Abstract
EphA2 is a receptor tyrosine kinase and is frequently overexpressed in a wide array of advanced cancers. We demonstrate in the current study that the EphA2 protein is restrictedly expressed in primary glioblastoma multiforme and anaplastic astrocytoma tissues in comparison to normal brain tissues. To evaluate the possibility of targeting EphA2 in glioma vaccine strategies, we stimulated human leukocyte antigen (HLA) A2+ peripheral blood mononuclear cells (PBMCs) obtained from healthy donors and glioma patients with autologous dendritic cells (DCs) loaded with synthetic EphA2883–891 peptide (TLADFDPRV), which has previously been reported to induce interferon-γ in HLA-A2+ PBMCs. Stimulated PBMCs demonstrated antigen-specific cytotoxic T lymphocyte (CTL) responses as detected by specific lysis of T2 cells loaded with the EphA2883 peptide as well as HLA-A2+ glioma cells, SNB19 and U251, that express EphA2. Furthermore, in vivo immunization of HLA-A2 transgenic HHD mice with the EphA2883–891 peptide resulted in the development of an epitope-specific CTL response in splenocytes, despite the fact that EphA2883–891 is an autoantigen in these mice. Taken together, these data suggest that EphA2883–891 may be an attractive antigen epitope for molecularly targeted glioma vaccines.
Keywords: EphA2, glioma, cancer vaccine, cytotoxic T lymphocytes, human leukocyte antigen (HLA) A2
Introduction
Immunotherapy, particularly active vaccination, may be developed as an effective and safe treatment modality for malignant gliomas, which continue to have a poor prognosis, despite advances in surgical techniques and adjuvant chemotherapy and radiotherapy [1]. Indeed, several groups, including ours, have demonstrated the safety and preliminary efficacy of whole glioma cell-based vaccine approaches [2,3]. However, the use of autologous glioma lysates or glioma cells as the antigen source may limit the feasibility and safety of the approach due to the cumbersome preparation procedures and theoretical concerns for inducing autoimmune encephalitis [4]. Although the number of well-defined glioma-specific cytotoxic T lymphocyte (CTL) epitopes has been limited, our recent report describing a human leukocyte antigen (HLA) A2-restricted CD8+ T-cell epitope derived from the GAA interleukin-13 receptor α2-chain (IL-13Rα2) demonstrates the feasibility of identifying such loci, particularly when targeting a protein that is differentially expressed in glioma cells versus normal tissues [5]. Given the marked antigenic heterogeneity of gliomas, however, immunotherapy with a single tumor-specific T-cell epitope might merely promote transient stabilization of disease, prior to the progression of antigen loss variants [6]. We have therefore been dedicated to broaden the list of available CTL epitopes for integration into multiepitope-based vaccine strategies for glioma therapy. EphA2 [7–9] is a member of the Eph family of receptor tyrosine kinases, comprised of two major classes (EphA and EphB), which are distinguished by their specificities for ligands (ephrin-A and ephrin-B, respectively) [7–9]. It has been reported that EphA2 is frequently overexpressed and often functionally dysregulated in advanced cancers, such as metastatic lesions (reviewed in Ref. [10]). Because of the aggressive and invasive nature of malignant gliomas, we hypothesized that EphA2 might be expressed in this tumor entity and might serve as an attractive target for glioma vaccines. Indeed, T-cell immunoepitopes in EphA2 have been identified and characterized as potential targets and surrogate markers for cancer immunotherapy [11,12]. In the current study, we have chosen to evaluate one of the HLA-A2-restricted epitopes, EphA2883–891, because it has been noted to elicit relatively high level interferon (IFN) γ responses in cancer patients, in comparison to the other epitopes [12]. Here, we show that EphA2 protein is expressed in human malignant glioma and that stimulation with EphA2883–891 can induce tumor-reactive CTLs in patient-derived HLA-A2+ peripheral blood mononuclear cells (PBMCs) in vitro and by in vivo vaccination of mice transgenic for modified HLA-A2.1-β2 microglobulin single chain (HHD mice).
