression to yield fully transformed cells.
Materials and Methods
Cell Culture
The base medium for all of the cells, except SUM-225, MDA-361, and MDA-468, was Ham's F12 media supplemented with 0.1% bovine serum albumin, 0.5 µg/ml fungizone, 5 µg/ml gentamycin, 5 mM ethanolamine, 10 mM HEPES, 5 µg/ml transferrin, 10 µM T3, 50 µM selenium, and 1 µg/ml hydrocortisone. H16N2-pTP, MCF-10HER2, and MCF-10HER2 E6 cell media were further supplemented with 10 ng/ml EGF. MCF-10 and MCF-10 E6 cell media were supplemented with 10 ng/ml EGF and 5 µg/ml insulin. SUM-190 cell medium was supplemented with 5 µg/ml insulin. The SUM-225 cell medium was Ham's F12 supplemented with 0.5 µg/ml fungizone, 5 µg/ml gentamycin, 1 µg/ml hydrocortisone, 5 µg/ml insulin, and 5% fetal bovine serum (FBS). MDA-361 and MDA-468 cells were grown in DMEM supplemented with 10% FBS, 0.5 µg/ml fungizone, and 5 µg/ml gentamycin. Cells infected with retroviral expression vectors for HPV-16 E6 and HPV E7 were selected for 2 weeks. All cell culture reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Protein Blots
Cells were lysed in a buffer containing 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 1% NP-40, 10% glycerol, 1 mM Na3VO4, 1 mM PMSF, 1% aprotinin, and 20 µg/ml leupeptin. Protein concentrations were equalized using the Løwry method. For whole cell lysates, Laemmli sample buffer was added and the samples were boiled. For immunoprecipitations, 1 µg of antibody was added to 1 mg of sample and incubated at 4°C for 1 hour. Immune complexes were then bound to protein A/G beads for 1 hour at 4°C. Immunoprecipitates were washed three times in lysis buffer. Laemmli sample buffer was added and the samples were boiled. Equal amounts of protein were separated by SDS-PAGE. The proteins were blotted to PVDF membranes and probed with the antiphosphotyrosine antibody, PY20 (MP Biochemicals, Irvine, CA). Antibodies used for immunoprecipitations were as follows: for HER-2, anti-HER-2 clone Ab-17 (Labvision, Fremont, CA); for epidermal growth factor receptor (EGFR), anti-EGFR clone Ab-5 (CalBiochem, LaJolla, CA); and for Her-3, anti-Her-3 clone Ab-4 (Labvision). All blots were repeated at least twice.
EGFR Multiplex Westerns
EGFR was immunoprecipitated as above. The immunoprecipitates were loaded on one lane across the top of a 7.5% SDS-PAGE gel, separated, and blotted to PVDF membrane. The membrane was loaded in a Miniblotter 28 (Immunetics, Cambridge, MA) and antibodies to different EGFR tyrosine phosphorylation sites (Cell Signaling, Beverly, MA and Biosource, Camarillo, CA) were used to probe different lanes of the membrane. Multiplex Westerns were repeated at least three times.
Propidium Iodide (PI) Staining
PI staining was used to determine cell cycle stage distribution and sub-G1 fraction of cycling cells. Cells were released from the plate by trypsinization, washed three times with PBS, fixed with 70% EtOH for 20 minutes at room temperature, and washed with PBS again. An amount of 0.1 ml of RNAse (1 mg/ml in PBS) was added for 30 minutes at 37°C. Cells were stained with PI (50 µg/ml in PBS) for at least 15 minutes and sorted. PI staining was done at least twice.
Growth and Survival Curves
For growth factor depletion/addition or growth with Iressa assays, cells were plated at 3.5 x 105 in triplicate in six-well plates. Plating efficiencies were obtained on day 1 after plating, after which the growth factors were removed from the media, and 2 ng/ml heregulin-β was added to the appropriate cells. Cells in complete media and cells in growth factor-depleted media were counted on day 7 after plating. For survival assays, cells were grown in their appropriate medium for 7 days, then switched to growth factor-depleted medium or growth factor-depleted medium containing 2 ng/ml heregulin-β. All cell counts were performed using a Coulter model Z1 (Coulter Corporation, Miami, FL).
Results
The Influence of HPV-16 E7 and E6 on HER-2 Expression and Growth Factor Independence
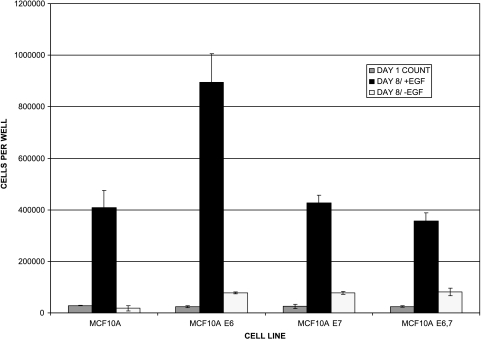
In previous studies, we demonstrated that the degree of growth factor independence of human breast cancer cells with a HER-2 gene amplification, and HME cells infected with a retroviral HER-2 expression vector, is proportional to the level of HER-2 protein overexpression [25,26]. Because the fully transformed H16N2-HER-2 cells were originally immortalized by infection with the HPV-16 viral oncoproteins E6 and E7, whereas the more moderately transformed MCF-10AHER-2 cells were derived from a spontaneously immortalized cell line, we sought to determine if infection of MCF-10HER2 cells with HPV-16 E7 and/or E6 would result in higher levels of expression of HER-2, and subsequent escape from their requirement for EGF. Accordingly, MCF-10HER2 cells or MCF-10A controls were infected with retroviral vectors for HPV-16 E7, E6, or both E6 and E7, and tested directly for the acquisition of EGF-independent growth capacity by direct selection in EGF-free medium. Table 1 shows the result of this experiment and indicates that MCF-10HER2 cells infected with the E6 retroviral vector failed to grow in EGF-free medium. By contrast, deletion of EGF from the medium of MCF-10HER2 cells infected with the E7 retroviral vector resulted in the emergence of numerous EGF-independent colonies that expanded slowly and also produced floating cells in the medium. Interestingly, MCF-10HER2 cells infected with both E6 and E7 vectors gave rise to many EGF-independent colonies that expanded rapidly, were readily subculturable, and did not produce floating cells. Infection of control MCF-10A cells with E7, E6, or both viral oncogenes never resulted in the emergence of EGF-independent cells, as shown in Figure 1. Thus, the MCF-10HER2/E6E7 cells expressed the same phenotype of EGF independence as the H16N2HER2 cells, indicating that HER-2 and HPV E6/E7 can cooperate to yield cells that are fully growth factor-independent.
