Abstract
McrBC from Escherichia coli K-12 is a restriction enzyme that belongs to the family of AAA+ proteins and cuts DNA containing modified cytosines. Two proteins are expressed from the mcrB gene: a full-length version, McrBL, and a short version, McrBS. McrBL binds specifically to the methylated recognition site and is, therefore, the DNA-binding moiety of the McrBC endonuclease. McrBS is devoid of DNA-binding activity. We observed that the quaternary structure of the endonuclease depends on binding of the cofactors. In gel filtration experiments, McrBL and McrBS form high molecular weight oligomers in the presence of Mg2+ and GTP, GDP or GTP-γ-S. Oligomerization did not require the presence of DNA and was independent of GTP hydrolysis. Electron micrographs of negatively stained McrBL and McrBS revealed ring-shaped particles with a central channel. Mass analysis by scanning transmission electron microscopy indicates that McrBL and McrBS form single heptameric rings as well as tetradecamers. In the presence of McrC, a subunit that is essential for DNA cleavage, the tetradecameric species was the major form of the endonuclease.
Keywords: AAA+ protein family/GTP/GTPase/McrBC restriction/scanning transmission electron microscopy
Introduction
Restriction–modification systems allow bacteria to detect and destroy foreign DNA, thus providing an efficient defense against bacteriophages. This phenomenon was first described in the early 1950s (Luria and Human, 1952; Bertani and Weigle, 1953). The molecular event underlying restriction was later shown to be cleavage of the phage DNA by endonucleases, and the modification was shown to be due to methylation of the DNA (Dussoix and Arber, 1962; Arber, 1965). In the classical restriction– modification systems, DNA modification and restriction work in concert to protect endogenous DNA and exclude exogenous DNA. A restriction endonuclease recognizes a specific DNA sequence and cleaves it if it is not modified. The associated DNA methyltransferase recognizes the same sequence and methylates a key residue within that sequence, thereby preventing cleavage of the host DNA by its own restriction enzyme(s) (Bickle and Krüger, 1993; Roberts and Macelis, 1993).
A fundamentally different way to distinguish between exogenous and endogenous DNA is to recognize a foreign modification pattern. This is accomplished by modification-dependent restriction systems. In this case, the host cell DNA avoids being cleaved because it is not modified. The modification-dependent restriction systems are, therefore, not associated with a methyltransferase (reviewed by Raleigh and Wilson, 1986; Bickle and Krüger, 1993). In fact, the first description of restriction–modification by Luria and Human (1952) is now known to be due to modification-dependent restriction (Revel, 1983).
In Escherichia coli K-12 there are at least four chromosomally encoded restriction activities. One, EcoKI, is a classical type I restriction–modification system that restricts unmodified DNA. The other three systems, mcrA, mrr and mcrBC, restrict DNA that is methylated at specific sequences (reviewed by Raleigh, 1992; Bickle and Krüger, 1993). McrBC is the best characterized of these methylation-dependent restriction enzymes. The mcrBC operon contains two genes, mcrB and mcrC. The mcrB gene shares sequence similarity with members of the AAA+ family of proteins (Neuwald et al., 1999). The key feature of the AAA+ family (AAA+ standing for ATPases associated with various cellular activities) is a conserved module of 230–250 amino acids that includes the Walker A and B motifs and other unique regions of similarity (reviewed by Patel and Latterich, 1998). Several mcrB-like genes have been identified based on sequence similarity to genes in different microorganisms. These form a subfamily among the large AAA+ family of proteins (Neuwald et al., 1999). The AAA+ domain is found in a wide variety of proteins that often perform chaperone-like functions that assist in the assembly, operation or disassembly of protein complexes. For example, the AAA+ domain is found in proteases such as the 19S cap complex of the eukaryotic 26S proteasome and the bacterial FtsH, as well as in the regulatory components of the Lon, Clp and HslVU proteases (Neuwald et al., 1999). The AAA+ domain is also associated with protein–DNA complexes; for example, it occurs in proteins involved in replication (DnaA and DnaB) and recombination (RuvB) (Neuwald et al., 1999). Frequently proteins that belong to the AAA+ family form hexameric or heptameric ring structures (Pamnani et al., 1997; Rohrwild et al., 1997; Wolf et al., 1998) and it has been proposed that they act as ATP-dependent protein clamps (Confalonieri and Duguet, 1995).
The mcrB gene encodes a large, full-length gene product termed McrBL of 53 kDa and a small McrBS protein of 34 kDa (Ross et al., 1989b; Dila et al., 1990; Krüger et al., 1992). McrBL and McrBS seem to be expressed at equimolar ratios (Ross and Braymer, 1987; Dila et al., 1990). McrBS lacks the N-terminal 161 amino acids but retains the C-terminal 287 residues (Ross et al., 1989b), which include the AAA+ domain. McrBS is produced by internal in-frame translational initiation rather than post-translational processing of the full-length product (Ross et al., 1989b; Krüger et al., 1992). Its role in vitro and in vivo seems to be to modulate McrBC activity (Beary et al., 1997; Panne et al., 1998).
