Abstract
Activated fatty acids stimulate budding and fusion in several cell-free assays for vesicular transport. This stimulation is thought to be due to protein palmitoylation, but relevant substrates have not yet been identified. We now report that Vac8p, a protein known to be required for vacuole inheritance, becomes palmitoylated when isolated yeast vacuoles are incubated under conditions that allow membrane fusion. Similar requirements for Vac8p palmitoylation and vacuole fusion, the inhibition of vacuole fusion by antibodies to Vac8p and the strongly reduced fusion of vacuoles lacking Vac8p suggest that palmitoylated Vac8p is essential for homotypic vacuole fusion. Strikingly, palmitoylation of Vac8p is blocked by the addition of antibodies to Sec18p (yeast NSF) only. Consistent with this, a portion of Vac8p is associated with the SNARE complex on vacuoles, which is lost during Sec18p- and ATP-dependent priming. During or after SNARE complex disassembly, palmitoylation occurs and anchors Vac8p to the vacuolar membrane. We propose that palmitoylation of Vac8p is regulated by the same machinery that controls membrane fusion.
Keywords: palmitoylation/Sec18p/SNARE complex/vacuole fusion/Vac8p
Introduction
Palmitoylation is the covalent attachment of fatty acids to cysteine residues of membrane proteins. The fatty acids can mediate protein–lipid and protein–protein interactions, thereby targeting the modified protein to membranes or membrane subdomains. In contrast to other hydrophobic modifications, i.e. myristoylation or isoprenylation, palmitoylation is often a dynamic event with cycles of acylation and deacylation (Resh, 1999).
It has been suggested that synthesis and consumption of palmitoyl-coenzyme A (Pal-CoA) are involved in intracellular transport. Activated fatty acids stimulate the budding of transport vesicles from the Golgi (Pfanner et al., 1989; Ostermann et al., 1993) as well as the Golgi and the yeast vacuole fusion assay (Glick and Rothman, 1987; Pfanner et al., 1990; Haas and Wickner, 1996). Using a non-hydrolyzable analog of Pal-CoA, it was suggested that this stimulatory effect might be due to palmitoylation of proteins, which thereby become activated to function in the fusion reaction. Inhibitors of fatty acid activation block the described stimulation, implicating an acyl-CoA synthetase in the reaction (Pfanner et al., 1990).
The enzymology of protein palmitoylation is poorly understood. A putative palmitoyl transferase was purified, but it subsequently turned out to be a peroxisomal thiolase, an enzyme unlikely to be involved in protein palmitoylation in vivo (Liu et al., 1996). Furthermore, several enzymatic activities extracted from microsomal membranes have been characterized, but none has been purified to homogeneity (Berthiaume and Resh, 1995; Dunphy et al., 1996; Veit et al., 1998). In contrast, some proteins are palmitoylated autocatalytically in vitro (Berger et al., 1984; Duncan and Gilman, 1996; Veit, 2000).
We are analyzing the homotypic fusion of yeast vacuoles as a model system to understand late steps in membrane trafficking. Vacuole fusion depends on a cascade of events that can be subdivided into a priming, docking and fusion step. A multisubunit SNARE complex, consisting of the SNAREs Vam3p, Vam7p, Nyv1p, Ykt6p and Vti1p (Ungermann et al., 1999a) and the chaperones Sec18p, Sec17p and LMA1, is present on isolated vacuoles and initially associated with a tethering complex, termed HOPS (Price et al., 2000; Seals et al., 2000). During priming, ATP hydrolysis by Sec18p results in the disassembly of the SNARE complex into its subunits and the release of the HOPS complex. The HOPS complex, probably together with the SNAP-23 homolog Vam7p (Ungermann et al., 2000), then engages in an association with the GTP-bound form of Ypt7p to initiate the first docking step, called tethering (Price et al., 2000). This is followed by the assembly of the primed SNAREs into trans-complexes between opposing membranes (Ungermann et al., 1998b). Finally, fusion requires the action of calmodulin, Ca2+ and protein phosphatase 1, and the assembly of the proteolipid of the vacuolar ATPase (Peters and Mayer, 1998; Peters et al., 1999, 2001).
Additional proteins have been implicated in the fusion reaction. Among these is Vac8p, which was isolated as a protein required for vacuole inheritance (Wang et al., 1996; Fleckenstein et al., 1998; Pan and Goldfarb, 1998; Wang et al., 1998). Vac8p is also required for the cytosol to vacuole transport (CVT) pathway and the formation of nuclear–vacuole junctions (Wang et al., 1998; Pan et al., 2000; Scott et al., 2000), but is not involved in the transport of proteins from the endosome to the vacuole (Wang et al., 1996). Vac8p is both myristoylated and palmitoylated at its N-terminus. Myristoylation is a co-translational event and enhances post-translational palmitoylation on multiple cysteine residues. Interestingly, palmitoylation, but not myristoylation, is essential for vacuole inheritance, although both hydrophobic modifications are required for complete localization of Vac8p to the vacuole (Pan and Goldfarb, 1998; Wang et al., 1998). Recently, it was shown that a cold-sensitive mutant of Acc1p, a subunit of fatty acid synthase, eliminates Vac8p palmitoylation in vivo (Schneiter et al., 2000). However, the timing and the role of Vac8p palmitoylation during vacuole fusion, besides being required for vacuole localization and thus vacuole morphology, has not been addressed so far and is the main focus of this study.
Results
Identification of activators and inhibitors of Vac8p palmitoylation
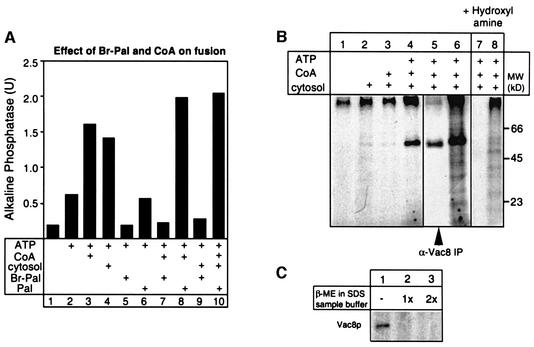
Vacuole fusion depends on CoA for optimal fusion (Figure 1A; Haas and Wickner, 1996; Ungermann et al., 1999b). This suggests that CoA could be a substrate for the synthesis of Pal-CoA on the vacuole, which can then be utilized for palmitoylation of proteins. Recently, 2-bromo-palmitate (Br-Pal) has been described as an inhibitor of protein palmitoylation in vivo (Webb et al., 2000). To analyze its effect on vacuole fusion, fusion reactions containing vacuoles from two different tester strains (see Materials and methods), cytosol and/or CoA were incubated at 26°C with or without Br-Pal for 90 min (Figure 1A). Br-Pal addition completely blocked vacuole fusion (Figure 1A, compare lanes 2 and 5, 3 and 7, and 4 and 9), whereas palmitate did not (Figure 1A, compare lanes 2 and 6, and 3 and 8). CoA alone (lane 3), and even more so together with palmitate, stimulates the reaction (compare lanes 3 and 6 with lane 8), indicating that synthesis of Pal-CoA by an acyl-CoA synthetase is involved in the reaction. Thus, vacuole fusion is blocked by inhibitors (Br-Pal) and stimulated by activators of protein palmitoylation (palmitate, CoA and Pal-CoA).