Materials and Methods
Cells and Cell Culture
The U251, SNB19, and A172 glioma cell lines [5] were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 10 mM l-glutamine (all reagents from Life Technologies, Inc., Grand Island, NY).
PBMCs were obtained from glioma patients and healthy donors under an Institutional Review Board-approved protocol. The HLA-A2 expression on the PBMCs was determined by flow cytometry as a double-positive staining with monoclonal antibodies (mAbs) MA2.1 (against HLA-A2, B17) and BB7.2 (against HLA-A2, Aw69) (both from ATCC, Manassas, VA; www.atcc.org). The murine EL-4HHD [Dbxβ2 microglobulin (β2M) null, and transgenic for modified HLA-A2.1-β2 microglobulin single chain (HHD gene)] and control EL-4S3-Rob cells (Dbxβ2M null) were generous gifts from Dr. François A. Lemonnier (Pasteur Institute, Paris, France) [13]. The HLA-A*0201-transfected transporter associated with antigen processing (TAP)-deficient T2 cell lines [5], EL-4HHD and EL-4S3-Rob cells, were maintained in RPMI 1640 medium (Life Technologies, Inc.) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 100 µg/ml streptomycin, and 10 mM l-glutamine (all reagents from Life Technologies, Inc.).
Peptides
The synthetic EphA2883–891 (TLADFDPRV) and control MART-127–35 (AAGIGILTV) were synthesized by FMOC chemistry in the University of Pittsburgh Cancer Institute (UPCI) Peptide Synthesis Facility, and were >95% pure as indicated by analytic high-performance liquid chromatography and mass spectrometric analysis performed by the University of Pittsburgh Cancer Institute's Protein Sequencing Facility. Peptides were dissolved in phosphate-buffered saline (PBS)/10% DMSO at a concentration of 2 mg/ml and stored at -20°C until use.
Western Blot
Tumor cells (5–10 x 106) were analyzed for EphA2 expression through Western blot analysis as described previously [12], with slight modifications. Cell lines were collected by scraping in PBS and were pelleted before lysis in a buffer containing the protease inhibitors, phenylmethylsulfonyl fluoride (100 µg/ml), aprotinin (1 µg/ml), and leupeptin (1 µg/ml) (all these reagents from Sigma, St. Louis, MO). Lysate protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Protein samples were denatured in gel-loading buffer at 99°C for 5 min. Equal amounts of proteins (40 µg) were applied to each well and electrophoresed on 7% polyacrylamide gel. Proteins were transferred to nitrocellulose membranes (Bio-Rad) and blocked with 5% low-fat skim milk for 1 hour at room temperature. Blots were incubated with the antihuman EphA2 polyclonal antibody (c-20; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or actin (ACTN05; NeoMarkers, Fremont, CA), followed by species-specific secondary antibodies (mouse and rabbit, respectively; Santa Cruz Biotechnology, Inc.). Blots were imaged on a Kodak X-Omat Blue XB-1 film (NEN Life Science Products, Boston, MA) using an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ).
Immunohistochemistry for EphA2 in Glioma Tissue
Glioma specimens were obtained surgically under an Institutional Review Board-approved protocol and paraffin-embedded. Normal brain sections were obtained from the Human Brain Tissue Bank, Evelyn F. and William L. McKnight Brain Institute of the University of Florida. Eight-micrometer sections were deparaffinized, rehydrated, and subjected to antigen retrieval by electric pressure cooker (DC2000; Biocare Medical, Walnut Creek, CA) in DakoCytomation Target Retrieval Solution (DakoCytomation, Glostrup, Denmark). Endogenous peroxidase was blocked with 3% H2O2 in PBS. Anti-EphA2 mAb (Ab 208, mIgG1; MedImmune, Inc., Gaithersburg, MD) or isotype-matched control mAb was incubated on sections for 2 hours at room temperature. After PBS washing, sections were incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 20 minutes at room temperature, then incubated with avidin-biotin complex horseradish peroxidase (Vectastatin ABC kits; Vector Laboratories) for 10 minutes, developed with a Nova Red substrate kit (Vector Laboratories), then nuclei were counterstained with hematoxylin. The expression intensity of EphA2 immunostaining (negative, lightly diffuse, moderately diffuse, focally moderately diffuse, or strongly diffuse) was evaluated independently with a microscope under x20 to x40 magnification.