The McrBL subunit confers specificity to the endonuclease by binding to the methylated recognition site RmC (Krüger et al., 1995; Stewart et al., 2000). The DNA-binding domain resides in the N-terminal 161 amino acids; evidence for this includes lack of DNA binding by McrBS and by a mutant with a missense mutation in the N-terminal region (W49C), and retention of DNA-binding ability by an N-terminal fragment of 190 amino acids (Gast et al., 1997; Panne et al., 1998, 1999). Therefore, McrBs alone or in the presence of McrC cannot support restriction either in vivo (D.Dila and E.A.Raleigh, unpublished results; Beary et al., 1997) or in vitro (Panne et al., 1998). In vitro, McrBL, McrC, GTP and Mg2+ are required for DNA cleavage (Sutherland et al., 1992). The mcrC gene directs the synthesis of a 40 kDa mcrC gene product (Ross et al., 1989a). McrC lacks DNA-binding activity but interacts directly with either McrBL or McrBS and stimulates their GTPase activity (Pieper et al., 1997; Panne et al., 1999; Stewart et al., 2000). McrBC is, to our knowledge, the only member of the AAA+ family that uses GTP rather than ATP as a cofactor.
McrBC recognizes and cleaves DNA containing at least two RmC sites. These recognition sites can be separated by 40 bp–3 kb (Stewart and Raleigh, 1998). Cleavage occurs ∼30 bp from either recognition site, independent of the spacing between the two sites (Stewart and Raleigh, 1998; Panne et al., 1999). Recent results support the model that communication between distant recognition sites is accomplished by GTP hydrolysis-driven DNA translocation by the McrBC complex (Panne et al., 1999). The stalling of such a translocating complex, either by the convergence of two translocating complexes or by a non-specific physical block such as a DNA-bound protein, can induce DNA cleavage (Panne et al., 1999).
Subunit titration experiments suggest that the active McrBC endonuclease contains McrBL and McrC subunits at a ratio of 3–5:1 (Panne et al., 1998). To analyze the subunit stoichiometry of the AAA+ type McrBC endonuclease further, we have investigated the quaternary structure of McrBL, McrBS and the complex McrBLC.
Results
Gel filtration experiments
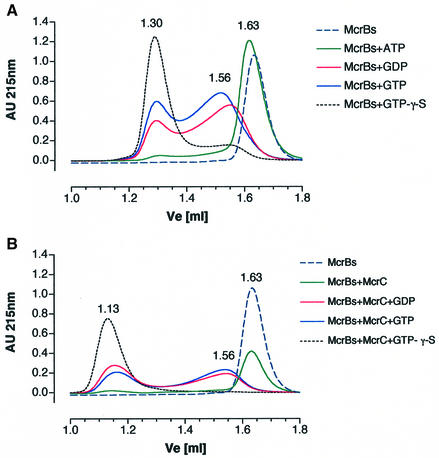
Previous studies have shown that the optimal ratio for DNA cleavage is 3–5 McrBL per McrC, suggesting that DNA cleavage is accomplished by a multisubunit complex (Panne et al., 1998). To investigate the nature of this complex further, we performed gel filtration experiments with McrBS or McrBL in the presence and absence of the cofactors and analogs GTP, GDP or GTP-γ-S. As shown in Figure 1A, McrBS alone eluted at 35.7 kDa, which is close to its predicted mol. wt of 34.6 kDa. When McrBS was pre-incubated in buffer containing GTP or GDP, the protein eluted in two peaks corresponding to mol. wts of ∼52 and 217 kDa, respectively. In the presence of GTP-γ-S, most of the protein eluted with a mol. wt of ∼217 kDa, with only a small shoulder remaining at 52 kDa. It is possible that the species eluting at 52 kDa represents an intermediate oligomer such as a dimer (see below). Assembly of the 217 kDa species was stabilized by the presence of the non-hydrolyzable GTP-γ-S. Pre-incubation in the presence of ATP did not lead to oligomerization (see Table I).
Fig. 1. Gel filtration. (A) Elution profile of McrBS in the presence of the indicated nucleotide cofactors. Without nucleotide cofactor the McrBS eluted at Ve = 1.63 ml. When 1 mM GTP or GDP was included, two peaks at Ve = 1.30 and 1.56 were observed. Including 1 mM GTP-γ-S shifted almost all of the protein to the Ve = 1.30 peak. In the presence of 1 mM ATP, the protein eluted as a monomer at Ve = 1.62. (B) Elution profile in the presence of McrC. An additional peak at Ve = 1.13 was observed.
Table I. Apparent relative masses (kDa) of McrBS and McrBSC complexes by gel filtration.
| Cofactor | McrBS | McrBS + McrC |
|---|---|---|
| None | 36 | 36 |
| GTP | 52 and 217 | 52 and 505 |
| GDP | 52 and 217 | 52 and 505 |
| GTP-γ-S | 52 and 217 | 505 |
| ATP | 36 | n.d. |
n.d., not determined.
Similar elution profiles were obtained in gel filtration experiments with the McrBL subunit (data not shown). In the absence of nucleotide cofactor, the protein eluted as a monomer with a mol. wt of 53 kDa and a very small shoulder at 78 kDa. The size of the major peak was close to the calculated mol. wt of 53.1 kDa. After pre-incubation in the presence of GTP, GDP or GTP-γ-S, the protein eluted with a mol. wt of 344 and 78 kDa (Table II). The species eluting at 344 kDa was predominant in the presence of the non-hydrolyzable GTP-γ-S, whereas, in the presence of GTP or GDP, significant amounts of the 78 kDa intermediate were observed.
Table II. Apparent relative masses (kDa) of McrBL and McrBLC complexes by gel filtration.
| Cofactor | McrBL | McrBL + McrC |
|---|---|---|
| None | 52 | n.d. |
| GTP | 78 and 344 | 78 and 736 |
| GDP | 78 and 344 | 78 and 736 |
| GTP-γ-S | 78 and 334 | 736 |
n.d., not determined.