Fig. 1. Identification of Vac8p as a target of palmitoylation on isolated vacuoles. (A) Vacuole fusion depends on palmitoylation. Vacuoles (6 µg) from yeast strains BJ3505 and DKY6281 were incubated in a 30 µl reaction in the presence of ATP for 90 min at 26°C. Where indicated, cytosol (15 µg), CoA (10 µM), palmitate (200 µM) or Br-Pal (200 µM) was added to the reaction. Then, fusion activity was measured (Haas et al., 1994). (B) Vac8p is palmitoylated during the fusion reaction. Vacuoles from DKY6281 (60 µg) were labeled with [3H]palmitate (150 µCi) in a 300 µl volume at 30°C in the absence or presence of ATP (1 mM), cytosol (0.5 µg/µl) and CoA (10 µM). After 90 min, vacuoles were isolated by centrifugation (5 min, 4°C, 12 000 g), washed with 500 µl of PS buffer and resuspended in SDS sample buffer without 2-mercaptoethanol (lanes 1–4). Lane 5 shows an immuno precipitation of one complete reaction. The sample shown in lane 6 was labeled in parallel to lane 5, but analyzed without immunoprecipitation. Palmitoylated proteins were identified by SDS–PAGE and fluorography. After film exposure, the gel shown in lanes 5 and 6 was rehydrated, treated with 1 M hydroxylamine pH 6.8 overnight, dried and analyzed by fluorography (lanes 7 and 8) as before. (C) Vac8p palmitoylation is sensitive to reducing agents. Vacuoles were incubated for 90 min in the presence of ATP, cytosol, CoA and [3H]palmitate as in (B). After reisolation, three identical vacuole pellets were resuspended in SDS sample buffer without (lane 1), with 5% (lane 2) or with 10% (lane 3) 2-mercaptoethanol. Samples were boiled for 4 min at 95°C prior to SDS–PAGE analysis and fluorography.
To identify proteins that would be palmitoylated during the fusion reaction, vacuoles were incubated in the presence of [3H]palmitate and cytosol, CoA or ATP for 90 min (Figure 1B). After the incubation, vacuoles were reisolated and subjected to SDS–PAGE and fluorography. Only in the presence of ATP did we detect a strong labeling of one 64 kDa band on the fluorogram (Figure 1B, lane 4). The vacuolar armadillo repeat protein Vac8p had been shown previously to be palmitoylated in vivo (Wang et al., 1998). Indeed, by immunoprecipitation of a detergent extract of 3H-labeled vacuoles with Vac8p-specific antiserum, we confirmed that the labeled 64 kDa band on the vacuole was Vac8p (Figure 1B, lane 5).
We then analyzed the chemical nature of the fatty acid bond in Vac8p. Treatment of the gel with hydroxylamine removed [3H]palmitate labeling from Vac8p, but not from protein aggregates running at the top of the gel (Figure 1B, lanes 7 and 8). Furthermore, labeling of Vac8p is sensitive to boiling with mercaptoethanol prior to SDS–PAGE (Figure 1C). Sensitivity to hydroxylamine and mercaptoethanol are hallmarks of authentic protein palmitoylation (Resh, 1999) and indicates a covalent fatty acid bond to cysteine residues, which have been identified as palmitoylation sites in Vac8p (Wang et al., 1998). Labeling of Vac8p with [3H]palmitate is reduced when fusion reactions are labeled on ice (see Figure 3A, lane 0 min) and is dependent on ATP (see Figure 1B), suggesting that at least one enzymatic activity is involved in the reaction. Thus, palmitoylation of Vac8p might be the target for the observed effects of CoA, palmitate and Br-Pal on vacuole fusion.
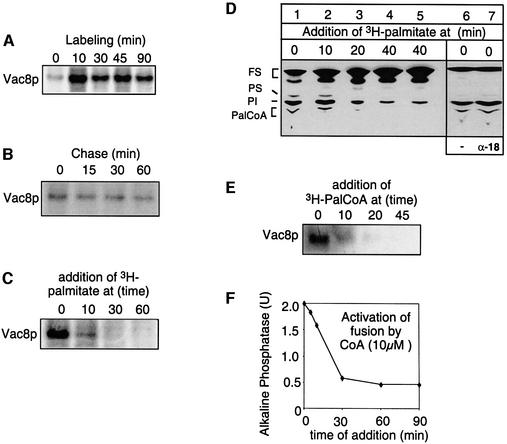
Fig. 3. Vac8p is labeled in the beginning of the fusion reaction. (A) Kinetics of Vac8p palmitoylation. Vacuole fusion reactions (300 µl) containing ATP, cytosol and CoA (10 µM) were labeled with [3H]palmitate for 0, 10, 30, 45 or 90 min. Palmitoylation of Vac8p was then analyzed as described in Figure 1B. Normalized intensities of labeled bands including standard deviations (n = 3): 100% (10 min), 79 ± 8.5% (30 min), 85 ± 10.5% (45 min), 68 ± 14% (90 min). (B) Vac8p palmitoylation cannot be chased by the addition of cold palmitate. Vacuolar fusion reactions containing ATP, cytosol and CoA (10 µM) were labeled with [3H]palmitate for 10 min. Palmitoylation was chased by addition of excess unlabeled palmitate (30 µM) and incubated for 0, 15, 30 or 60 min. Normalized intensities of labeled bands including standard deviations (n = 3): 100% (0 min), 88 ± 5.5% (15 min), 86 ± 4.5% (30 min), 79 ± 9% (60 min). (C) Vac8p is palmitoylated only within the first 10 min of the fusion reaction. Vacuolar fusion reactions containing ATP, cytosol and CoA (10 µM) were labeled with [3H]palmitate at 0, 10, 30 or 60 min after the start of the reaction and incubations were continued for 15 min. (D) Synthesis of Pal-CoA is restricted to the start of the reaction. Vacuoles from DKY6281 were incubated at 30°C in the presence of cytosol, ATP and CoA. [3H]palmitate was added at the indicated times and labeling was carried out for 15 min. In lane 5, additional CoA (10 µM) and ATP (1 mM) were added together with [3H]palmitate. In lane 7, vacuoles received anti-Sec18p to block priming. Lane 6 is the control without antibodies. Labeling with [3H]palmitate was carried out for 15 min. Reactions were then placed on ice, and lipids were extracted and analyzed by TLC and fluorography as described in Materials and methods. The identity of bands was verified with marker lipids run on the same TLC plate. FS = fatty acids and neutral lipids, PS = phosphatidylserine, PI = phosphatidylinositol. (E) [3H]Pal-CoA was added at 0, 10, 20 or 45 min after the start to fusion reactions without CoA and the incubations were continued for 15 min. (F) CoA addition stimulates fusion only within the first 10 min. Vacuoles from both tester strains were incubated in a 30 µl volume in the presence of ATP. At the indicated time points, 10 µM CoA was added and the incubation was continued to a total of 90 min. Then fusion was determined.