In Vitro Induction of CTL with the EphA2883–891 Peptide
Induction of CTL responses in HLA-A2+ PBMCs against HLA-A2-binding peptides was evaluated with a previously described method by us [5], with slight modifications. Briefly, autologous dendritic cells (DCs) (2 x 105 per well), which were preincubated with each peptide (10 µg/ml) for 2 hours, were cocultured with 2 x 106 CD8+ T cells in a final volume of 2 ml (each well of 24-well plates) of AIM-V media supplemented with 10%human serum(both reagents from Life Technologies, Inc.) and 20 U/ml human rhIL-2 (R&D Systems, Minneapolis, MN). On day 7, lymphocytes were restimulated with autologous DCs pulsed with the peptide, and the stimulated T cells were analyzed for their specific CTL activity on day 14 by standard 51Cr release assays. For the cold target (CT) inhibition assays, 51Cr-labeled SNB19 cells (1 x 103 cells/well) and cold T2 cells (1 x 104 cells/well), pulsed with or without EphA2883–891, were incubated with the effector cells.
Vaccination of HHD Mice with EphA2883–891 Peptide
HHD mice received (on days 0 and 7) subcutaneous (s.c.) injections of 100 µg of EphA2883–891 peptide emulsified in incomplete Freund's adjuvant (IFA) in the presence of 140 µg of I-Ab-restricted HBVcore128 (TPPAYRPPNAPIL) T-helper epitope, which stimulates a CD4+ helper T-cell response [14]. Control animals received IFA containing HBV helper peptide only. On day 14, the animals were sacrificed, and 5 x 107 splenocytes (SPCs) were stimulated in vitro with the same peptide that was used for in vivo stimulation (10 µM). On day 6 of culture, the bulk populations were tested for specific cytotoxicity against the EL4-HHD cells pulsed with EphA2883–891, and control unpulsed EL4-HHD or EL4S3-Rob cells. All animal experiments were performed under Institutional Animal Care and Use Committee (IACUC)-approved protocols.
Statistical Analyses
Statistical differences between groups were assessed using a Student's t test for two samples with unequal variances. Statistical significance was determined at the < .05 level.
Results
Expression of EphA2 Protein in Human Glioma Tissues
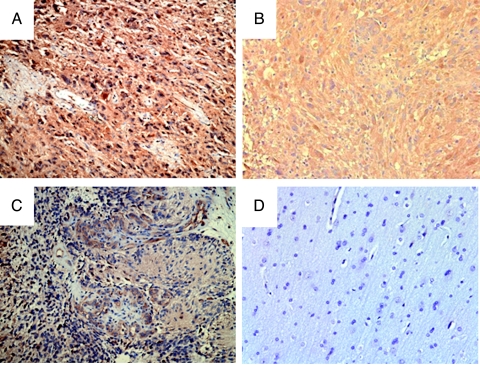
First, we evaluated the EphA2 expression in surgically resected human glioma tissues. Immunohistochemical analyses of paraffin-embedded specimens using anti-EphA2 mAb (Ab208 mIgG1) demonstrated a partly membranous, partly diffuse cytoplasmic staining pattern in EphA2+ cells (Figure 1). In comparison to the normal brain specimens (Figure 1D), which exhibited a negative or minimal, lightly diffuse pattern of EphA2 staining, high-grade gliomas, including 10 anaplastic astrocytoma (AA) and 9 glioblastoma multiforme (GBM), demonstrated variable levels of generally diffuse EphA2 expression in all cases (Figure 1, A–C and Table 1). In addition, one gemistocytic astrocytoma and one gliomatosis cerebri case, as well as one of two grade II astrocytoma (diffuse fibrillary astrocytoma) cases, demonstrated a diffuse EphA2 expression. Taken together, EphA2 appeared to be expressed in all malignant gliomas examined with a variety of intensity levels. However, the tumor grade did not appear to correlate with the staining intensity levels.