Because McrC stimulates GTP hydrolysis by McrBL and McrBS and is required for DNA cleavage (Pieper et al., 1997; Panne et al., 1999; Stewart et al., 2000), it is predicted that McrC interacts directly with these McrB subunits. When McrC and McrBS were pre-mixed in the presence of GTP-γ-S and applied to the gel filtration column, a single peak corresponding to a mol. wt of 505 kDa was observed (Figure 1B). In the presence of GTP or GDP, protein eluted in two peaks with mol. wts of 52 and 505 kDa. In the absence of nucleotide cofactor, McrBS eluted at its predicted mol. wt of 36 kDa (Table I). For McrBL, a high molecular weight species of 736 kDa was obtained if McrC and GTP-γ-S were present (Table II). The peak eluting at 736 kDa showed McrBLC endonuclease activity if the reaction mixture was supplemented with 1 mM GTP (data not shown). Assembly of the oligomers was dynamic in that re-injection into the column in the absence of nucleotide cofactor led to dissociation, with McrBL or McrBS migrating as monomers (data not shown).
From these results, we conclude that the formation of McrB oligomers is GTP dependent. Oligomerization did not require the presence of DNA and was independent of GTP hydrolysis.
Electron microscopy of McrB oligomers
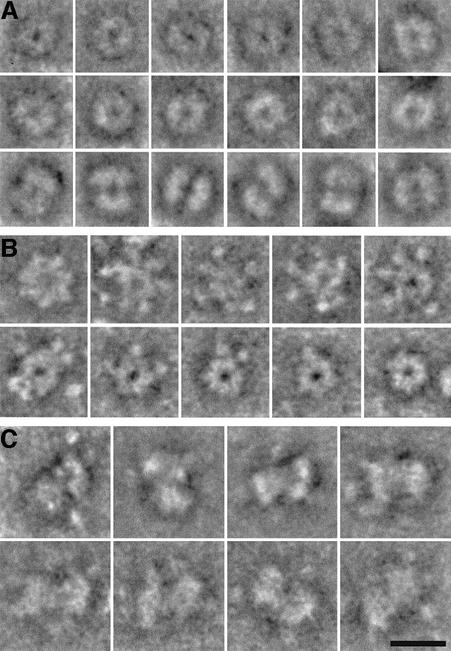
To understand further the nature of the enzyme oligomers, we analyzed McrBS, McrBL and McrBLC samples by scanning transmission electron microscopy (STEM). As shown in Figure 2, images recorded from negatively stained samples containing GTP-γ-S revealed the assembly of McrBL and McrBS into ‘doughnut’-shaped complexes. The presence of GTP-γ-S was essential for their formation; no such structures were seen in the absence of this nucleotide cofactor (data not shown). Thus, in agreement with the gel filtration experiments, GTP binding but not hydrolysis was found to be essential for McrBS and McrBL oligomerization. These ‘doughnuts’ are considered to be top-view projections (Figure 2A, top rows, and B). The few side-view projections also observed for the McrBS sample showed that sometimes the two rings are stacked (Figure 2A, bottom row). For the McrBL sample, the ‘doughnut’ structure exhibited up to seven protrusions (Figure 2B). Such protrusions were observed with different preparations of McrBL. Although their nature remains unclear, it is possible that they correspond to the 19 kDa N-terminal DNA-binding domain, which can exist in different conformations. No protrusions were observed for McrBS, which is missing this N-terminal region. Rotational and translational alignment of negatively stained McrBS or McrBL particle projections recorded by conventional transmission electron microscopy did not allow the rotational symmetry, and hence the subunit stoichiometry, for either oligomer to be defined unambiguously (data not shown).
Fig. 2. McrBC complexes formed in the presence of GTP-γ-S. The images were recorded from negatively stained samples by scanning transmission electron microscopy. (A) McrBS: the top views shown in the first two rows clearly reveal a ring-shaped structure. Side views of complexes formed from two McrBS oligomers are displayed in the third row. (B) McrBL: up to seven protrusions radiate from the central ring. Distinct side views were not detected on the micrographs. (C) McrBLC: the complexes are formed from two McrBL oligomers. The exact position of McrC cannot be defined. Scale bar, 15 nm.
The McrBLC complex examined was assembled in the presence of GTP-γ-S and purified by gel filtration. Negatively stained particles were visualized by STEM (Figure 2C). Most particles had an elongated shape, representing side views of the endonuclease. The association of two McrBL oligomers was clearly revealed.
Mass analysis by scanning transmission electron microscopy
To determine the subunit stoichiometry, the masses of freeze-dried, unstained McrBS, McrBL and McrBLC oligomers were determined by STEM. The McrBS and McrBL samples were prepared by diluting the protein into buffer containing 1 mM GTP-γ-S (see Materials and methods). For the analysis of McrBLC complexes, McrBL was pre-mixed with McrC and GTP-γ-S and the resulting McrBLC oligomers were purified by gel filtration. After elution from the column, the McrBLC sample was supplemented immediately with 1 mM GTP-γ-S and the grid was prepared. The McrBLC particles were less homogenous when GTP-γ-S was not included after elution of the particles from the gel filtration column (data not shown). In each case, the microscopy grids were washed with quartz double-distilled water and freeze-dried.