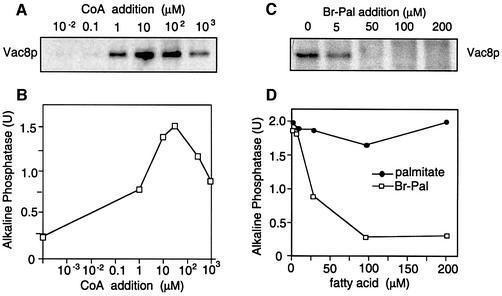
We therefore tested whether the concentration dependencies of CoA and Br-Pal for Vac8p palmitoylation and for the fusion reaction are similar (Figure 2). To measure the CoA dependence, vacuoles were incubated in the presence of [3H]palmitate with increasing concentrations of CoA. In a parallel experiment, vacuole fusion was determined. Both vacuole fusion and palmitoylation of Vac8p have an optimum of 10 µM CoA (Figure 2A and B). Higher CoA concentrations did not further stimulate, but rather inhibited Vac8p palmitoylation and the fusion reaction. This effect might be due to excessive production of Pal-CoA, which has an inhibitory effect on vacuole fusion and on other in vitro transport assays at a concentration of 100 µM (our unpublished observations; Glick and Rothman, 1987; Haas and Wickner, 1996). Addition of Br-Pal to the assay completely blocked [3H]palmitate labeling of Vac8p as well as the fusion assay at comparable concentrations (Figure 2C and D). The observation that the block in Vac8p palmitoylation (Figure 2C) already occurs at lower Br-Pal concentrations than the block in fusion (Figure 2D) suggests that even a low amount of palmitoylated Vac8p is enough to drive fusion. In fact, vacuole inheritance is rescued by a myristoylation mutant of Vac8p that is palmitoylated very poorly (Wang et al., 1998). Only if palmitoylation is completely blocked is fusion stalled. Therefore, the correlation of vacuole fusion requirements and the requirements for Vac8p palmitoylation suggest that these reactions are tightly linked.
Fig. 2. Requirements for Vac8p palmitoylation and vacuole fusion coincide. (A) Vac8p palmitoylation requires an optimal CoA concentration. A fusion reaction (300 µl) containing 60 µg of vacuoles from DKY6281 was incubated for 60 min in the presence of cytosol, ATP, [3H]palmitate and increasing amounts of CoA. Palmitoylated Vac8p was identified by SDS–PAGE and fluorography as described in Figure 1. (B) Vacuoles from both tester strains were incubated in a 30 µl reaction volume under similar conditions with varying CoA concentrations to determine fusion activity. (C) Addition of Br-Pal blocks Vac8p palmitoylation. Vacuoles (60 µg) were incubated in a 300 µl volume for 60 min in the presence of ATP, cytosol, CoA and [3H]palmitate. Br-Pal was added at the indicated concentrations to the fusion reaction. Palmitoylated Vac8p was analyzed as in (A). (D) Fusion is blocked by Br-Pal. Vacuoles from both tester strains were incubated at 26°C in the presence of ATP, CoA, cytosol and Br-Pal or palmitate at the indicated concentrations. Fusion was determined after 90 min incubation. Note that palmitate has no stimulatory effect on vacuole fusion if cytosol is added to the assay (see also lanes 8 and 10 in Figure 1A).
Palmitoylation of Vac8p occurs early in the reaction
We then searched for a kinetic linkage between the palmitoylation of Vac8p and specific events of the vacuole fusion reaction. First, fusion reactions were incubated with [3H]palmitate, CoA and ATP for increasing periods of time (Figure 3A). Vac8p was labeled after 10 min. No linear increase in the labeling intensity was seen with longer labeling times. We then tested whether palmitoylation of Vac8p is dynamic (Figure 3B). Vacuoles were labeled for 10 min with [3H]palmitate and the reaction was chased by addition of excess cold palmitate. However, no significant cleavage of fatty acids was detectable. Next, we asked whether Vac8p was a substrate for palmitoylation throughout the reaction (Figure 3C). Fusion reactions were started, [3H]palmitate was added at the indicated time points and labeling was continued for 10 min before placing the sample on ice. Labeling of Vac8p is strong when [3H]palmitate is present at the start of the reaction, greatly reduced after 10 min of incubation and almost not detectable when palmitate was added 30 or 60 min after the start of the reaction, indicating that Vac8p labeling is an early event.
Since we labeled Vac8p with [3H]palmitate, we asked whether synthesis of Pal-CoA is regulated during the vacuole fusion assay. TLC analysis of [3H]palmitate-labeled lipids shows that Pal-CoA is also synthesized only at the start of the reaction (Figure 3D, lanes 1–3). Lack of Pal-CoA synthesis at later time points is not due to exhaustion of ATP or CoA pools, because addition of both compounds together with [3H]palmitate does not reactivate Pal-CoA synthesis (Figure 3D, lanes 4 and 5). Freshly synthesized [3H]Pal-CoA apparently is metabolized rapidly. At each time point, more of the [3H]palmitate label is detectable in phospholipids than in Pal-CoA. However, even if activation of palmitate is not required, by using [3H]Pal-CoA for labeling, [3H]palmitoylation of Vac8p still occurs only at the beginning of the fusion reaction (Figure 3E). Consistent with the kinetics of fatty acid activation and Vac8p palmitoylation, stimulation of the fusion reaction with CoA (Figure 3F) and Pal-CoA (data not shown) is detectable only if they are added during the first 10 min of the reaction. Therefore, we conclude that (i) palmitoylation of Vac8p occurs only at the beginning of the fusion reaction and (ii) that the fatty acids remain attached throughout.
Inhibition of palmitoylation and vacuole fusion
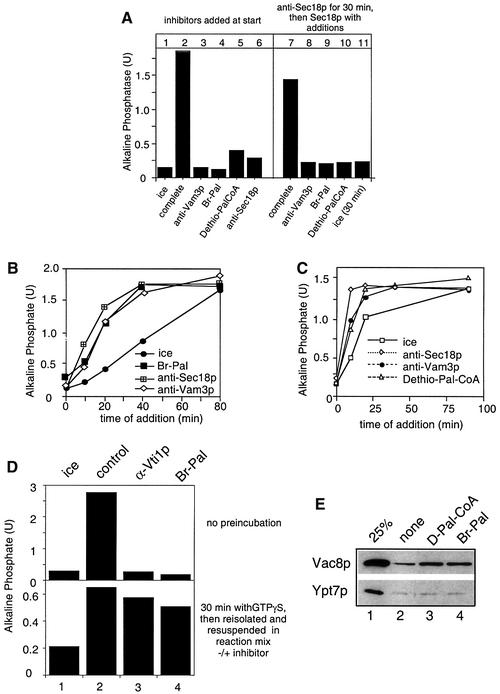
To analyze the function of palmitoylation in vacuole fusion, we first tested whether the requirement for palmitoylation can be fulfilled prior to Sec18p-mediated priming (Figure 4A). Vacuoles were incubated in the presence of ATP and antibodies to Sec18p for 30 min, then reisolated and resuspended in the presence of Sec18p to relieve the antibody block. In addition, antibodies to Vam3p or palmitoylation inhibitors (Br-Pal or the non-hydrolyzable Pal-CoA analog dethio-Pal-CoA; Pfanner et al., 1989) were added in the second incubation, which in a parallel experiment inhibited fusion completely (lanes 3–6). Neither of these inhibitors was bypassed in the first incubation (lanes 8–11), indicating that Sec18p function is required to initiate palmitoylation. We also asked at which point in the fusion reaction palmitoylation inhibitors would act (Figure 4B and C). Inhibitors were added to an ongoing fusion reaction and samples were incubated until the end of a 90 min incubation. At each indicated time point, an aliquot was withdrawn and placed on ice to monitor the progression of the fusion reaction up to this time point. All inhibitors block the reaction when added at the start. Inhibitors of priming such as anti-Sec18p only inhibit within the first 15 min, those that block docking only until 40 min, and fusion inhibitors block the reaction coincident with the ice curve (Mayer et al., 1996; Ungermann et al., 1998a). We observed that both Br-Pal (Figure 4B) and dethio-Pal-CoA (Figure 4C) inhibit the fusion reaction similarly to anti-Vam3p antibodies. We then asked whether palmitoylation inhibitors would interfere with fusion (Figure 4D). Vacuoles from both tester strains were incubated with ATP, cytosol and GTPγS for 30 min. This allows the reaction to proceed beyond the docking step (Mayer et al., 1996; Eitzen et al., 2000). Then, vacuoles were reisolated to remove GTPγS and resuspended in a new reaction mix containing ATP and cytosol. Antibodies to Vti1p or Br-Pal were added where indicated, and the reaction was incubated for an additional 60 min. Both inhibitors block fusion completely when added at the start, but do not do so once docking is completed. Therefore, palmitoylation inhibitors exert their effect only after Sec18p-mediated priming (Figure 4A) and until docking is completed (Figure 4B–D). These kinetics parallel the kinetics observed for palmitoylation of Vac8p (Figure 3).