Figure 1.
EphA2 expression in GBM tissues. Paraffin-embedded GBM (A–C) or normal brain (D) sections were examined for EphA2 expression using anti-EphA2-specific antibody (Ab208; MedImmune, Inc.) as described in Materials and Methods section. The case in (A) was scored as strongly diffuse, and cases in (B) and (C) were scored as moderately diffuse in Table 1 (original magnification, x20).
Table 1.
Tumor Grades and EphA2 Expression Levels.
| Histologic Diagnosis and Grade | Staining Evaluation of Slide | Number of Cases |
| Astrocytoma grade II | Negative | 1 |
| (diffuse fibrillary astrocytoma) | Strongly diffuse | 1 |
| AA | Lightly diffuse | 5 |
| Moderately diffuse | 3 | |
| Strongly diffuse | 2 | |
| GBM | Lightly diffuse | 4 |
| Moderately diffuse | 3 | |
| Strongly diffuse | 2 | |
| Gemistocytic astrocytoma | Strongly diffuse | 1 |
| Gliomatosis cerebri | Moderately diffuse, diffusely infiltrating tumor only | 1 |
Induction of EphA2883–891 Peptide-Specific CTL Lines
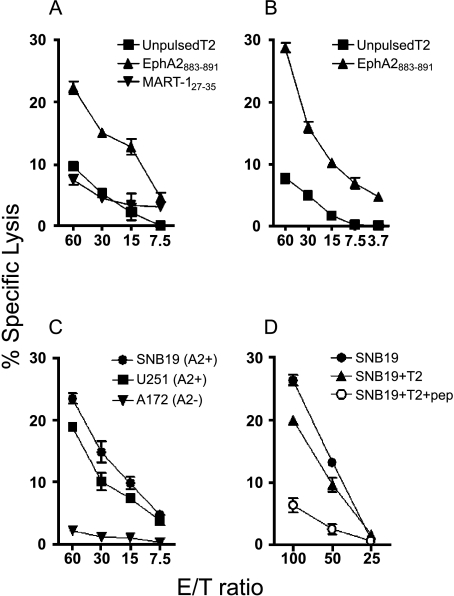
We have previously reported EphA2-derived T-cell epitopes that are able to induce IFN-γ responses in CD8+ cells from HLA-A2+ patients [12]. Among five HLA-A2-binding peptides described in this reference study, EphA2883–891 produced higher IFN-γ induction levels than the others [12]. We therefore chose to examine this peptide for its ability to induce CTL responses in CD8+ PBMCs derived from HLA-A2+ glioma patients. Human glioma cell lines SNB19 (HLA-A2+), U251 (HLA-A2+), and A172 (HLA-A2-), but not normal PBLs, expressed a high level of EphA2 proteins based on Western blot analysis (Figure 2), and thus were used as target cells in parallel with peptide-pulsed T2 cells. Figure 3 depicts specific CTL reactivities in one healthy donor (Figure 3A) and one representative glioma patient (Figure 3, B–D) following two cycles of stimulation with autologous DCs loaded with EphA2883–891. Responder T cells efficiently lysed T2 target cells pulsed with the EphA2883–891 peptide, whereas only low background lysis was observed in the absence of the peptide or T2 cells loaded with irrelevant control MART-127–35 (Figure 3, A and B). These results demonstrated that the CTL line induced with EphA2883–891 recognized the relevant antigen peptide specifically and was able to lyse cells presenting the peptide.
Figure 2.