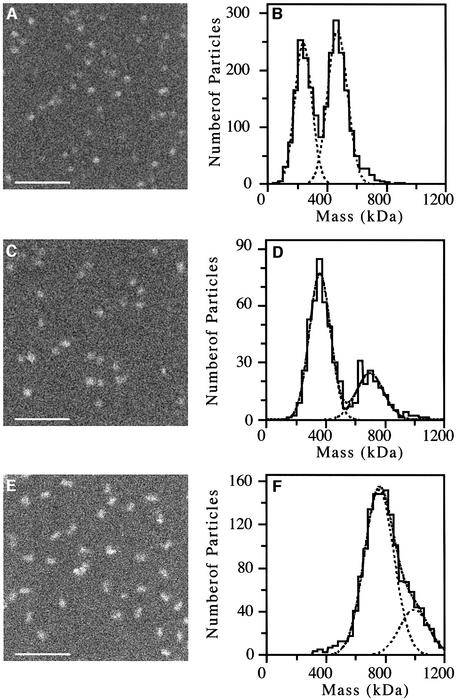
Structural details of the complexes could rarely be distinguished on the low dose, dark-field images recorded. For McrBS and McrBL, the oligomers generally had a circular projection, although a few elongated particles were also observed (Figure 3A and C). In contrast, the McrBLC complexes mostly had an elongated shape, presumably representing side views of the endonuclease (Figure 3E). In all, 2183 McrBS, 572 McrBL and 1518 McrBLC particles were analyzed. The resulting mass histograms are shown in Figure 3 and the masses corresponding to the Gaussian curves fitted are summarized in Table III. For the McrBS sample, the data fall into two peaks with masses of 239 (± 57) kDa and 468 (± 66) kDa (Figure 3B). As each McrBS subunit has a mass of 34.6 kDa, the mass of the first peak corresponds well to that of a heptamer, whereas the second peak has a mass close to that of a tetradecamer (Table IV). Similarly, two peaks were obtained for the McrBL sample with masses of 361 (± 71) kDa and 698 (± 93) kDa (Figure 3D). The McrBL subunit has a mass of 53.1 kDa and, therefore, the mass of the first peak is again close to that of a heptamer and the mass of the second peak is close to that of a tetradecamer (Table IV). For the McrBLC complex, there is a single major peak at 759 (± 100) kDa (Figure 3F). The relatively large standard deviation of the measurement reflects protein heterogeneity. This, combined with the 5% absolute calibration uncertainty of the instrument, prohibits the precise definition of the McrC, McrBL stoichiometry since McrC has a mass of only 40 kDa. Accordingly, the result indicates the association of a McrBL tetradecamer with one or two molecules of McrC. A small (n ∼264) number of particles in this sample were of higher mass, giving rise to the minor mass peak at 994 (± 100) kDa. Only very few particles had masses in the range of McrBL heptamers. Thus, in agreement with the results obtained by gel filtration, McrC seems to stabilize the association of the two heptameric McrBS or McrBL rings.
Fig. 3. STEM mass measurements. (A) Image of unstained McrBS particles. (B) Histogram showing the masses of the 2415 selected McrBS particles. The Gaussian curves fitted indicate two peaks with masses of 239 (± 57) and 468 (± 66) kDa. (C) Image of unstained McrBL particles. (D) Histogram showing the masses of the 572 selected McrBL particles. The Gaussian curves fitted indicate two peaks with masses of 361 (± 71) and 698 (± 93) kDa. (E) Image of unstained McrBLC particles. (F) Histogram showing the masses of the 1518 selected McrBLC particles. The Gaussian curves fitted indicate a main peak with a mass of 759 (± 100) kDa. An additional smaller peak with a mass of 994 (± 100) kDa was obtained. Correction has been made for beam-induced mass loss. Scale bars, 100 nm.
Table III. Masses (kDa) of McrBC complexes determined by scanning transmission electron microscopy.
| No. of particles |
Mass |
SD |
SE |
|||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | |
| McrBS | ∼1053 | ∼1318 | 239 | 468 | 57 | 66 | 2 | 2 |
| McrBL | 402 | 166 | 361 | 698 | 71 | 93 | 4 | 7 |
| McrBLC | ∼1162 | ∼265 | 759 | 994 | 100 | 100 | 3 | 6 |
The number of particles in each peak was estimated by measuring up to and away from curve crossover. The standard deviation (SD) as well as the standard error (SE) for each mass measurement is indicated.
Table IV. Stoichiometry of the McrBC endonuclease.
| Monomer kDa | Peak 1 kDa | Ratio | Peak 2 kDa | Ratio | |
|---|---|---|---|---|---|
| McrBS | 34.6 | 239 | 7.0 | 468 | 13.7 |
| McrBL | 53.1 | 361 | 6.8 | 698 | 13.1 |
| McrBLC | – | 759 | 14BL + 1(2)C | 994 | ? |
The ratio of subunits in each complex was obtained by dividing the measured mass of each complex by the calculated molecular weight for each subunit.
Discussion
Here we have analyzed the assembly of the methyl-dependent restriction endonuclease McrBC from its constituent subunits. McrBL and McrBS both form heptameric ring structures upon incubation with nucleotide cofactor. These heptameric rings can then stack to form tetradecameric cylindrical structures. McrC binding stabilized the tetradecameric form of the endonuclease. These results favor the model that DNA restriction is accomplished by a multisubunit McrBC complex (Panne et al., 1998).
Overall, the masses of the particles measured by STEM compare well with those obtained by gel filtration. However, there are some important differences. In the STEM experiments, McrBS and McrBL formed tetradecameric as well as heptameric complexes. In the gel filtration experiments, we did not observe the formation of the tetradecameric species in the absence of McrC. Presumably the tetradecamer dissociates during gel filtration unless McrC is included. Together, the results show that although the tetradecameric McrBL complex can form in the absence of McrC, it is much more stable when McrC is present; note the absence of an ∼400 kDa peak in the STEM histogram from McrBLC (Figure 3F). In agreement with this, the ratios between heptamer and tetradecamer varied between experiments; in one STEM experiment with McrBL, only a minimal amount of the tetradecamer was observed (data not shown).