Table 1.
Emergence of EGF-Independent Colonies Following Transduction of MCF-10A Cells or MCF-10HER2 Cells with HPV-16 E7, E6, or E6 and E7 Expression Vectors.
| Colonies Emerging in EGF-Free Medium | ||||
| Control | E6 | E7 | E6/E7 | |
| MCF-10A | None* | None | None | None |
| MCF-10HER2 | None | None | Many/floating† | Many‡ |
No colonies emerged after 4 weeks in EGF-free medium.
Many colonies emerged in EGF-free medium. Colonies shed floating cells into the medium.
Many colonies emerged in EGF-free medium. No floating cells were shed.
Figure 1.
EGF requirement for growth of MCF-10A cells and their E6-and E7-transduced derivatives. The control cell lines for these experiments include MCF-10A cells themselves, MCF-10AE6 cells, MCF-10AE7 cells, and MCF-10AE6E7 cells. To examine the EGF dependency of these control cell lines, cells were plated at low density in serum-free medium, cultured for 8 days in the presence or absence of EGF, and counted. Data indicate the mean number of cells per plate for triplicate plates, and the error bars indicate the range in the data.
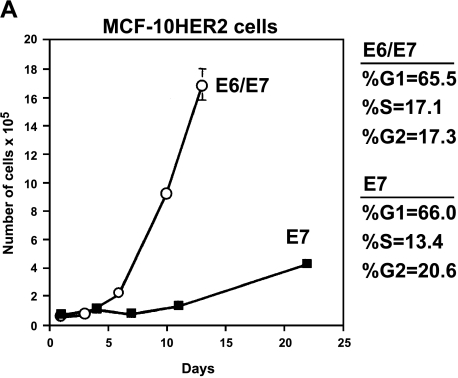
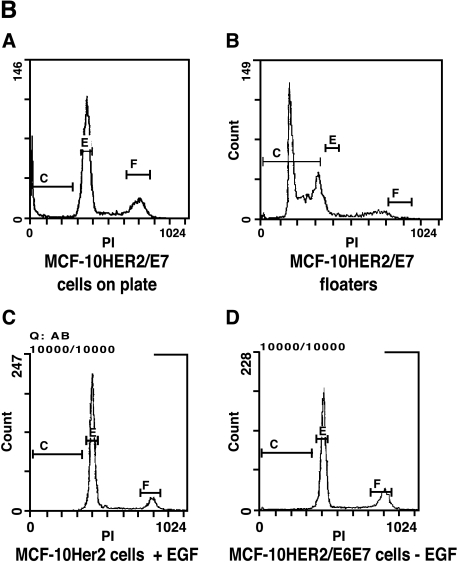
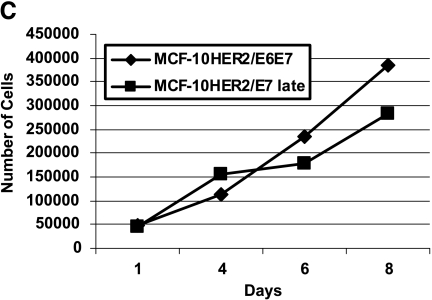
Because early-passage MCF-10HER-2/E7 cells grew slowly as a population and produced many floating cells in EGF-free medium, we analyzed the cell cycle stage distribution of these cells and the MCF-10HER-2/E6E7 cells, which were growing much more rapidly. Figure 2A shows the difference in the growth rate of the population of MCF-10HER-2/E7 cells compared to that of the HER-2/E6E7 cells, and also shows the results of the flow cytometry analysis. Because the cell cycle stage distribution was essentially the same in both populations, but the doubling times of the two cell types were very different, we hypothesized that the MCF-10HER-2/E7 cells were undergoing EGF-independent cell cycle progression similar to the HER-2/E6E7 cells, but were much less able to survive under these conditions and, as a result, were producing floating cells in the medium. Confirmation that floating cells present in the medium of the MCF-10HER2-E7 cells were apoptotic was obtained by flow cytometric analysis, which demonstrated that floating cells had sub-G1 DNA content, whereas the attached cells retained a normal DNA histogram (Figure 2B). Not surprisingly, the apoptosis that occurred under EGF-free conditions was relieved by HPV-E6 expression, which is known to disrupt p53 function in normal cells, and similar phenomena have been observed in other experimental systems [29,30]. It is important to note that the growth rate of the MCF-10HER2/E7 cell population in EGF-free medium increased with time in culture such that by after approximately 10 passages in EGF-free medium, these cells were growing as rapidly as the cells infected with both the E7 and E6 constructs and no longer produced floating cells in the medium. The growth curves for the late-passage MCF-10HER2/E7 cells compared to the HER-2/E6E7 cells are shown in Figure 2C. At present, there is no evidence that the MCF-10HER2/E7 cells acquired alterations in p53 that explain their increased growth rate with passage. Rather, our data suggest that the levels of activated EGFR increased steadily with passage, resulting in a level of EGF-independent EGFR signaling that was sufficient to maintain viability and cell cycle progression.
Figure 2.