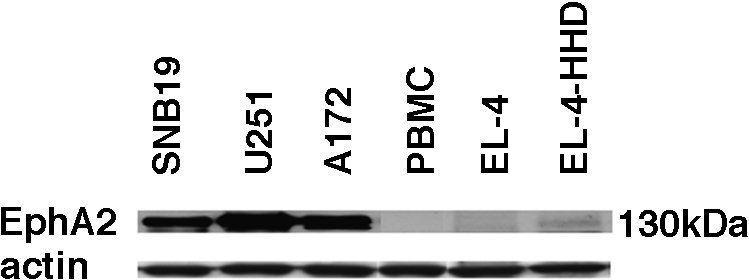
Human glioma cell lines express the EphA2 protein at high levels. Expression of EphA2 protein was analyzed in three human glioma cell lines (SNB19, U251, and A172), healthy donor-derived PBLs, and two mouse lymphoma cell lines (EL-4 and EL-4-HHD) by Western blot analysis using anti-EphA2 polyclonal antibody (C-20) and anti-pan-actin mAb (ACTN05) as internal controls.
Figure 3.
Stimulation of HLA-A2+ donor-derived PBMCs with EphA2883–891-induced antigen-specific CTL responses. (A and B) T-cell lines generated from a healthy donor (A) or patient 1 (B) by stimulation with EphA2883–891 peptide were tested for their ability to lyse 51Cr-labeled T2 pulsed with EphA2883–891 (▲) or irrelevant MART-127–35 (AAGIGILTV) (▼) or without peptides (■). P < .05 at all E/T ratios for EphA2883–891-pulsed T2 versus other groups. (C) T-cell lines generated from patient 2 with EphA2883–891 peptide were incubated for 4 hours with 51Cr-labeled human glioma cell lines SNB19 (●; HLA-A2+, EphA2+), U251 (■; HLA-A2+, EphA2+), and A172 (▼; HLA-A2-, EphA2+) at the indicated E/T ratios for evaluation of specific lytic ability. P < .05 at all E/T ratios for A172 vs U251 or SNB19. For the CT inhibition assay (D), 51Cr-labeled tumor target cells (5 x 103 cells) and cold T2 cells pulsed with (○) or without (▲) EphA2883–891 peptide were incubated with CTLs from patient 3. P < .05 at E/T ratios 50 and 100 for peptide-pulsed T2 (cold inhibition) versus other groups. These data are representative of at least three independent experiments with each of the three different donors. Bars = SD.
More importantly, we examined whether the patient-derived responder cells were able to recognize and lyse HLA-A2+ human glioma cells that endogenously expressed and presented EphA2883–891 epitope. The standard 51Cr release assays revealed the HLA-A2+ SNB19 and cell U251 lines were highly susceptible to lysis by the CTL line, whereas the HLA-A2- A172 cells were not susceptible to lysis by the responder cells, suggesting that the CTL reaction is HLA-A2-restricted (Figure 3C). To determine the specificity of the lytic activity, CT competition experiments were performed by addition of nonradiolabeled T2 cells pulsed with EphA2883–891 peptide in the 51Cr release assay (Figure 3D). The lytic activity of the CTL line against these glioma cell lines was almost completely inhibited by the addition of the cold T2 pulsed with EphA2883–891 peptide, but not by nonpulsed cold T2 cells, demonstrating that the lytic ability was specific for the epitope EphA2883–891. These data indicated that HLA-A2+ donor-derived PBMCs stimulated with EphA2883–891 peptide were capable of recognizing and lysing HLA-A2+ glioma cell lines that endogenously expressed EphA2, suggesting that the EphA2883–891 peptide might be useful for inducing antiglioma immunoreactivity.