Fig. 4. Palmitoylation inhibitors block vacuole docking. (A) Requirement for palmitoylation cannot be fulfilled before Sec18p action. Vacuoles were incubated for 30 min at 26°C in the presence of ATP, cytosol and CoA and in the absence (lanes 1–6) or presence (lanes 7–11) of antibodies to Sec18p. The indicated inhibitors were added to the reactions shown in lanes 1–6 before the start. After 30 min, reactions 7–11 were placed on ice, diluted with 300 µl of PS buffer, centrifuged (8000 g, 4 min, 4°C) and resuspended in 30 µl of reaction buffer containing ATP, cytosol, CoA and 100 ng of Sec18p. Br-Pal (200 µM), dethio-Pal-CoA (30 µM) or antibodies to Vam3p were added where indicated. Incubations were for 90 min at 26°C. Then fusion was assayed. (B) Time-of-addition experiment with Br-Pal. A 30× scale fusion reaction was started in the presence of ATP, CoA (10 µM) and cytosol at 26°C. Aliquots (30 µl) were removed at the indicated time, antibodies (200 ng/µl) to Sec18p, Vam3p or Br-Pal (200 µM) were added and samples were incubated further at 26°C for a total of 90 min before being assayed for fusion activity. Ice = samples were placed on ice at the indicated times. (C) As for (B) except that dethio-Pal-CoA (30 µM) was added instead of Br-Pal. (D) The fusion step is insensitive to inhibitors of palmitoylation. Upper panel: fusion reactions (30 µl) containing ATP, cytosol and inhibitors [antibodies to Vti1p (200 ng/µl) or Br-Pal (200 µM) as indicated] were incubated for 90 min at 26°C or on ice. Lower panel: fusion reactions (30 µl) containing ATP, cytosol and 2 mM Mg-GTPγS were incubated at 26°C for 30 min. Samples were then diluted with 150 µl of PS buffer containing 150 mM KCl, and vacuoles were reisolated (3 min, 8000 g, 4°C) and resuspended in 30 µl of reaction buffer containing ATP, cytosol and the inhibitors as indicated. Fusion was determined after an additional 60 min incubation at 26°C or on ice. (E) A block in palmitoylation causes release of Vac8p from the vacuole. BJ3505 vacuoles (15 µg) were incubated in a 75 µl reaction for 30 min at 26°C in the presence of ATP, and Br-Pal (200 µM) or dethio Pal-CoA (30 µM) where indicated. Vacuoles were then reisolated (10 min, 8000 g, 4°C) and proteins in the reaction supernatant were precipitated by 13% trichloroacetic acid (v/v). Proteins were then analyzed by SDS–PAGE and immunoblotting with antibodies to Vac8p and Ypt7p. Bands were quantified by laser densitometry. In lanes 2–4, release to the supernatant was 4.3, 12 and 11% for Vac8p, and 2.6, 3.1 and 2.7% for Ypt7p, respectively.
We asked whether a block in palmitoylation would cause a release of Vac8p from the membrane (Figure 4E). Fusion reactions were started in the presence of cytosol and ATP, and the inhibitors were added as indicated. Both Br-Pal and dethio-Pal-CoA cause a significant release of Vac8p to the reaction supernatant. Br-Pal was ineffective in the absence of ATP (not shown), indicating that release of Vac8p requires priming. The yeast rab homolog Ypt7p, which is membrane anchored by an isoprenoid group, was not released from the membrane. Thus, Vac8p requires palmitoylation for its stable association with the vacuole after priming.
Vac8p is palmitoylated upon activation of vacuoles by Sec18p and ATP
The data in Figure 4A suggest that palmitoylation is linked to the priming reaction. We therefore tested whether inhibitors of the vacuole fusion reaction would interfere with palmitoylation of Vac8p (Figure 5A). Vacuole fusion reactions were labeled with [3H]palmitate in the presence of antibodies to the t-SNARE Vam3p, which inhibit docking, or antibodies to Sec18p (yeast NSF), which inhibit only the priming reaction. Whereas our anti-Vam3p antibodies had only a modest effect on the labeling of Vac8p (lane 3), addition of anti-Sec18p caused a striking block (lane 2). Other inhibitors of docking, such as anti-Vti1p, anti-Nyv1p or neomycin, and fusion inhibitors such as BAPTA did not or only slightly blocked the labeling (Figure 5B, see Discussion). The effect of Sec18p antibodies on palmitoylation is not due to inhibition of fatty acid activation, because synthesis of Pal-CoA was not altered by anti-Sec18p (Figure 3D, lanes 6 and 7). There fore, Sec18p-mediated priming is required for Vac8p palmitoylation.
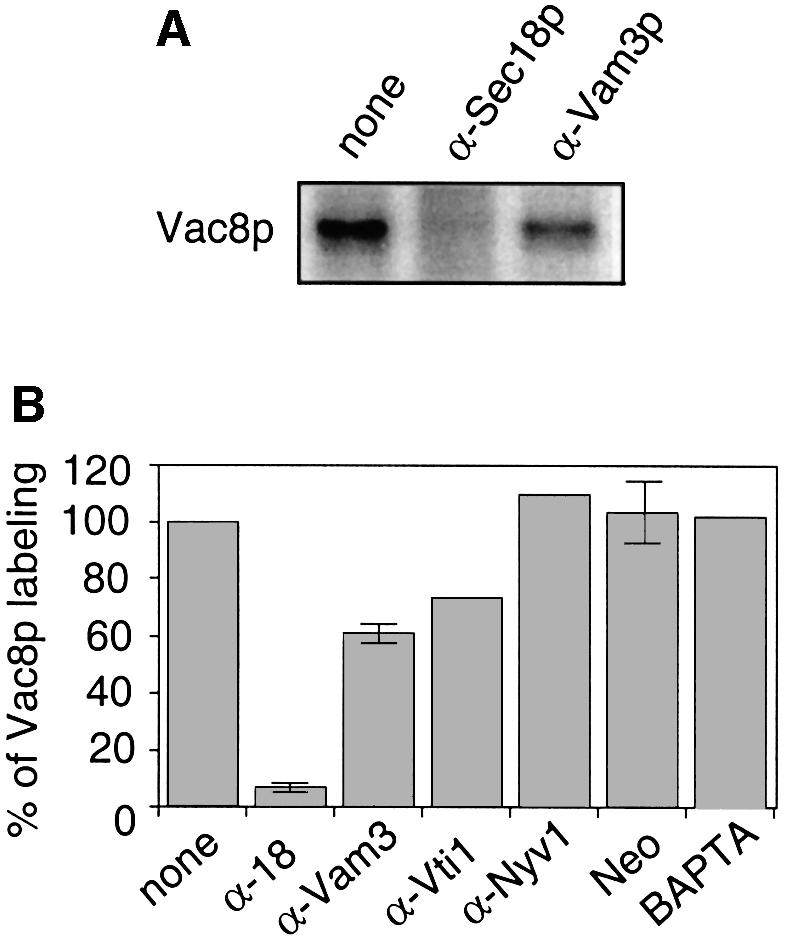
Fig. 5. Vac8p palmitoylation depends on Sec18p. (A) Fusion reactions were labeled with [3H]palmitate in the presence or absence of antibodies to Sec18p or Vam3p (200 ng/µl) for 15 min as described in Figure 1B. Then, vacuoles were reisolated and analyzed by SDS–PAGE and fluorography. (B) Quantification of the inhibition of Vac8p palmitoylation from independent experiments. Labeling of vacuoles with [3H]palmitate was carried out as in (A). Control incubation was set to 100%. Inhibition of Vac8 palmitoylation with antibodies (200 ng/µl) to Sec18p (n = 3), Vam3p (n = 3), Vti1p (n = 1), Nyv1p (n = 1), 5 mM BAPTA (n = 1) and 300–500 µM neomycin (n = 4) was quantified by laser densitometry. Control incubation was set to 100%. All inhibitors blocked the fusion reaction completely (not shown).