Growth of MCF-10HER2 cells expressing HPV-16 E7 or E6E7 in EGF-free medium. (A) Cells were infected with retroviruses carrying the E7 and/or E6 genes of HPV-16, EGF was withdrawn, and the cells remaining on the plate were counted. MCF-10HER2 cells or MCF-10HER2/E6 cells did not grow in EGF-free medium. PI staining was performed on actively growing cells to obtain a cell cycle analysis. MCF-10HER2/E7 cells appeared to grow slower than MCF-10HER2/E6E7 cells; however, both cell types exhibited similar cell cycle stage parameters. (B) DNA histograms of MCF-10HER-2/E7 cells attached to the dish and floating in the medium compared to MCF-10AHER-2 cells growing with EGF, or to the EGF-independent MCF-10HER-2/E6E7 cells. Indicated cells were stained with PI and analyzed by flow cytometry. Note that the G1 peak for the attached cells and fully EGF-independent cells occurs at approximately channel 400. A G1 peak at a similar channel is seen in the floating cell population; however, a large fraction of this population is present in a sub-G1 peak that occurs between channels 200 and 350. (C) MCF-10HER2/E7 cells after 10 passages in EGF-free medium were grown without EGF for the indicated days and counted. Late-passage MCF-10HER2/E7 cultures grew at the same rate as MCF-10HER2/E6E7 cultures.
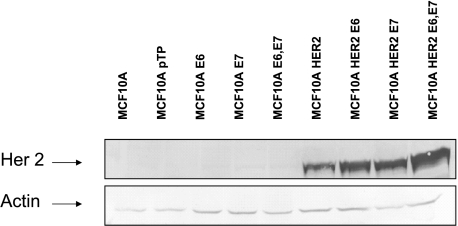
Western blot analysis (Figure 3) of the expression levels of HER-2 in the MCF-10HER2 cell lines and their E6/E7 transduced derivatives demonstrated that MCF-10HER-2/E6 cells expressed higher HER-2 levels than the EGF-independent HER-2/E7 cells. The highest HER-2 expression levels were found in the cells infected with both E6 and E7. Thus, the level of HER-2 expression did not directly correlate with growth factor independence. Rather, coexpression of HER-2 with E7 was able to drive EGF-independent proliferation, whereas coexpression of HER-2 with E6 resulted in higher overall levels of HER-2 expression. Thus, combining HER-2 with both E6 and E7 yielded EGF-independent cells expressing very high levels of HER-2.
Figure 3.
HER-2 expression levels in control MCF-10A cells, and in MCF-10HER-2 cells with E6, E7, or both. Whole cell lysates from the panel of cell lines were separated on 7.5% SDS-PAGE, blotted, and probed with anti-HER-2 antiserum. Blots were also probed with antiactin antibody to control for loading.
EGF and β-Heregulin Dependence for Growth and Survival of MCF-10HER2 Cells and Their Derivatives
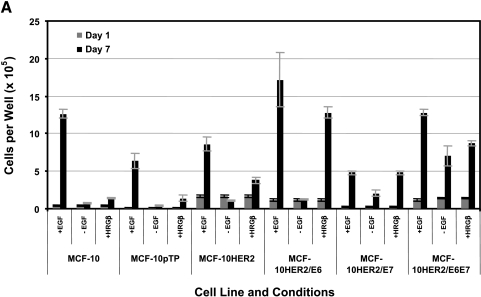
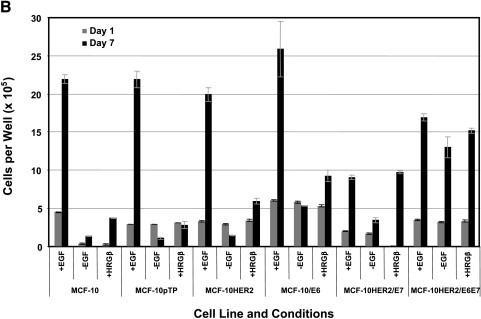
The results described above prompted experiments aimed at further defining the influence of HER-2, E6, and E7 on the requirements for EGF or β-heregulin on the growth and survival of MCF-10A cells. To measure the effect of these growth factors on cell proliferation, cells were seeded at low density and the media changed 24 hours later to defined media supplemented with or without EGF, or in EGF-free medium supplemented with β-heregulin, and cells were counted 7 days later (Figure 4A). To assess the survival effects of these growth factors, cells were seeded at low density, cultured for 7 days in their regular medium, and then switched to defined medium containing the same factor combinations as above, and then counted 7 days later (Figure 4B). The data shown in Figure 4, A and B demonstrate that MCF-10HER2 cells are dependent on EGF for both growth and survival, as both experiments showed a net cell loss over the culture period in the absence of EGF. Further, replacement of EGF with β-heregulin yielded only small increases in cell number, indicating that even when HER-2 is moderately overexpressed, heregulin is a relatively weak EGF-like growth factor, consistent with our previously published results [19]. MCF-10HER2/E6 cells showed an even greater dependency on EGF for survival than control MCF-10 or MCF-10HER-2 cells, but the E6-transduced cells also had a more robust response to b-heregulin. This result is consistent with the elevated levels of HER-2 expressed by the E6-transduced cells (Figure 5A). The MCF-10HER2/E7 cells were able to survive and proliferate in EGF-free medium, and these cells were also responsive to addition of either EGF or β-heregulin to the medium. Finally, MCF-10HER2/E6E7 cells were completely independent of EGF, and did not respond to exogenous EGF or β-heregulin with increased growth or improved survival.
Figure 4.
Growth and survival of various cells ± EGF or + heregulin (HRGβ). (A) To analyze cell proliferation, cells were plated in the indicated media and three plates were counted on day 1; the remaining plates were counted on day 7. (B) To estimate the influence of growth factors on cell survival, cells were plated and allowed to grow for 7 days, and then counted. Cells were then switched to experimental media, cultured for another 7 days, and then counted. Bars indicate the mean number of cells per 35-mm well and the error bars indicate the range in the data.
Figure 5.