In gel filtration experiments, an intermediate with a mass between that of a monomer and a dimer (52 kDa for McrBS and 78 kDa for McrBL) was observed for both McrBS and McrBL in the presence of GTP or GDP. These masses are below the range of STEM mass measurements at the recording doses employed. Since SDS–PAGE followed by silver staining did not reveal any additional proteins (data not shown), these species appear to be true assembly intermediates, possibly dimers, which, due to their non-ideal shape, elute with an apparent low relative molecular mass.
The oligomeric ring structures formed by McrBL and McrBS are reminiscent of those formed by hexameric helicases such as T7 gp4 (Patel and Hingorani, 1993), RuvB (Stasiak et al., 1994), DnaB (Reha-Krantz and Hurwitz, 1978), the SV40 T antigen (Mastrangelo et al., 1989) and the heptameric Rad52 (Stasiak et al., 2000). Like the eukaryotic SV40 T antigen, T7 gp4 and DnaB, which form hexameric complexes upon incubation with Mg2+ and nucleotide cofactor, oligomerization of McrBL and McrBS is dependent on the nucleotide cofactor. Assembly did not require GTP hydrolysis since both GDP and the non-hydrolyzable analog GTP-γ-S supported oligomerization. The N-terminal methylation-specific DNA-binding domain of McrBL targets the endonuclease to assemble on the DNA. It remains unclear whether the DNA-binding domain resides in the channel region or on the exterior of the complex, as suggested at first glance by the additional protrusions exhibited by the McrBL oligomers (Figure 2B).
McrBL can bind to DNA in the absence of GTP-γ-S with an ∼7-fold lower affinity than in its presence (Stewart et al., 2000). Therefore, it seems that G nucleotide-induced ring formation is not a prerequisite for DNA binding. The DNase I footprint of McrBL in the absence or presence of GTP-γ-S is identical, covering 16–18 bp around the methylated recognition site (Stewart et al., 2000). This indicates that either the McrBL monomer protects the same amount of DNA as the oligomer or that the DNA substrate induces McrBL oligomerization. Such DNA-induced oligomerization has been observed, for example, in the case of the hexameric E1 helicase (Fouts et al., 1999).
The location and exact stoichiometry of McrC in the McrBC endonuclease are still unclear. McrC interacts with the McrBL–DNA complex as monitored by gel shift experiments (Stewart et al., 2000). According to DNase I footprint experiments in the presence of GTP-γ-S, McrC does not interact with the DNA (Stewart et al., 2000). The STEM data show that although the McrBL tetradecameric species is stabilized by McrC binding, both McrB subunits can form tetradecameric complexes in the absence of McrC (Figure 3). In addition, the mass measurements indicate the presence of up to two McrC proteins. Therefore, in our model for the assembly of the McrBC endonuclease, we tentatively place McrC outside of the two assembled heptameric rings (Figure 4).
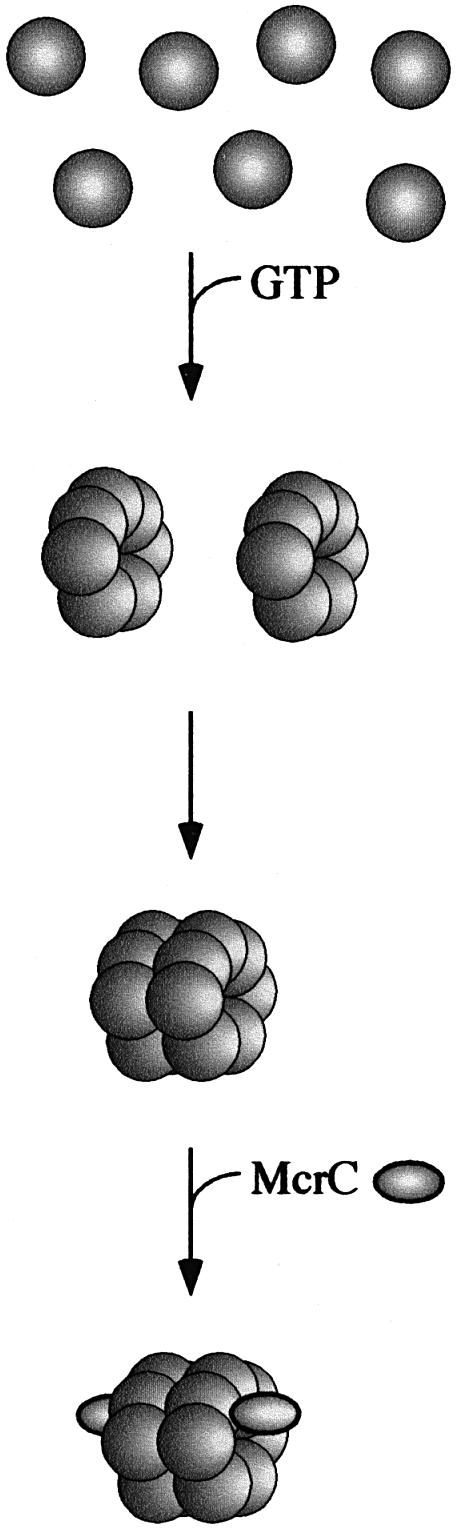
Fig. 4. Model for the assembly of the McrBC endonuclease complex. McrBL or McrBS assemble into heptameric rings in the presence of GTP or GDP. These rings can spontaneously dimerize into a tetradecamer. McrC binding stabilizes the tetradecameric form of the endonuclease.