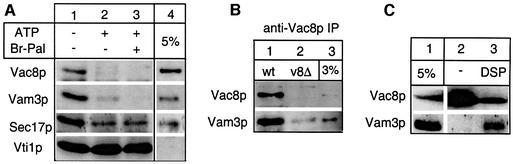
This suggested that Vac8p was either activated parallel to the SNARE disassembly or was in fact part of the SNARE complex. We tested the latter assumption by immunoprecipitation of the cis-SNARE complex with antibodies to the v-SNARE Vti1p (Ungermann et al., 1999a). Vacuoles were incubated with or without ATP prior to the analysis (Figure 6A). We did observe that a portion of Vac8p is associated with the cis-SNARE complex in the absence of ATP. When ATP was added, Vac8p was dissociated from the complex, similarly to Vam3p and Sec17p. However, Br-Pal together with ATP did not block the SNARE complex disassembly (lane 2 versus lane 3), indicating that Vac8p palmitoylation is not a prerequisite for SNARE complex disassembly. We also performed the reverse co-immunoprecipitation to prove the specificity of the interaction. Antibodies to Vac8p precipitated Vam3p from wild-type detergent extracts but not from those made from vac8 deletion vacuoles (Figure 6B). Finally, Vam3p was found in a complex with Vac8p after cross-linking, implying that both proteins are in close proximity on the membrane (Figure 6C). Thus, a fraction of Vac8p is associated with the SNARE complex on isolated vacuoles. We suggest that only during or after its release from the SNARE complex does Vac8p become palmitoylated and activates Vac8p for its function in homotypic fusion.
Fig. 6. Vac8p is part of the SNARE complex. (A) Co-immunoprecipitation of Vac8p with anti-Vti1p antiserum.Vacuoles (50 µg) were incubated in a 250 µl volume with or without ATP and Br-Pal (200 µM) for 15 min at 26°C, pelleted (5 min, 8000 g, 4°C) and solubilized. The detergent extract was incubated overnight with pre-immune IgGs, and then immunoprecipitated for 2 h with antibodies to Vti1p. Retained proteins were eluted and analyzed (see Materials and methods). Immunoblots were decorated with antibodies to Vam3p, Sec17p, Vac8p and Vti1p. Whereas all Vti1p is immunoprecipitated in this experiment, 30% of Vam3p and Sec17p and 2% of Vac8p is found in association with Vti1p (lane 1) as quantified by laser densitometry. (B) Immunoprecipitation of Vam3p with anti-Vac8p antiserum. Vacuoles (50 µg) from DKY6281 containing Vac8p with a C-terminal GFP tag (lane 1) or DKY vacuoles lacking Vac8p (lane 2) were detergent solubilized as in (A) and immunoprecipitation was carried out overnight with protein A–Sepharose-linked antibodies to Vac8p. Western blots were decorated with antibodies to Vac8p and Vam3p. Tagged Vac8p was used in this experiment because of occasional cross-reactivity of the anti-Vac8p antibody with the 60 kDa region on the blot. (C) Cross-linking of Vac8p and Vam3p. Vacuoles from BJ3505 containing a Vac8 protein with a C-terminal TAP tag (see Materials and methods) were incubated in 250 µl of reaction buffer for 30 min on ice with or without 200 µM dithiobis-succinimidyl propionate (DSP, Pierce). Then, 20 mM Tris pH 7.9 was added, vacuoles were reisolated (5 min, 8000 g, 4°C) and detergent solubilized in 1 ml of 1% Triton X-100, 20 mM HEPES–KOH pH 7.9, 300 mM NaCl, 1 mM PMSF, 0.5× PIC. Immunoprecipitation with 30 µl of IgG–Sepharose (Amersham Pharmacia), washing and elution of bound proteins are described in Materials and methods. Blots were decorated with antibodies to Vac8p and Vam3p. Due to high salt washing of the beads, association of Vam3p with Vac8p was lost without DSP pre-treatment. Some Vac8p signal in lane 3 was lost due to cross-linking into high molecular weight complexes.
Vac8p is required for homotypic vacuole fusion
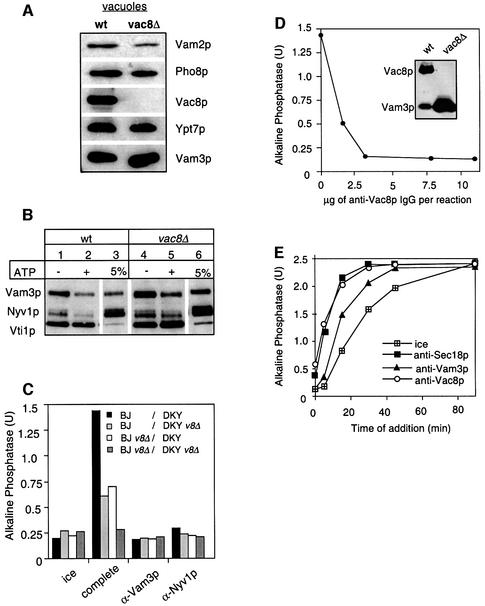
The observation that palmitoylation inhibitors blocked fusion (Figures 1 and 4) and that Vac8p palmitoylation was linked to the priming reaction (Figure 5) implied that Vac8p had a role in the fusion reaction. Therefore, we deleted the VAC8 gene from our tester strains and analyzed the morphology and fusion competence of isolated vacuoles. Unexpectedly, vacuoles from wild-type or mutant strains were indistinguishable under the microscope (not shown), even though vac8Δ vacuoles are multilobed in vivo (Fleckenstein et al., 1998; Pan and Goldfarb, 1998; Wang et al., 1998). The mutant vacuoles also contain the same amount of marker proteins on their membranes (Figure 7A). The only reproducible difference was a reduction in Vam2p, a component of HOPS. Total levels of Vam2p were, however, unaltered in the cell (not shown). This may indicate a functional relationship between Vac8p and HOPS that will be addressed in future experiments. Next, we asked if the integrity of the SNARE complex is impaired if Vac8p is missing from the vacuole. Detergent extracts of isolated wild-type and vac8Δ vacuoles were prepared and subjected to immunoprecipitation with antibodies to Vti1p (Figure 7B). We observed that the stability and integrity of the SNARE complex were unaltered regardless of whether Vac8p was present or not. Therefore, the reduced fusion activity of the mutant vacuoles is more probably linked to a lack of coordination within the fusion cascade. We then tested whether the different combinations of vacuoles isolated from wild-type and vac8Δ strains were fusion competent (Figure 7C). Fusion of wild-type vacuoles with those lacking Vac8p resulted in a reduction of the fusion activity to 50–70% (light gray, white bars). Fusion of vac8Δ vacuoles was reduced to background levels (dark gray bars). All reactions were sensitive to fusion inhibitors such as antibodies to the SNAREs Vam3p and Nyv1p, showing that authentic fusion was measured. The same observations were made when the fusion reaction was assayed with vacuoles from a strain with a Vac8 protein in which the three N-terminal cysteine residues were replaced by alanine (not shown). However, this mutant Vac8p localizes only poorly to the vacuole (Wang et al., 1998), and it is therefore not surprising that these vacuoles exhibit the same phenotype as the vac8Δ vacuoles. Thus, Vac8p is required at least on one membrane for fusion. In the absence of Vac8p, fusion is blocked. Finally, we analyzed whether IgGs to Vac8p would inhibit the fusion assay. The antibodies used in previous experiments did not inhibit fusion (not shown). We therefore raised new antibodies against Vac8p that recognize Vac8p specifically on western blots (see inset of Figure 7D). Purified IgGs to Vac8p, used at concentrations similar to those for anti-Vam3p in Figure 4B, inhibit vacuole fusion completely (Figure 7D). Furthermore, in a time-of-addition experiment (similar to Figure 4B), we observed that antibodies to Vac8p inhibit the fusion reaction coincident with priming (Figure 7E). This would suggest that Vac8p is not required later in the reaction, though an accumulation of Vac8p at docking sites has been described (Pan and Goldfarb, 1998; see Discussion). Taking the results together, Vac8p is essential for homotypic vacuole fusion as fusion is sensitive to specific antibodies and deletion of Vac8p.