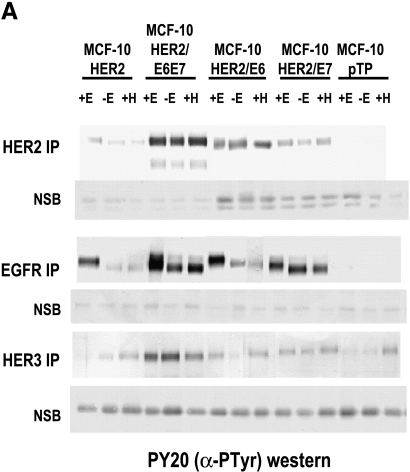
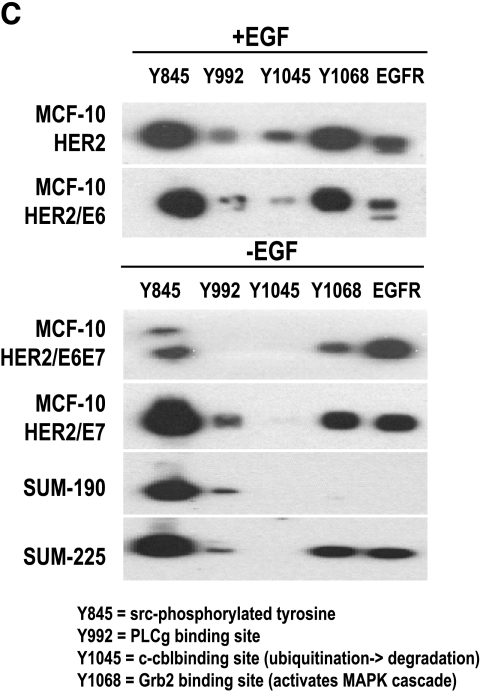
Analysis of erbB receptors in MCF-10HER-2 and derivative cell lines. (A) HER-2, EGFR, and HER3 were immunoprecipitated, separated on 7.5% SDS-PAGE, blotted, and probed for P-Tyr. Nonspecific bands (NSB) from the blots are shown to indicate equivalent loading of immunoprecipitates per cell type within the experiments. (B) HER-2 and EGFR were immunoprecipitated, separated on 7.5% SDS-PAGE, blotted, and probed for HER-2 or EGFR. Nonspecific bands (NSB) from the blots are shown to indicate equivalent loading of immunoprecipitates within each experiment. (C) EGFR was immunoprecipitated from the indicated cells. The proteins from one immunoprecipitation were electrophoresed, blotted, and fixed in a multiplex blotting apparatus. The immunoprecipitate was probed with antibodies to the indicated EGFR phosphotyrosines.
Expression and Activation of EGFR, HER-2, and HER-3 in MCF-10HER2 Cells and Their Derivatives
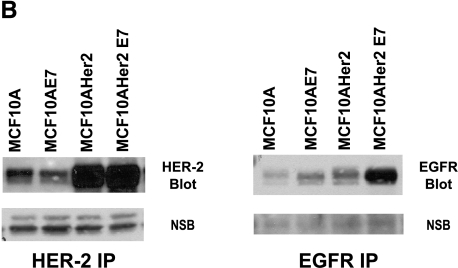
To compare the growth and survival responses of the HER-2-overexpressing MCF-10A cell populations to the expression and activation status of HER-1, HER-2, and HER-3 in these cells, immunoprecipitation/Western blot analysis experiments were performed and the results are shown in Figure 5A. Consistent with their EGF dependence, MCF-10HER2 cells only expressed high levels of tyrosine-phosphorylated EGFR when cultured in the presence of EGF, and the levels of activated HER-2 and HER-3 in these cells were higher than in vector control cells. Tyrosine-phosphorylated HER-2 was increased slightly in the presence of EGF, and tyrosine-phosphorylated HER-3 was slightly elevated in the presence of heregulin. By contrast, the fully EGF-independent MCF-10HER2/E6E7 cells expressed high levels of activated EGFR, HER-2, and HER-3 regardless of the presence or absence of growth factors. MCF10HER2/E6 cells had elevated levels of HER-2 expression and activation, yet EGFR activation was only maintained to high levels in the presence of EGF, consistent with their EGF dependency. The elevated levels of activated HER-2 in these cells are also consistent with their enhanced responsiveness to β-heregulin, as noted earlier. The results obtained with the MCF-10HER2/E7 cells were most interesting. These cells, which are the minimally perturbed cells that acquired EGF independence, expressed only moderate levels of activated HER-2, but expressed constitutively tyrosine-phosphorylated EGFR when grown in the absence of EGF. Furthermore, the electrophoretic mobility of the activated EGFR in both the MCF-10HER2/E7 cells and MCF-10HER2/E6E7 cells was consistently faster than for the cells cultured in the presence of EGF. The data shown in Figure 5B demonstrate that the MCF-10HER2/E7 cells, in addition to overexpressing HER-2, also expressed elevated levels of EGFR compared to their EGF-dependent counterparts. Thus, acquisition of EGF independence was associated with increased EGFR protein expression. Figure 5C shows that activated EGFR in the cells grown in EGF-free medium has fewer specific phosphotyrosine sites than the cells grown with EGF. Notably, phosphorylation of tyrosine 1045, the c-cbl-binding site that is responsible for ubiquitination of EGFR [31], was consistently absent in the EGF-independent cells. Differential tyrosine phosphorylation of EGFR in the EGF-dependent versus the independent cells could explain, at least in part, the faster electrophoretic mobility of activated EGFR that occurs in the absence of exogenous growth factor.
Taken together, these results suggest that expression of HER-2 and E7 acts to mediate the EGF independence phenotype, which is driven by the constitutive activation of EGFR. It is important to note that this interpretation stands in contrast to the mechanism suggested by the previously published correlative data, which indicated that HER-2 expression levels were the primary driver of EGF-independent growth. The current data now demonstrate that, whereas high HER-2 levels can augment the EGF independence phenotype, high HER-2 levels alone are insufficient to make cells EGF-independent. Rather, molecular alterations induced by the HPV-16 E7 oncoprotein lead to the constitutive activation of EGFR that is required for EGF-independent proliferation, and this interaction requires the presence of elevated levels of HER-2.