McrBL and McrBS both have a weak intrinsic GTPase activity that is stimulated by McrC (data not shown; Pieper et al., 1997; Panne et al., 1999). In the case of McrBL (but not McrBS) this McrC-stimulated GTPase activity is stimulated moderately by substrate DNA (Panne et al., 1999). DNA cleavage is dependent on GTP hydrolysis and is strongly inhibited by GTP-γ-S (data not shown; Sutherland et al., 1992). DNA cleavage requires the presence of two suitably modified recognition elements separated by 40–3000 bp in the DNA substrate (Sutherland et al., 1992; Stewart and Raleigh, 1998; Panne et al., 1999). Recent data support the model that GTP hydrolysis fuels DNA translocation by the endonuclease (Panne et al., 1999). DNA translocation is accomplished while the enzyme remains bound to the recognition sequence. Once translocation is stalled, by collision with either a second translocating complex or a non-specific physical block, the enzyme cleaves the DNA in a region ∼30 bp away from its recognition site (Panne et al., 1999). The ring structure of McrBL may link the endonuclease topologically to the DNA and thereby ensure processive translocation of the DNA along the nuclease. The fact that two recognition sites are normally required for DNA cleavage suggests that two complexes, each assembled on a single recognition site, collaborate in DNA cleavage (Sutherland et al., 1992; Stewart and Raleigh, 1998; Panne et al., 1999). It is possible that a heptameric McrBL complex assembles on a single recognition site and that McrC binding then stimulates GTP hydrolysis and DNA translocation. Once two such complexes are brought into proximity, they engage to form the tetradecameric McrBLC cleavage complex. However, since it has been demonstrated that a single recognition site can be sufficient for assembly of a cleavage-competent complex (Panne et al., 1999) and because the tetradecameric complex forms in the absence of DNA, further experiments are required to reveal the stoichiometry of the enzyme complex on DNA.
The McrBC endonuclease is unique in several respects: it is the only endonuclease among the large and functionally divergent AAA+ family of proteins and it is the only restriction endonuclease with a ring-like structure. It is quite possible that the AAA+ proteins not only share sequence and structural similarities but that there are mechanistic similarities underlying their diverse biochemical functions. It has been suggested that the regulatory 19S cap complex of the eukaryotic 26S proteasome is involved in the unfolding and translocation of the substrate protein into the proteolytic core (Braun et al., 1999). Similarly, the role of the regulatory subunit ClpA in proteolysis by ClpAP is that of substrate unfolding and translocation into the proteolytic core of ClpP (Hoskins et al., 1998). Among the DNA-binding proteins of the AAA+ family, RuvB as well as DnaB and McrBC require DNA translocation at one stage of their reaction mechanism. The translocation of DNA on the one hand and that of substrate protein on the other may provide an unexpected link between these unrelated transport processes and reveal a common function for the AAA+ domain.
Materials and methods
Gel filtration
Gel filtration was carried out at 10°C on a Superdex 200 PC3.2/30 column using the Pharmacia SMART system. The column was equilibrated with buffer 1 [20 mM Tris–HCl pH 7.5 (21°C), 50 mM NaCl, 1 mM dithiothreitol (DTT)]. The same results were obtained using buffer 1 with 150 mM NaCl. The proteins were prepared as described previously (Panne et al., 1998) and stored at –20°C in storage buffer [10 mM Tris–HCl pH 7.5 (21°C), 200 mM NaCl, 0.1 mM Na2EDTA, 1 mM DTT and 50% glycerol]. The sample was prepared by diluting the protein 10-fold into 100 µl of buffer 2 [10 mM Tris–HCl pH 7.9 (25°C), 10 mM MgCl2, 50 mM NaCl, 1 mM DTT] with or without 1 mM nucleotide cofactor. The samples were incubated for 5 min at room temperature. For McrBS, 50 µl of a 4.1 µM and for McrBL, 50 µl of a 2.8 µM solution were injected into the column. For experiments with McrC, McrBL or McrBS was incubated as above and then McrC was added to a final concentration of 1.2 µM. The column was run at a flow rate of 40 µl/min. Absorbance was monitored at the wavelengths 215, 253 and 280 nm. Fractions of 80 µl were collected and the presence of protein was confirmed by SDS–PAGE and/or western blot analysis (data not shown). The column was calibrated under the same buffer conditions as above, using Blue 2000 dextran (Pharmacia) to determine the void volume V0 = 0.94. The molecular weight standards were thyroglobulin (669 kDa) Ve = 1.04, ferritin (440 kDa) Ve = 1.17, aldolase (158 kDa) Ve = 1.36, bovine serum albumin (67 kDa) Ve = 1.49, ovalbumin (43 kDa) Ve = 1.57 and RNase A (13.7 kDa) Ve = 1.83. The Kav values were calculated using: Kav = Ve – V0/Vt – V0 and the total column volume Vt of 2.4 ml.
Scanning transmission electron microscopy and mass analysis
For both the McrBS and the McrBL experiments, 7.5 µl aliquots of 0.1 µM protein in buffer 2 with 1 mM GTP-γ-S were adsorbed for 1 min to glow-discharged thin carbon films. These spanned a thick fenestrated carbon layer covering 200 mesh/inch, gold-plated copper grids. For negative stain microscopy, the grids were blotted, washed with three drops of quartz double-distilled water, stained with 2% (w/v) uranyl formate for 20 s and air dried. For mass measurements, the grids were blotted and washed with five drops of quartz double-distilled water to remove buffer salts. A blotting step followed each wash. They were left unstained, frozen in liquid nitrogen and freeze-dried at –80°C at 5 × 10–8 Torr overnight in the microscope. McrBL–McrC complexes were prepared by mixing 2 µM McrBL, 1.2 µM McrC and 1 mM GTP-γ-S in buffer 2. The oligomers were purified by gel filtration as described above. The protein eluted in a single peak, was immediately supplemented with 1 mM GTP-γ-S and the sample was applied directly to the microscope grids, which were prepared for either negative stain microscopy or mass measurement as outlined above.