Fig. 7. Vac8p is essential for fusion. (A) Protein composition of vacuoles from wild-type or vac8Δ mutants. Vacuoles (10 µg) from DKY6281 and the corresponding deletion mutant were solubilized in SDS sample buffer, boiled for 4 min at 95°C and analyzed by SDS–PAGE and western blotting. The immunoblot was decorated with antibodies to Vam3p, Vac8p, Pho8p, Vam2p and Ypt7p. (B) Deletion of Vac8p does not influence the integrity of the SNARE complex. Vacuoles from BJ3505 or the corresponding strain lacking Vac8p were purified, incubated for 10 min in the presence or absence of ATP and processed for co-immunoprecipitation with anti-Vti1p antibodies as described in Figure 6A. (C) Fusion of vac8Δ vacuoles. Vacuoles from the tester strains and the respective vac8Δ strains were incubated for 90 min at 26°C in the presence of cytosol, Sec18p (20 ng), CoA (10 µM) and ATP in the combinations shown. Inhibitors such as IgGs to Vam3p or Nyv1p (both at 200 ng/µl) were added where indicated. After the incubation, fusion was assayed as described. v8Δ stands for vac8Δ. (D) Sensitivity of vacuole fusion to antibodies against Vac8p. Fusion reactions (30 µl) containing vacuoles from both tester strains, ATP and cytosol were incubated for 90 min at 26°C. Increasing amounts of purified IgGs against full-length Vac8p (see Materials and methods) were added where indicated. After 90 min, fusion was measured. The inset shows an immunoblot of vacuoles (wt = 5 µg, vac8Δ = 50 µg) decorated with Vac8p (new) and Vam3p antibodies. (E) Vac8p antibodies inhibit at priming. Reactions were incubated and aliquots removed as described in Figure 4B, except that IgGs to Sec18, Vam3p and Vac8p (200 ng/µl) were added as inhibitors.
Discussion
Our results suggest that the requirement for Pal-CoA for a membrane fusion reaction is indeed linked to the palmitoylation of a target protein (Glick and Rothman, 1987; Pfanner et al., 1990; Haas and Wickner, 1996). We have identified Vac8p as the major protein palmitoylated during the vacuole fusion reaction (Figure 1). Br-Pal, an inhibitor of protein palmitoylation, blocks vacuole fusion and palmitoylation of Vac8p at comparable concentrations (Figure 2), suggesting that this hydrophobic modification is an essential step in the vacuole fusion reaction. This is consistent with published data showing that the palmitoylation of Vac8p is essential for vacuole inheritance in vivo (Wang et al., 1998; Schneiter et al., 2000). Our assumption is supported further by the observation that palmitoylation of Vac8p occurs only at a certain time point during the fusion reaction (Figure 3). Vac8p is essential for vacuole fusion (Figure 7) and initially is associated with the cis-SNARE complex (Figure 6A). In addition, palmitoylation requires Sec18p-mediated priming (Figures 4 and 5). Inhibitors of docking or fusion had no strong effect on Vac8p palmitoylation. The slight inhibition of palmitoylation with antibodies against Vam3p might be due to its interference with Sec17p release (Ungermann et al., 1998a). Thus, palmitoylation of Vac8p occurs upon priming. Blocking palmitoylation releases Vac8p from the vacuolar membrane (Figure 4E), indicating that the fatty acids serve as a membrane anchor after the association of Vac8p with the cis-SNARE complex is lost. Only a small fraction of all Vac8p molecules are bound initially to the cis-SNARE complex (Figure 6A). However, ∼50% of all Vam3p molecules are found in association with Vac8p (Figure 6B). Vac8p is at least 10 times more abundant than Vam3p (R.Laage and C.Ungermann, unpublished) and, therefore, is present in large molar excess compared with the SNARE proteins. The priming dependency of Vac8p acylation suggests that only the SNARE-bound subpopulation of Vac8p becomes palmitoylated and thus is released from the membrane if palmitoylation is blocked (Figure 4E).
Although we have been able to link palmitoylation of Vac8p to the Pal-CoA requirement of the fusion reaction, the machinery that performs palmitoylation remains to be discovered. On vacuoles, it probably consists of several components. First, vacuoles must contain a fatty acid-activating enzyme (FAA) that activates palmitate to Pal-CoA, as shown for GLUT4 and synaptic vesicles (Sleeman et al., 1998; Veit et al., 2000). Several FAA proteins are known (Johnson et al., 1994; Choi and Martin, 1999), and it will be challenging to identify the synthetase required for vacuole fusion. Secondly, a protein-acyl-CoA transferase (PAT), a postulated, though not yet purified enzyme, could be required for the acylation, though acylation can also occur autocatalytically in vitro (Duncan and Gilman, 1996; Bano et al, 1998; Veit, 2000). The yeast vacuolar fusion assay might be a promising system to clarify this issue. The requirement for priming for Vac8p palmitoylation suggests that the palmitoylation machinery is only active if Sec18p is functional. It is also possible that the machinery is active all the time, but the cysteines of Vac8p are hidden prior to priming. Identifying the relevant components will be the important next step.
There is certainly the question of whether Vac8p is the only target of palmitoylation. Although we did not observe other proteins labeled with [3H]palmitate reproducibly we cannot rule out that less abundant proteins were not detected. Likewise, acylation of specific lipids, a process known to be involved in vesicular transport (Huttner and Schmidt, 2000), may also occur during the vacuole fusion reaction (Figure 3D).
The requirement for palmitoylation of Vac8p upon priming implies that Vac8p must be depalmitoylated at some point during the fusion reaction. Although we could not detect turnover in palmitoylation of Vac8p during the vacuolar fusion assay, possibly due to limiting amounts of thioesterase, it is likely that depalmitoylation of Vac8p occurs in vivo. Recently, an enzyme has been purified from vertebrate cells that depalmitoylates α-subunits of heterotrimeric G-proteins and the Ras protein (Duncan and Gilman, 1998). We currently are trying to identify a putative homolog on yeast vacuoles that could perform this role. We postulate that Vac8p is retrieved into the SNARE complex after depalmitoylation and is then activated again upon priming and palmitoylation.