Role of EGFR Versus HER-2 Activation for Growth and Survival of HER-2-Overexpressing Cells
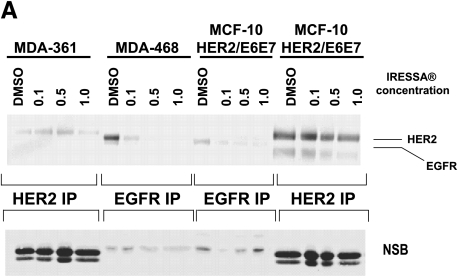
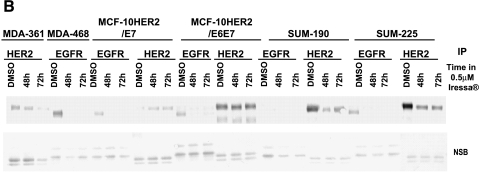
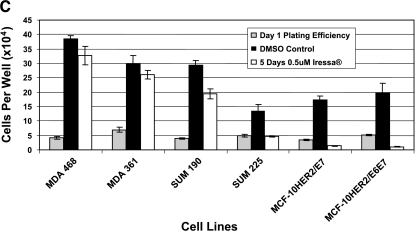
To examine further the separate roles of EGFR and HER-2 in mediating the EGF independence phenotype, experiments were performed in which the MCF-10HER2 cells and their derivatives were exposed to concentrations of ZD1839 (Iressa) that are able to block EGFR activation while having little or no effect on HER-2 tyrosine phosphorylation [32–34]. To confirm the appropriate concentrations of ZD1839 to use for these experiments, MDA-361 cells, which overexpress HER-2 but do not express EGFR, and MDA-468 cells, which overexpress EGFR but do not express HER-2, were exposed to varying concentrations of ZD1839 and analyzed 24 hours later for HER-2 or EGFR tyrosine phosphorylation (Figure 6A). These experiments demonstrated that exposure of MDA-468 cells to 0.5 µM ZD1839 resulted in the complete loss of tyrosine-phosphorylated EGFR, and had little or no effect on HER-2 tyrosine phosphorylation in the MDA-361 cells. Similarly, exposure of the EGF-independent MCF-10HER2/E6E7 cells to ZD1839 under the same conditions resulted in a complete loss of activated EGFR, but little or no reduction in tyrosine-phosphorylated HER-2. Experiments were next performed to evaluate the activation status of EGFR and HER-2 in the MCF-10HER2 cells and their E6-and E7-transduced counterparts following exposure to 0.5 µM ZD1839 for 48 and 72 hours. Two breast cancer cell lines with a HER-2 gene amplification that express different levels of EGFR were included in this analysis (SUM-190 and SUM-225). The data in Figure 6B show that exposure of MCF-10HER2/E7 cells and MCF-10HER2/E6E7 cells to 0.5 µM ZD1839 resulted in the complete loss of EGFR tyrosine phosphorylation but only partially reduced HER-2 phosphorylation. Similar results were obtained with the SUM-190 and SUM-225 cells. We next examined the effect of daily administration of 0.5 µM ZD1839 on the growth of these EGF-independent cell lines. The data in Figure 6C show that 0.5 µM ZD1839 was able to completely inhibit the growth of MCF-10HER2/E7 cells, MCF-10HER2/E6E7 cells, and SUM-225 cells, whereas SUM-190 cells were only partially growth-inhibited by this concentration of ZD1839.
Figure 6.
Effects of blocking EGFR on HER-2 activation. (A) MDA-361 cells (containing HER-2 and not EGFR), MDA-468 cells (containing EGFR and not HER-2), and MCF-10HER2/E6E7 cells were treated with DMSO or 0.1, 0.5, or 1.0 µM Iressa. HER-2 and EGFR were immunoprecipitated, separated on 7.5% SDS-PAGE, blotted, and probed for PTyr. At a concentration of 0.5 µM, Iressa had no effect on HER-2 activation, whereas that concentration abrogated EGFR activation. (B) Cells were treated with DMSO or 0.5 µM Iressa for 48 or 72 hours. HER-2 and EGFR were immunoprecipitated, separated on 7.5% SDS-PAGE, blotted, and probed for PTyr. Nonspecific bands are once again presented to show an equivalent loading of immunoprecipitates from each experiment. (C) Cells were plated and a sample was counted at day 1. Another sample was treated with 0.5 µM Iressa or DMSO for 5 days and counted.
These results are consistent with the Western blot results shown in Figures 3A and 5A, and demonstrate that the EGF-independent growth phenotype in MCF-10HER2/E7 cells and MCF-10HER2/E6E7 cells is primarily the result of the constitutive activation of EGFR that occurs in the context of HER-2 and E7 expression. EGF-independent growth of the SUM-225 human breast cancer cells, which express high levels of EGFR in addition to HER-2, is also driven primarily by EGFR. However, the SUM-190 breast cancer cells that overexpress HER-2 but express relatively low EGFR levels were much less affected by ZD1839 concentrations that preferentially inhibit EGFR signaling, whereas the EGFR-negative, HER-2-amplified MDA-361 cells were unaffected by ZD1839.
Discussion
The HER-2 oncogene undoubtedly plays an important role in breast cancer progression in a significant fraction of cases. However, HER-2 becomes amplified and overexpressed in a broader genetic context, and genetic and epigenetic alterations that accompany HER-2 gene amplification are very likely to interact with HER-2 to mediate the complete transformed phenotypes expressed by breast cancer cells. It is known from clinical studies that the prognostic significance of HER-2 overexpression is influenced by other factors, specifically the EGFR and p53 status of the breast cancers [35–38]. Indeed, one can make the argument based on the literature that the overexpression of HER-2, EGFR, and mutant p53 combines to yield highly aggressive breast cancer cells [39].