Vaccination of HHD Mice with EphA2883–891 Peptide-Induced Antigen-Specific CTL Response
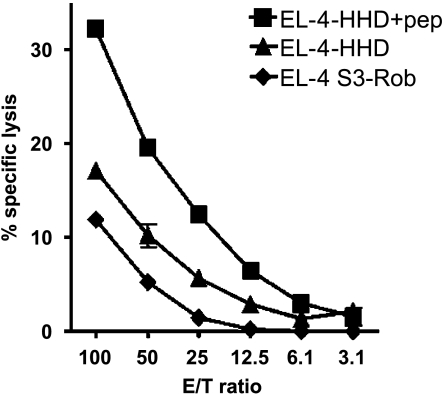
To evaluate the immunogenic potential of EphA2883 peptide in vivo, HHD mice were immunized with EphA2883–891 peptide. As the amino acid sequence for human EphA2883–891 is completely identical to the corresponding portion in mouse (m) EphA2 (NCBI accession nos. AAH37166 and NP_034269, respectively), vaccination with EphA2883–891 in this mouse model may serve as an attractive and clinically relevant model in the light of overcoming potential partial tolerance against an autoantigen epitope. The EphA2883–891-specific CTL activity in the SPCs from the immunized HHD mice was examined with EL-4-HHD cells pulsed with EphA2883–891 by standard 51Cr release assays. As EL-4HHD cells express only a modest level of EphA2 protein (Figure 2), nonpulsed EL-4-HHD cells were used as controls. As depicted in Figure 4, the responder bulk SPCs demonstrated a significantly higher lytic activity against EphA2883–891-loaded EL-4HHD cells in comparison to nonpulsed EL-4-HHD cells, supporting the peptide-specific nature of the effector cell response. Interestingly, nonpulsed EL-4-HHD cells demonstrated slightly higher susceptibility to the SPCs than EL-4 S3Rob cells that do not express the HHD gene [P < .05 at all effector-to-target ratios (E/T ratios) higher than 10], suggesting the EphA2883–891 epitope derived from endogenously expressed EphA2, albeit low levels, may still be presented in the context of HHD. Control animals that received mock immunizations demonstrated only background CTL reactivities under the same conditions (data not shown). These data suggest EphA2883–891 epitope may serve as an efficient vaccine antigen in vivo.
Figure 4.
In vivo vaccination of HHD mice with EphA2883–891 peptide induced an antigen-specific CTL activity. SPCs obtained from HHD mice that had been immunized with EphA2883891 peptide were tested their specific lytic activity against EL4-HHD cells pulsed with EphA2883–891 peptide (■), nonpulsed EL-4-HHD (▲), and control EL-4S3-Rob (◆) cells by standard 4-hour 51Cr release assays. P < .05 at all E/T ratios higher than 10 for unpulsed EL-4-HHD (▲) versus other groups. These data are representative of at least two independent experiments performed. Bars = SD.
Discussion
The most significant findings in the present study are that human malignant gliomas express EphA2 protein and that EphA2883–891 epitope can induce an antigen-specific, antiglioma CTL response in HLA-A2+ patient-derived PBMCs. These findings strongly support the rationale for targeting EphA2 in peptide-based vaccine trials for patients with AA and GBM.
Our immunohistochemical analyses demonstrated variable levels of EphA2 expression in all high-grade (grade III or IV) gliomas examined. However, unlike many other cancer types in which EphA2 expression levels predict malignant behaviors of cancers [10,15], expression levels in gliomas did not correlate with the tumor grade, as both AA and GBM cases had variable expression levels and one of two grade II astrocytoma (diffuse fibrillary astrocytoma) cases expressed a high level of EphA2. This may be due to the fact that both AA and GBM are highly malignant tumors with aggressive invasiveness into the surrounding normal brain tissue. Further evaluation with additional low-grade astrocytoma cases and other gliomas, such as ependymoma, is warranted. Nevertheless, EphA2 appeared to be expressed widely in high-grade gliomas and is relatively restricted in tumor tissues as normal brain samples examined had little or no EphA2 expression. In our previous study with a mouse model [16], EphA2 expression was not detected in the mouse brain based on Western blot analysis, supporting the finding that the EphA2 expression in the central nervous system is restricted to gliomas.
We also demonstrated that EphA2883–891 peptide induced a specific CTL response in HLA-A2+ donor-derived PBMCs in vitro. It was noteworthy that the levels of CTL activity observed in patient-derived PBMCs were as high as those observed in healthy donor-derived PBMCs in most cases, unless the donor patient suffered from clinical lymphopenia (defined as an absolute lymphocyte count < 800 µl-1) due to concurrent chemotherapy and/or radiotherapy. We are currently optimizing the conditions for the fluorescence-conjugated tetramer for EphA2883–891, which will allow us to evaluate the frequency of EphA2883–891, recognizing CD8+ T cells in glioma patients undergoing our perspective vaccine trials.