Vac8p requires palmitoylation only for the vacuole inheritance reaction and for vacuole morphology (Wang et al., 1998). This suggests that the recently identified complex of Apg13 and Vac8p (Scott et al., 2000), which is required for the CVT pathway, performs an entirely different function from that required for vacuole fusion. Vac8p has been shown to interact with actin in vitro (Wang et al., 1998). Furthermore, it was demonstrated that Vac8p interacts with a nuclear protein, Nvj1p, and participates in the formation of nuclear–vacuolar junctions (Pan et al., 2000). Although the role of this interaction remains unclear, it may also be mediated by cytoskeletal interactions. We have tested whether actin plays a role in vacuole fusion, but could not identify any effect using a wide panel of inhibitors (C.Ungermann, unpublished), suggesting that this interaction does not have a role in the fusion cascade.
Why is palmitoylation an essential prerequisite for Vac8p function? Deletion of the myristoylation and/or palmitoylation motif causes mislocalization to the cytosol (Wang et al., 1998). However, only palmitoylation minus mutants show defective vacuole inheritance and multilobed vacuoles, indicating a further, more specific function of Vac8p palmitoylation. Fatty acids bound to different cysteines near the Vac8p N-terminus may perform different functions, i.e. involvement in targeting, vacuolar retention and/or localization to subdomains of the lipid bilayer (rafts). Palmitoylation adds additional hydrophobicity to Vac8p, which could be required for interaction with other proteins that associate with Vac8p after priming. Alternatively, palmitoylation could inactivate Vac8p by removing it from the SNARE complex. Also, palmitoylation of Vac8p at priming may induce a conformational change that makes the modified Vac8p inaccessible to antibodies later on. Either assumption would explain why IgGs to Vac8p would block at priming (Figure 7E). We favor, however, the idea that Vac8p has a post-priming function, as Vac8p accumulates at docking sites (Pan and Goldfarb, 1998), and palmitoylation is necessary for retention of Vac8p on the vacuole (Figure 4). Characterizing the role of palmitoylated Vac8p in the fusion reaction will be the main focus of future studies.
Materials and methods
Reagents and strains
[9,10-3H]palmitic acid (50 Ci/mmol) was obtained from Hartmann Analytic (Braunschweig, Germany). 2-bromo-palmitate was from ACROS, Belgium. All other reagents were purchased from Sigma. The non-hydrolyzable Pal-CoA analog, termed here dethio-Pal-CoA, was a kind gift of Professor Jens Knudsen (University of Odense). Antibodies to Vac8p were raised in White New Zealand rabbits against the His6-tagged full-length Vac8 protein purified from Escherichia coli.
The yeast strains used in this study were as described (Haas et al., 1994; Price et al., 2000). The strains BJ3505 vac8::TRP1 and DKY6281 vac8::HIS3 were created by transformation of PCR fragments containing the gene for TRP1 or HIS3 with flanking regions of the VAC8 promoter and terminator. Colonies that grew on SC-trp and SC-his plates, respectively, were restreaked and analyzed for loss of the VAC8 gene by PCR and immunoblotting with antibodies against Vac8p. Integration of the C-terminal green fluorescent protein (GFP) tag or TAP tag to VAC8 was carried out by PCR amplification of a cassette containing the respective tag and a HIS or TRP marker and flanking regions of the C-terminus of VAC8 (Rigaut et al., 1999). The TAP tag consists of the IgG-binding domain of protein A combined with a calmodulin-binding domain.
Vacuole fusion assay
Vacuole fusion is measured by a biochemical complementation assay (Conradt et al., 1992; Haas et al., 1994). Standard fusion reactions were performed as described (Mayer et al., 1996; Ungermann et al., 1998a).
Labeling of vacuole fusion assay with [3H]palmitate and [3H]Pal-CoA
[3H]Pal-CoA was prepared using acyl-CoA synthetase and [3H]palmitate as described (Veit, 2000). The preparation was then bound to a Sep-Pak C18 column (Waters), equilibrated in 50 mM ammonium acetate pH 5.5. The column was washed twice with 10% acetonitrile and 20% acetonitrile (2 ml each wash, dissolved in 50 mM ammonium acetate pH 5.5). [3H]Pal-CoA was eluted with 60% acetonitrile. Acetonitrile was then evaporated in a speed vac. Purity of the preparation was checked by TLC as described below.
Ten-fold standard fusion reactions (300 µl) with DKY6281 vacuoles (60 µg) and 10 µM CoA were labeled with [3H]palmitate (3 µl in ethanol, 50 Ci/mmol, 500 µCi/ml and 10 µM final concentration) or [3H]Pal-CoA (1.5 µCi/ml, 30 nM final concentration) at 30°C for the indicated times. Labeling was similar if carried out at 26°C or if the BJ vacuoles or both vacuoles were used (not shown). Vacuoles were pelleted (5 min, 12 000 g), resuspended in 500 µl 10 mM PIPES–KOH pH 6.8, 200 mM sorbitol (PS buffer) containing 0.5× protein inhibitor cocktail (PIC; 7.5 µM pefabloc SC, 7.5 ng/ml leupeptin, 3.75 µM o-phenanthroline and 37.5 ng/ml pepstatin; Xu et al., 1996), and again pelleted. Vacuoles were boiled immediately in 20 µl of 2× concentrated non-reducing SDS–PAGE buffer. Proteins were separated in 12% SDS–polyacrylamide gels. Gels were stained with Coomassie, destained, washed with H2O (2× 15 min) and treated with 1 M salicylate (30 min). The dried gel was exposed to X-ray film for 4–10 days. Quantification of bands was carried out with an Epson scanner GT 9000 and Scan-Pack 3.0 software (Biometra, Göttingen, Germany).
TLC analysis of [3H]Pal-CoA synthesis
[3H]palmitate-labeled fusion reactions (100 µl) were stopped with 500 µl of acetonitrile/phosphoric acid (9:1). Precipitated proteins were pelleted (14 000 g, 15 min) and aliquots of the supernatant were separated on Silicagel 60 TLC plates with butanol/acetic acid/distilled water (8:3:3) as solvent system. The plates were then sprayed with En3Hance (Amersham) and exposed to X-ray film. Bands were identified with radioactive reference substances ([3H]Pal and [3H]Pal-CoA) or unlabeled phospholipids (phosphatidylserine, phosphatidylethanolamine and phosphatidylinositol), which were run in parallel. The latter were visualized with iodine vapor.
Immunoprecipitation experiments
After the reaction, vacuoles were pelleted (8000 g, 5 min, 4°C), washed with 500 µl of PS buffer and reisolated as before. Vacuoles were detergent solubilized by the addition of 1 ml of 0.1% Triton X-100, 50 mM KCl, 20 mM HEPES–KOH pH 7.4, 0.5× PIC and 1 mM phenylmethylsulfonyl fluoride (PMSF). Immunoprecipitation with protein A–Sepharose bead-coupled antibodies and analysis of protein complexes were carried out as described (Ungermann et al., 1999a). For the identification of 3H-labeled Vac8p, the protocol was altered as follows. Solubilization and washing were carried out in 0.5% Triton X-100, 300 mM NaCl, 1× phosphate-buffered saline, 1 mM PMSF and 0.5× PIC. Bound proteins were eluted by the addition of SDS sample buffer without 2-mercaptoethanol and analyzed by SDS–PAGE and fluorography.