In previous work, we set out to recapitulate in immortalized HME cells the HER-2 expression levels found in human breast cancer cells with a HER-2 gene amplification. We found that the only way to accomplish high-level HER-2 overexpression was to transduce HER-2 into cells that were also engineered to coexpress HPV-16 viral oncoproteins [26]. Thus, the cells that we eventually obtained were similar to breast cancer cell lines such as SKBR3, SUM-190, SUM-225, 21MT1, and 21MT2 that express high levels of HER-2. Interestingly, these breast cancer cell lines also express EGFR and mutant p53. Further studies with the high-level HER-2 overexpressers demonstrated that the altered signaling that resulted from HER-2 overexpression in this context was directly responsible for the phenotypes of growth factor-independent proliferation, anchorage-independent growth, motility, and invasive capacity [26,27,40,41]. The results obtained from the current studies now allow us to begin to examine the relative contribution of HER-2, EGFR, E7, and E6 on the expression of the completely transformed phenotypes described above.
Data from the clinical literature indicate that amplification of HER-2 in the setting of EGFR positivity results in more aggressive disease than when HER-2 is amplified in EGFR-negative cells [37,38]. A number of laboratory studies have examined the potential for interaction between HER family members, and it has become widely accepted that HER-2 is the preferred heterodimerization partner for EGFR and HER-3, and that the HER-2 oncoprotein largely acts as a signaling coreceptor [6–9]. However, most of the studies on which this interpretation is based were performed in highly artificial systems using cells engineered to express equivalent levels of HER family members. Thus, these experiments did not take into account the fact that, in most breast cancers, widely different levels of HER family members that can have a significant impact on signaling are expressed. Recently though, Hendriks et al. [42] examined this question, and their results help us to interpret our data in light of the widely different levels of EGFR and HER-2 expressed in our cell lines. From their data, as well as our own, a model begins to emerge in which the relative proportion of EGFR homodimers, EGFR/HER-2 heterodimers, HER-2/HER-3 heterodimers, and HER-2 homodimers depends on the relative expression of each of the receptors in different cells. In cells that express low levels of p185HER-2, such as normal mammary epithelial cells and MCF-10A cells, EGFR homodimers that are activated by EGF and other ligands of the family predominate. As HER-2 levels increase, EGFR/HER-2 heterodimers begin to predominate such that when HER-2 is overexpressed relative to EGFR, the majority of EGFR molecules are associated with HER-2. As HER-2 levels increase still further, as is the case when the HER-2 gene becomes amplified, the absolute levels of HER-2 homodimers increase, and these homodimers can come to represent the majority of the signaling complexes in cells with a HER-2 gene amplification. The Western blot data shown in Figure 6 are consistent with such a model, and indicate that when ZD1839 is used at concentrations specific for EGFR, tyrosine-phosphorylated EGFR becomes undetectable, but the levels of tyrosine-phosphorylated HER-2 are only decreased slightly. We interpret this finding to indicate that ZD1839 at 0.5 µM blocks the activity of EGFR associated with HER-2 in heterodimers, but has little or no effect on the activity of HER-2 homodimers. Thus, SUM-225 breast cancer cells that express high levels of EGFR are completely growth-inhibited by ZD1839 at these concentrations, whereas the SUM-190 cells, which express lower levels of EGFR, are only partially inhibited. MDA-361 cells, which do not express EGFR at all, are unaffected by ZD1839. With regard to the SUM-225 cells, it is noteworthy that exposure of these cells to 0.5 µM ZD1839 was able to block proliferation, but appeared to have no effect on survival. This is in stark contrast to the effect that the pan-erbB kinase inhibitor, CI-1033, has on SUM-225 cells. Exposure of SUM-225 and SUM-190 cells to 0.1 to 1.0 µM CI-1033 eliminates tyrosine phosphorylation of EGFR, HER-2, and HER-3, and elicits both growth arrest and a profound apoptotic response (Molecular Cancer Therapeutics, in preparation). Thus, we propose that in cells expressing both HER-2 and EGFR, heterodimeric complexes of these receptors play an important role in driving cell cycle progression, and that activation of HER-2 homodimers plays an important role in mediating survival. Experiments are underway to directly test this hypothetical model.
The interaction of HER-2 overexpression with the HPV-16 oncoproteins E7 and E6 is interesting and likely reflects important cooperating genetic events that occur during breast cancer progression. Similar observations have recently been made using an oral epithelial cell model system in which E6 and E7 enhanced the transforming potential of HER-2 [43]. In past studies, we found that breast cancer cell lines with a HER-2 gene amplification express levels of HER-2 message and protein 10 to 50 times higher than physiological levels. It is probably not coincidental that all of these cell lines have p53 mutations, and our experiments with HER-2-transduced cells showed that coexpression of HBV-16 E6 resulted in elevated levels of tyrosine-phosphorylated p185HER-2. We do not yet know the mechanism by which p53 inactivation results in enhanced expression of HER-2 protein. We do know that expression of HPV-16 E6 and E7 resulted in increases in HER-2 mRNA levels without influencing the copy level of the transgene (data not shown). Thus, it is possible that loss of functional p53 relieves some negative constraints on HER-2 gene transcription. Alternatively, expression of very high HER-2 levels could induce a growth arrest or apoptotic signal that prevents cells from expressing very high levels of HER-2 in the context of wild-type p53.
The cooperation of HER-2 with HPV-16 E7 to yield the growth factor independence phenotype is particularly intriguing. Expression of E7 in the context of HER-2 overexpression results in the growth factor-independent activation of EGFR, which is the primary driver of cell growth. Because E7 only induces EGF independence in the context of HER-2 overexpression, we hypothesize that activated EGFRs in these cells are in heterocomplexes with HER-2. Indeed, activation of EGFR as a heterodimer with HER-2 likely explains the differential phosphorylation on specific tyrosines, and the molecular weight shift that we have observed in growth factor-independent cells. Future experiments will seek to determine the proximate mechanisms of EGFR activation in HER-2- and E7-expressing cells. In addition, it will be very important to determine what aspect of breast cancer progression is being mimicked by E7 expression in our experimental system. Based on our current understanding of the transforming properties of E7, the most likely candidates are centered around the Rb pathway of cell cycle regulation [44]. Some breast cancer cells lose the expression of p21 and p27 during cancer progression [35,45,46]. Other breast cancer cells overexpress cyclin D1 either as a result of gene amplification or transcriptional activation of the gene [47–51]. Although rare, some breast cancer cells have point mutations in the Rb gene itself [52]. These observations have prompted some investigators to argue that genetic alterations epistatic to the Rb pathway occur in virtually all cancer cells [30]. Therefore, all of these genetic alterations are candidates to be tested for their interaction with HER-2 in mediating the growth factor independence phenotype. Thus, we hypothesize that expression of HPV-16 E7 in our experimental system mimics one or more of the genetic alterations epistatic to the Rb pathway, which occur in different types of breast cancers and which interact with HER-2 to drive cell transformation.