A Vacuum Generators STEM HB-5 interfaced to a modular computer system (Tietz Video and Image Processing Systems GmbH, D-8035 Gauting) was employed. Details of the instrument’s calibration and use for mass measurement can be found in Engel (1978) and Müller et al. (1992). Series of 512 × 512 pixel, digital dark-field images were recorded from the unstained samples at an accelerating voltage of 80 kV. The nominal magnification was 200 000× and recording doses were in the range of 300 electrons/nm2. In addition, repeated low dose scans were recorded from several grid regions of the samples to assess beam-induced mass loss.
The images recorded for mass measurement were evaluated using the program package IMPSYS as described (Müller et al., 1992). Accordingly, complexes were selected in circular boxes. The total scattering within the box was then calculated and the contribution arising from the carbon support film subtracted. The latter was determined from randomly selected regions on the same image. The resulting mass values were corrected for beam-induced mass loss, displayed in histograms and described by Gaussian curves. The mass loss relationship was determined experimentally from the behavior of both enzyme oligomers within the repeatedly imaged regions of the sample, by monitoring their mass as the total exposure dose increased up to 2000 electrons/nm2 (Müller et al., 1992). The correction factors were 1.052 for the McrBS sample (recording dose 377 ± 49 electrons/nm2), 1.043 for the McrBL sample (recording dose 315 ± 32 electrons/nm2) and 1.035 for the McrBLC sample (recording dose 343 ± 21 electrons/nm2). Series of 512 × 512 pixel, digital dark-field images were recorded at a nominal magnification of 500 000× from the corresponding negatively stained samples. In this case, the STEM was operated at an accelerating voltage of 100 kV and recording doses ranged from 4000 to 6000 and from 8000 to 10 000 electrons/nm2.
Acknowledgments
Acknowledgements
We thank Fiona J.Stewart for providing us with purified McrC, and Elisabeth A.Raleigh for critical reading and suggestions for the improvement of the manuscript. This work was supported by the Swiss National Science Foundation grant number 31-42435.94 to A.E. and the M.E.Müller Foundation of Switzerland and grant number 31-46768.96 to T.A.B.
References
- Arber W. (1965) Host specificity of DNA produced by Escherichia coli: V. The role of methionine in the production of host specificity. J. Mol. Biol., 11, 247–256. [DOI] [PubMed] [Google Scholar]
- Beary T.P., Braymer,H.D. and Achberger,E.C. (1997) Evidence of participation of McrBs in McrBC restriction in Escherichia coli K-12. J. Bacteriol., 179, 7768–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. and Weigle,J.J. (1953) Host controlled variation in bacterial viruses. J. Bacteriol., 65, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T.A. and Krüger,D.H. (1993) The biology of DNA restriction. Microbiol. Rev., 57, 434–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun B.C., Glickman,M., Kraft,R., Dahlmann,B., Kloetzel,P.M., Finley,D. and Schmidt,M. (1999) The base of the proteasome regulatory particle exhibits chaperone-like activity. Nature Cell Biol., 1, 221–226. [DOI] [PubMed] [Google Scholar]
- Confalonieri F. and Duguet,M. (1995) A 200-amino acid ATPase module in search of a basic function. BioEssays, 17, 639–650. [DOI] [PubMed] [Google Scholar]
- Dila D., Sutherland,E., Moran,L., Slatko,B. and Raleigh,E.A. (1990) Genetic and sequence organization of the mcrBC locus of Escherichia coli K-12. J. Bacteriol., 172, 4888–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussoix D. and Arber,W. (1962) Host specificity of DNA produced by Escherichia coli: II. Control over acceptance of DNA from infecting phage λ. J. Mol. Biol., 5, 37–49. [DOI] [PubMed] [Google Scholar]
- Engel A. (1978) Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy, 3, 273–281. [DOI] [PubMed] [Google Scholar]
- Fouts E.T., Yu,X., Egelman,E.H. and Botchan,M.R. (1999) Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J. Biol. Chem., 274, 4447–4458. [DOI] [PubMed] [Google Scholar]
- Gast F.U., Brinkmann,T., Pieper,U., Krüger,T., Noyer-Weidner,M. and Pingoud,A. (1997) The recognition of methylated DNA by the GTP-dependent restriction endonuclease McrBC resides in the N-terminal domain of McrB. Biol. Chem., 378, 975–982. [DOI] [PubMed] [Google Scholar]
- Hoskins J.R., Pak,M., Maurizi,M.R. and Wickner,S. (1998) The role of the ClpA chaperone in proteolysis by ClpAP. Proc. Natl Acad. Sci. USA, 95, 12135–12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger T., Grund,C., Wild,C. and Noyer-Weidner,M. (1992) Characterization of the mcrBC region of Escherichia coli K12 wild-type and mutant strains. Gene, 114, 1–12. [DOI] [PubMed] [Google Scholar]