Acknowledgments
Acknowledgements
We would like to thank Bill Wickner for his very generous help with reagents, and Felix Wieland and Ed Hurt for their support at the BZH. Thanks to Nils Faergeman, Jens Knudsen and Bertrand Seraphin for discussions and reagents, and Douglas Cyr and Christine Boeddinghaus for critical comments on the manuscript. M.V. is grateful to Michael F.G.Schmidt for encouragement and for providing excellent facilities in his institute, and to Christiane Palissa and Ellen Lyhs for expert technical assistance. This work was supported by grants from the DFG (to C.U. and M.V.).
References
- Bano M.C., Jackson,C.S. and Magee,A.I. (1998) Pseudo-enzymatic S-acylation of a myristoylated Yes protein kinase peptide in vitro may reflect non enzymatic S-acylation in vivo. Biochem. J., 330, 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. and Schmidt,M.F. (1984) Identification of acyl donors and acceptor proteins for fatty acid acylation in BHK cells infected with Semliki Forest virus. EMBO J., 3, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume L. and Resh,M.D. (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J. Biol. Chem., 270, 22399–22405. [DOI] [PubMed] [Google Scholar]
- Choi J.Y. and Martin,C.E. (1999) The Saccharomyces cerevisiae FAT1 gene encodes an acyl-CoA synthetase that is required for maintenance of very long chain fatty acid levels. J. Biol. Chem., 274, 4671–4683. [DOI] [PubMed] [Google Scholar]
- Conradt B., Shaw,J., Vida,T., Emr,S. and Wickner,W. (1992) In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J. Cell Biol., 119, 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.A. and Gilman,A.G. (1996) Autoacylation of G protein α subunits. J. Biol. Chem., 271, 23594–23600. [DOI] [PubMed] [Google Scholar]
- Duncan J.A. and Gilman,A.G. (1998) A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein α subunits and p21(RAS). J. Biol. Chem., 273, 15830–15837. [DOI] [PubMed] [Google Scholar]
- Dunphy J.T., Greentree,W.K., Manahan,C.L. and Linder,M.E. (1996) G-protein palmitoyltransferase activity is enriched in plasma membranes. J. Biol. Chem., 271, 7154–7159. [DOI] [PubMed] [Google Scholar]
- Eitzen G., Will,E., Gallwitz,D., Haas,A. and Wickner,W. (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J., 19, 6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein D., Rohde,M., Klionsky,D.J. and Rudiger,M. (1998) Yel013p (Vac8p), an armadillo repeat protein related to plakoglobin and importin α is associated with the yeast vacuole membrane. J. Cell Sci., 111, 3109–3118. [DOI] [PubMed] [Google Scholar]
- Glick B.S. and Rothman,J.E. (1987) Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature, 326, 309–312. [DOI] [PubMed] [Google Scholar]
- Haas A. and Wickner,W. (1996) Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF). EMBO J., 15, 3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A., Conradt,B. and Wickner,W. (1994) G-protein ligands inhibit in vitro reactions of vacuole inheritance. J. Cell Biol., 126, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W.B. and Schmidt,A. (2000) Lipids, lipid modification and lipid–protein interaction in membrane budding and fission—insights from the roles of endophilin A1 and synaptophysin in synaptic vesicle endocytosis. Curr. Opin. Neurobiol., 10, 543–551. [DOI] [PubMed] [Google Scholar]
- Johnson D.R., Knoll,L.J., Levin,D.E. and Gordon,J.I. (1994) Saccharo myces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J. Cell Biol., 127, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Dudler,T. and Gelb,M.H. (1996) Purification of a protein palmitoyltransferase that acts on H-Ras protein and on a C-terminal N-Ras peptide [published erratum appears in J. Biol. Chem., 274, 3252]. J. Biol. Chem., 271, 23269–23276. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Orci,L., Tani,K., Amherdt,M., Ravazzola,M., Elazar,Z. and Rothman,J.E. (1993) Stepwise assembly of functionally active transport vesicles. Cell, 75, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Pan X. and Goldfarb,D.S. (1998) YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuole fusion and inheritance. J. Cell Sci., 111, 2137–2147. [DOI] [PubMed] [Google Scholar]
- Pan X., Roberts,P., Chen,Y., Kvam,E., Shulga,N., Huang,K., Lemmon,S. and Goldfarb,D.S. (2000) Nucleus–vacuole junctions in Saccharo myces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p. Mol. Biol. Cell, 11, 2445–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Buhler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Orci,L., Glick,B.S., Amherdt,M., Arden,S.R., Malhotra,V. and Rothman,J.E. (1989) Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell, 59, 95–102. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Glick,B.S., Arden,S.R. and Rothman,J.E. (1990) Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J. Cell Biol., 110, 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M.D. (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta, 1451, 1–16. [DOI] [PubMed] [Google Scholar]
- Rigaut G., Shevchenko,A., Rutz,B., Wilm,M., Mann,M. and Seraphin,B. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol., 17, 1030–1032. [DOI] [PubMed] [Google Scholar]
- Schneiter R., Guerra,C.E., Lampl,M., Tatzer,V., Zellnig,G., Klein,H.L. and Kohlwein,S.D. (2000) A novel cold-sensitive allele of the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase, affects the morphology of the yeast vacuole through acylation of Vac8p. Mol. Cell. Biol., 20, 2984–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S.V. et al. (2000) Apg13p and Vac8p are part of a complex of phosphoproteins that are required for cytoplasm to vacuole targeting. J. Biol. Chem., 275, 25840–25849. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W.T. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman M.W., Donegan,N.P., Heller-Harrison,R., Lane,W.S. and Czech,M.P. (1998) Association of acyl-CoA synthetase-1 with GLUT4-containing vesicles. J. Biol. Chem., 273, 3132–3135. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Nichols,B.J., Pelham,H.R. and Wickner,W. (1998a) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998b) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., von Mollard,G.F., Jensen,O.N., Margolis,N., Stevens,T.H. and Wickner,W. (1999a) Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol., 145, 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Wickner,W. and Xu,Z. (1999b) Vacuole acidification is required for trans-SNARE pairing, LMA1 release and homotypic fusion. Proc. Natl Acad. Sci. USA, 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Price,A. and Wickner,W. (2000) A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc. Natl Acad. Sci. USA, 97, 8889–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M. (2000) Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem. J., 345, 145–151. [PMC free article] [PubMed] [Google Scholar]
- Veit M., Sachs,K., Heckelmann,M., Maretzki,D., Hofmann,K.P. and Schmidt,M.F. (1998) Palmitoylation of rhodopsin with S-protein acyltransferase: enzyme catalyzed reaction versus autocatalytic acylation. Biochim. Biophys. Acta, 1394, 90–98. [DOI] [PubMed] [Google Scholar]
- Veit M., Becher,A. and Ahnert-Hilger,G. (2000) Synaptobrevin 2 is palmitoylated in synaptic vesicles prepared from adult, but not from embryonic brain. Mol. Cell. Neurosci., 15, 408–416. [DOI] [PubMed] [Google Scholar]
- Wang Y.X., Zhao,H., Harding,T.M., Gomes de Mesquita,D.S., Woldringh,C.L., Klionsky,D.J., Munn,A.L. and Weisman,L.S. (1996) Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol. Biol. Cell, 7, 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Catlett,N.L. and Weisman,L.S. (1998) Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol., 140, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Y., Hermida-Matsumoto,L. and Resh,M.D. (2000) Inhibition of protein palmitoylation, raft localization and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem., 275, 261–270. [DOI] [PubMed] [Google Scholar]
- Xu Z. and Wickner,W. (1996) Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J. Cell Biol., 132, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]