In summary, the results of experiments in which we have attempted to recapitulate the transformed phenotypes expressed by breast cancer cells with a HER-2 gene amplification have led us to appreciate better how HER-2 overexpression cooperates with other genetic alterations to yield complete malignant transformation. Future studies will be aimed at teasing apart the individual contributions of HER-2, EGFR, p53, and RB in driving the expression of altered phenotypes. These studies will help us to understand better the transforming function of HER-2 gene amplification when present in different genetic contexts. Such an understanding will be important for determining the true prognostic significance of HER-2 overexpression and for predicting the response of breast cancer cells from different patients to specific targeted therapeutics.
Acknowledgements
We thank Denise Galloway for the gift of the E6- and E7-containing retroviruses. We also thank Jason Chien for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health grant no. CA 70354-05 (“Altered signaling in transformed breast epithelial cells”).
References
- 1.Thor AD, Liu S, Edgerton S, Moore D, II, Kasowitz KM, Benz CC, Stern DF, DiGiovanna MP. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): a study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18:3230–3239. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda HH. Prognostic and predictive value of c-erbB-2 (HER2/neu) gene amplification in human breast cancer. Breast Cancer. 2001;8:38–44. doi: 10.1007/BF02967476. [DOI] [PubMed] [Google Scholar]
- 3.Borg A, Tandon AK, Sigurdsson H, Clark GM, Ferno M, Fuqua SAW, Killander D, McGuire WL. HER2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4332–4337. [PubMed] [Google Scholar]
- 4.Marte BM, Grausporta D, Jeschke M, Fabbro D, Hynes NE, Taverna D. NDF/heregulin activates MAP kinase and p70/p85 S6 kinase during proliferation or differentiation of mammary epithelial cells. Oncogene. 1995;10:167–175. [PubMed] [Google Scholar]
- 5.Beerli RR, Grausporta D, Woodscook K, Chen XM, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karunagaran D, Tzahar E, Beerli RR, Chen XM, Grausporta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- 7.Pinkaskramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, et al. Diversification Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 8.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin B, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GrausPorta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alroy I, Yarden Y. The erbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 11.Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly JM, Olayioye MA, Wong AM, Neve R, Lane HA, Maurer FG, Hynes NE. NDF/heregulin-induced cell cycle changes and apoptosis in breast tumour cells: role of PI3 kinase and p38 MAP kinase pathways. Oncogene. 1999;18:3440–3451. doi: 10.1038/sj.onc.1202700. [DOI] [PubMed] [Google Scholar]
- 14.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, III, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. PNAS. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethier SP. Primary culture and serial passage of normal and carcinogen-treated rat mammary epithelial cells in vitro. J Natl Cancer Inst. 1985;74:1307–1318. [PubMed] [Google Scholar]
- 17.Ethier SP, Summerfelt RM, Cundiff KC, Asch BB. The influence of growth factors on the proliferative potential of normal and primary breast cancer-derived human breast epithelial cells. Breast Cancer Res Treat. 1990;17:221–230. doi: 10.1007/BF01806371. [DOI] [PubMed] [Google Scholar]
- 18.Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous reentry into the cell cycle. Exp Cell Res. 1993;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- 19.Ram TG, Kokeny KE, Dilts CA, Ethier SP. Mitogenic activity of neu differentiation factor/heregulin mimics that of epidermal growth factor and insulin-like growth factor-I in human mammary epithelial cells. J Cell Physiol. 1995;163:589–596. doi: 10.1002/jcp.1041630320. [DOI] [PubMed] [Google Scholar]
- 20.Gollahon LS, Shay JW. Immortalization of human mammary epithelial cells transfected with mutant p53 (273(his)) Oncogene. 1996;12:715–725. [PubMed] [Google Scholar]
- 21.Wright WE, Brasiskyte D, Piatyszek MA, Shay JW. Experimental elongation of telomeres extends the lifespan of immortal x normal cell hybrids. EMBO J. 1996;15:1734–1741. [PMC free article] [PubMed] [Google Scholar]
- 22.Ethier SP, Cundiff KC. Importance of extended growth potential and growth factor independence on in vivo neoplastic potential of primary rat mammary carcinoma cells. Cancer Res. 1987;47:5316–5322. [PubMed] [Google Scholar]
- 23.Ethier SP, Moorthy R, Dilts CA. Secretion of an epidermal growth factor-like growth factor by epidermal growth factor-independent rat mammary carcinoma cells. Cell Growth Differ. 1991;2:593–602. [PubMed] [Google Scholar]
- 24.Ethier SP, Langton BC, Dilts CA. Growth factor independent proliferation of rat mammary carcinoma cells by autocrine secretion of neu-differentiation factor/heregulin and transforming growth factor alpha. Mol Carcinog. 1996;15:134–143. doi: 10.1002/(SICI)1098-2744(199602)15:2<134::AID-MC6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Ram TG, Dilts CA, Dziubinski ML, Pierce LJ, Ethier SP. Insulin-like growth factor and epidermal growth factor independence in human mammary carcinoma cells with c-erbB-2 gene amplification and progressively elevated levels of tyrosine phosphorylated erbB-2. Mol Carcinog. 1996;15:227–2386. doi: 10.1002/(SICI)1098-2744(199603)15:3<227::AID-MC8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Woods Ignatoski KM, LaPointe AJ, Radany EH, Ethier SP. ErbB-2 overexpression in human mammary epithelial cells confers growth factor independence. Endocrinology. 1999;140:3615–3622. doi: 10.1210/endo.140.8.6939. [DOI] [PubMed] [Google Scholar]