- Krüger T., Wild,C. and Noyer-Weidner,M. (1995) McrB: a prokaryotic protein specifically recognizing DNA containing modified cytosine residues. EMBO J., 14, 2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S.E. and Human,M.L. (1952) A nonhereditary, host-induced variation of bacterial viruses. J. Bacteriol., 64, 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I.A., Hough,P.V., Wall,J.S., Dodson,M., Dean,F.B. and Hurwitz,J. (1989) ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature, 338, 658–662. [DOI] [PubMed] [Google Scholar]
- Müller S.A., Goldie,K.N., Bürki,R., Häring,R. and Engel,A. (1992) Factors influencing the precision of quantitative scanning transmission electron microscopy. Ultramicroscopy, 46, 317–334. [Google Scholar]
- Neuwald A.F., Aravind,L., Spouge,J.L. and Koonin,E.V. (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation and disassembly of protein complexes. Genome Res., 9, 27–43. [PubMed] [Google Scholar]
- Pamnani V., Tamura,T., Lupas,A., Peters,J., Cejka,Z., Ashraf,W. and Baumeister,W. (1997) Cloning, sequencing and expression of VAT, a CDC48/p97 ATPase homologue from the archaeon Thermoplasma acidophilum. FEBS Lett., 404, 263–268. [DOI] [PubMed] [Google Scholar]
- Panne D., Raleigh,E.A. and Bickle,T.A. (1998) McrBs, a modulator peptide for McrBC activity. EMBO J., 17, 5477–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panne D., Raleigh,E.A. and Bickle,T.A. (1999) The McrBC endonuclease translocates DNA in a reaction dependent on GTP hydrolysis. J. Mol. Biol., 290, 49–60. [DOI] [PubMed] [Google Scholar]
- Patel S.S. and Hingorani,M.M. (1993) Oligomeric structure of bacteriophage T7 DNA primase/helicase proteins. J. Biol. Chem., 268, 10668–10675. [PubMed] [Google Scholar]
- Patel S. and Latterich,M. (1998) The AAA team: related ATPases with diverse functions. Trends Cell Biol., 8, 65–71. [PubMed] [Google Scholar]
- Pieper U., Brinkmann,T., Kruger,T., Noyer,W.M. and Pingoud,A. (1997) Characterization of the interaction between the restriction endonuclease McrBC from E.coli and its cofactor GTP. J. Mol. Biol., 272, 190–199. [DOI] [PubMed] [Google Scholar]
- Raleigh E.A. (1992) Organization and function of the mcrBC genes of Escherichia coli K-12. Mol. Microbiol., 6, 1079–1086. [DOI] [PubMed] [Google Scholar]
- Raleigh E.A. and Wilson,G. (1986) Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc. Natl Acad. Sci. USA, 83, 9070–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reha-Krantz L.J. and Hurwitz,J. (1978) The dnaB gene product of Escherichia coli. I. Purification, homogeneity and physical properties. J. Biol. Chem., 253, 4043–4050. [PubMed] [Google Scholar]
- Revel H.R. (1983) DNA modification: glucosylation. In Mathews,C.K., Kutter,E.M., Mosig,G. and Berget,P. (eds), Bacteriophage T4. American Society for Microbiology, Washington, DC, pp. 156–165.
- Roberts R.J. and Macelis,D. (1993) The restriction enzymes. In Linn,S.M., Lloyd,R.S. and Roberts,R.J. (eds), Nucleases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 439–444.
- Rohrwild M., Pfeifer,G., Santarius,U., Müller,S.A., Huang,H.C., Engel, A., Baumeister,W. and Goldberg,A.L. (1997) The ATP-dependent HslVU protease from Escherichia coli is a four-ring structure resembling the proteasome. Nature Struct. Biol., 4, 133–139. [DOI] [PubMed] [Google Scholar]
- Ross T.K. and Braymer,H.D. (1987) Localization of a genetic region involved in McrB restriction by Escherichia coli K-12. J. Bacteriol., 169, 1757–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T.K., Achberger,E.C. and Braymer,H.D. (1989a) Identification of a second polypeptide required for McrB restriction of 5-methylcytosine-containing DNA in Escherichia coli K-12. Mol. Gen. Genet., 216, 402–407. [DOI] [PubMed] [Google Scholar]
- Ross T.K., Achberger,E.C. and Braymer,H.D. (1989b) Nucleotide sequence of the McrB region of Escherichia coli K-12 and evidence for two independent translational initiation sites at the mcrB locus. J. Bacteriol., 171, 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak A., Tsaneva,I.R., West,S.C., Benson,C.J., Yu,X. and Egelman, E.H. (1994) The Escherichia coli RuvB branch migration protein forms double hexameric rings around DNA. Proc. Natl Acad. Sci. USA, 91, 7618–7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak A.Z., Larquet,E., Stasiak,A., Müller,S., Engel,A., Van Dyck,E., West,S.C. and Egelman,E.H. (2000) The human Rad52 protein exists as a heptameric ring. Curr. Biol., 10, 337–340. [DOI] [PubMed] [Google Scholar]
- Stewart F.J. and Raleigh,E.A. (1998) Dependence of McrBC cleavage on distance between recognition elements. Biol. Chem., 379, 611–616. [PubMed] [Google Scholar]
- Stewart F.J., Panne,D., Bickle,T.A. and Raleigh,E.A. (2000) Methyl-specific DNA binding by McrBC, a modification-dependent restriction enzyme. J. Mol. Biol., 298, 611–622. [DOI] [PubMed] [Google Scholar]
- Sutherland E., Coe,L. and Raleigh,E.A. (1992) McrBC: a multisubunit GTP-dependent restriction endonuclease. J. Mol. Biol., 225, 327–358. [DOI] [PubMed] [Google Scholar]
- Wolf S., Nagy,I., Lupas,A., Pfeifer,G., Cejka,Z., Müller,S.A., Engel,A., De Mot,R. and Baumeister,W. (1998) Characterization of ARC, a divergent member of the AAA ATPase family from Rhodococcus erythropolis. J. Mol. Biol., 277, 13–25. [DOI] [PubMed] [Google Scholar]