It has been well recognized that the CTL repertoire against high-affinity epitopes is often partially tolerized [13], and this mechanism may explain the limited levels of CTL responses against a majority of tumor-associated autoantigens. However, our data employing HHD mice indicated that vaccinations with IFA containing the EphA2883–891 peptide effectively mounted an antigen-specific CTL response that was demonstrated not only against peptide-loaded EL-4-HHD cells but also against EL-4-HHD cells that endogenously express EphA2, suggesting that the EphA2883–891 peptide is capable of inducing a specific CTL response in vivo with the assistance of effective adjuvants.
There is a theoretical possibility that vaccinations of patients with EphA2-derived peptides can induce autoimmune reactions because of their expression in the normal lung, spleen, and liver [16]. However, EphA2 is not expressed in mouse brain [16], and we have not observed any inflammatory pathology in normal organs in HHD mice following vaccinations with the EphA2883–891 (data not shown). This novel CTL epitope may therefore serve as an attractive component of peptide-based vaccines to treat patients with high-grade glioma [3,17] and as a surrogate marker of T-cell immune responses in patients before and after immunotherapy.
Acknowledgements
We thank François A. Lemonnier (Pasteur Institute) and Pravin T. P. Kaumaya (The Ohio State University) for their kind assistance in obtaining HHD mice.
Abbreviations
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- E/T ratio
effector-to-target ratio
- HLA
human leukocyte antigen
- IL
interleukin
- PBMC
peripheral blood mononuclear cell
Footnotes
This work was supported by P01 NS40923 (I.F.P. and H.O.), a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (H.O.), a 21st Century Scientist Award from the James S. McDonnell Foundation (H.O.), and the Copeland Fund of the Pittsburgh Foundation (H.O. and N.K.).
References
- 1.Wen PY, Kesari S. Malignant gliomas. Curr Neurol Neurosci Rep. 2004;4:218–227. doi: 10.1007/s11910-004-0042-4. [DOI] [PubMed] [Google Scholar]
- 2.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q, Elder EH, Whiteside TL, Schold SC, Jr, Pollack IF. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neuro-Oncol. 2003;64:13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- 4.Bigner DD, Pitts OM, Wikstrand CJ. Induction of lethal experimental allergic encephalomyelitis in non-human primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg. 1981;55:32–42. doi: 10.3171/jns.1981.55.1.0032. [DOI] [PubMed] [Google Scholar]
- 5.Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A*0201 restricted cytotoxic T lymphocyte epitope in a human glioma associated antigen, interleukin-13 receptor 2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 6.Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, Fetsch P, Lee KH, Steinberg S, Rosenberg S, et al. Immune selection after antigen-specific immunotherapy of melanoma. Surgery. 1999;126:112–120. [PubMed] [Google Scholar]
- 7.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 8.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 9.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 10.Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59–68. doi: 10.1023/a:1022546620495. [DOI] [PubMed] [Google Scholar]
- 11.Alves PM, Faure O, Graff-Dubois S, Gross DA, Cornet S, Chouaib S, Miconnet I, Lemonnier FA, Kosmatopoulos K. EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–8480. [PubMed] [Google Scholar]
- 12.Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E, Storkus WJ. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- 13.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Naceur-Griscelli O, Faure O, Guillaume P, Firat H, Chouaib S, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrem CJ, Tatsumi T, Olson KS, Shirai K, Finke JH, Bukowski RM, Zhou M, Richmond AL, Derweesh I, Kinch MS, et al. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically treated patients with renal cell carcinoma. Clin Cancer Res. 2005;11:226–231. [PubMed] [Google Scholar]
- 16.Hatano M, Kuwashima N, Tatsumi T, Dusak JE, Nishimura F, Reilly KM, Storkus WJ, Okada H. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada H, Pollack IF, Lieberman F, Lunsford LD, Kondziolka D, Schiff D, Attanucci J, Edington H, Chambers W, Kalinski P, et al. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther. 2001;12:575–595. doi: 10.1089/104303401300042528. [DOI] [PubMed] [Google Scholar]