- 27.Woods Ignatoski KM, Maehama T, Markwart SM, Dixon JE, Livant DL, Ethier SP. ERBB-2 overexpression confers PI 3′-kinase-dependent invasion capacity on human mammary epithelial cells. Br J Cancer. 2000;82:666–674. doi: 10.1054/bjoc.1999.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Y, Markwart SM, Gransden LA, Woods Ignatoski KM, Ethier SP, Livant DL. Integrin receptors regulate MMP1 dependent invasion by breast cancer cells and by mammary epithelial cells. Cancer Res. 2004;64:8674–8681. doi: 10.1158/0008-5472.CAN-04-0069. [DOI] [PubMed] [Google Scholar]
- 29.Chau BN, Wang JYJ. Coordinated regulation of life and death by Rb. Nat Rev Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- 30.Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 31.Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- 32.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 33.Moasser M, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 34.Anido J, Matar P, Albanell J, Guzmán M, Rojo F, Arribas J, Averbuch S, Baselga J. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9:1274–1283. [PubMed] [Google Scholar]
- 35.Lebeau A, Unholzer A, Amann G, Kronawitter M, Bauerfeind I, Sendelhofert A, Iff A, Löhrs U. EGFR, HER2/neu, Cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2003;79:187–198. doi: 10.1023/a:1023958324448. [DOI] [PubMed] [Google Scholar]
- 36.Hudelist G, Singer CF, Manavi M, Pischinger K, Kubista E, Czerwenka K. Co-expression of ErbB-family members in human breast cancer: HER2/neu is the preferred dimerization candidate in nodal-positive tumors. Breast Cancer Res Treat. 2003;80:353–361. doi: 10.1023/A:1024929522376. [DOI] [PubMed] [Google Scholar]
- 37.Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, Nesland JM. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol. 2002;196:17–25. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- 38.Bull SB, Ozcelik H, Pinnaduwage D, Blackstein ME, Sutherland DAJ, Pritchard KI, Tzontcheva AT, Sidlofsky S, Hanna WM, Qizilbash AH, et al. The combination of p53 mutation and neu/erbB-2 amplification is associated with poor survival in node-negative breast cancer. J Clin Oncol. 2004;22:86–96. doi: 10.1200/JCO.2004.09.128. [DOI] [PubMed] [Google Scholar]
- 39.Charafe-Jauffret E, Tarpin C, Bardou V-J, Bertucci F, Ginestier C, Braud A-C, Puig B, Geneix J, Hassoun J, Birnbaum D, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an “inflammatory signature”. J Pathol. 2004;202:265–273. doi: 10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- 40.Woods Ignatoski KM, Livant DL, Markwart S, Grewal NK, Ethier SP. The role of PI3′kinase and its downstream signals in erbB-2-mediated transformation. Mol Cancer Res. 2003;1:551–560. [PubMed] [Google Scholar]
- 41.Woods Ignatoski KM, Livant DL, Grewal NK, Markwart S, Ethier SP. p38MAPK induces cell surface a4 integrin down-regulation to facilitate erbB-2-mediated invasion. Neoplasia. 2003;5:128–134. doi: 10.1016/s1476-5586(03)80004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem. 2003;278:23343–23351. doi: 10.1074/jbc.M300477200. [DOI] [PubMed] [Google Scholar]
- 43.Moustafa A-EA, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron G, Batist G, Alpert L, Alaoui-Jamali MA. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene. 2004;23:350–358. doi: 10.1038/sj.onc.1207148. [DOI] [PubMed] [Google Scholar]
- 44.Helt A-M, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 45.Winters ZE, Leek RD, Bradburn MJ, Norbury CJ, Harris AL. Cytoplasmic p21WAF1/CIP1 expression is correlated with HER2/ neu in breast cancer and is an independent predictor of prognosis. Br Cancer Res. 2003;5:R242–R249. doi: 10.1186/bcr654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkarain A, Jordan R, Slingerland J. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J Mammary Gland Biol Neoplasia. 2004;9:67–80. doi: 10.1023/B:JOMG.0000023589.00994.5e. [DOI] [PubMed] [Google Scholar]
- 47.Jares P, Fernandez PL, Campo E, Nadal A, Bosch F, Aiza G, Nayach I, Traserra J, Cardesa A. Prad-1/cyclin D1 gene amplification correlates with messenger RNA overexpression and tumor progression in human laryngeal carcinomas. Cancer Res. 1994;54:4813–4817. [PubMed] [Google Scholar]
- 48.Lukas J, Aagaard L, Strauss M, Bartek J. Oncogenic aberrations of p16INK4/CDKN2 and cyclin D1 cooperate to deregulate G1 control. Cancer Res. 1995:4818–4823. [PubMed] [Google Scholar]
- 49.Michalides R, Hageman P, Vantinteren H, Houben L, Wientjens E, Klompmaker R, Peterse J. A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer. 1996;73:728–734. doi: 10.1038/bjc.1996.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, et al. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA. 1997;94:6380–6385. doi: 10.1073/pnas.94.12.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodén M, Stighall M, Nielsen NH, Roos G, Emdin SO, Östlund H, Landberg G. The cyclin D1 high and cyclin E high subgroups of breast cancer: separate pathways in tumorigenesis based on pattern of genetic aberrations and inactivation of the pRb node. Oncogene. 2002;21:4680–4690. doi: 10.1038/sj.onc.1205578. [DOI] [PubMed] [Google Scholar]
- 52.Fung YK, T'Ang A. The role of the retinoblastoma gene in breast cancer development. Cancer Treat Res. 1992;61:59–68. doi: 10.1007/978-1-4615-3500-3_4. [DOI] [PubMed] [Google Scholar]