ABSTRACT
Five unusual cases of posterior skull base tumors were treated through different skull base approaches. Two or more staged operations were required to achieve total or near-total excision and decompression of two extensive tumors. Total excision of an extensive en plaque meningioma of the foramen magnum that encircled the brain stem and cervical spinal cord could not be achieved through the extreme lateral and suboccipital craniectomy approach. However, the vital structures were decompressed and the patient's postoperative morbidity was acceptable. An extended middle fossa approach was required to excise a hemangiopericytoma of the middle and posterior fossae in a 12-year-old. Extension of the tumor into the posterior fossa precluded a retromastoid approach because the mass draped the lower cranial nerves posteriorly. Two men had undifferentiated adenocarcinomas involving the jugular foramen and middle to posterior fossa, respectively. The origin of one was renal in a 37-year-old man. A 63-year-old man survived 1.5 years after a good decompression of his extensive tumor and irradiation. The histological diagnosis of paraganglioma of the occipital bone was a surprise in a 25-year-old man with pure bony involvement. These cases indicate that the appropriate selection of skull base approaches and their combination can provide the needed access to achieve adequate excision or decompression of masses located in challenging anatomical regions of the skull base. Furthermore, good surgical excision improves palliation in radioresistant metastatic tumors of the skull base.
Keywords: Skull base tumors, metastasis, hemangiopericytoma
The posterior skull base surrounds the foramen magnum (FM) and includes the clivus below the spheno-occipital synchondrosis, the petrous temporal bone, and the pars lateralis and squama of the occipital bone.1 Cranial skull base lesions that involve the posterior fossa are the most complex. The different types of tumors occur in different locations, and their incidence varies from site to site. Among the clival and petroclival tumors, the most common benign tumors are meningiomas and the most common destructive clival masses are the chordomas and metastases.2 In the cerebellopontine angle (CPA), the most common tumors are acoustic neuromas followed by meningiomas.3 However, among jugular foramen tumors, the common tumors are schwannomas, paragangliomas, and meningiomas.4
We present the unusual tumors of the posterior skull base that we have treated during the last 4 years. These lesions include a metastasis from a renal cell carcinoma at the jugular foramen and FM region; an en plaque meningioma of the FM and lateral cerebellomedullary cistern, a hemangiopericytoma that straddled the middle fossa base and posterior fossa, a metastatic undifferentiated carcinoma of the petrous apex and posterior and middle fossae, and a paraganglioma of the occipitotemporal bone. Clinical profiles, operative approaches, and outcomes of these cases are discussed.
ILLUSTRATIVE CASES
Five unusual cases of posterior skull base tumors treated at Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow [India] between 1998 and January 2004 were analyzed retrospectively. The clinical profile, radiological findings, and operative approach of each case were recorded from the computer-based discharge summaries and the case sheet of each case. Considering the rarity of these cases, the interesting radiological features, operative findings, and follow-up data from each case were maintained by the principal author. Follow-up of these cases varied from 3 months to 3 years.
Case 1
A 25-year-old woman experienced progressive spastic quadriparesis for 1 year, neck pain for 7 months, and severe agonizing dysesthetic pain in the limbs and trunk for 1 week. On examination she had spasticity with 3/5 strength of the upper limbs and 4/5 strength of the lower limbs. Joint position and vibration sensation were impaired at the level of C2.
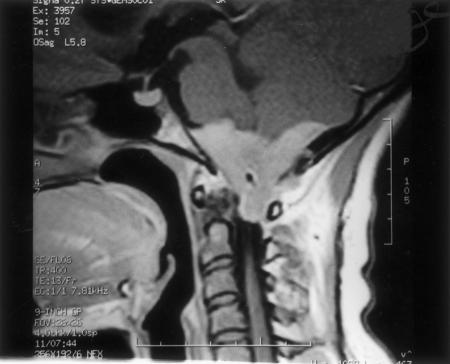
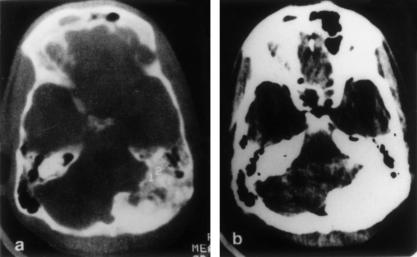
T1- and T2-weighted MRIs of the posterior fossa and upper cervical spine showed an extensive isointense mass attached to the dura around the FM and posterior fossa. The bulk of the mass was on the left side with an extension into the cerebellomedullary cistern on the left, compressing and shifting the medulla and cervical Spinal cord to the right (Fig. 1). The occipital squama was hyperostotic on the left side. Digital subtraction angiography (DSA) of the posterior circulation showed bilateral compression of the vertebral arteries by tumor, more on the left than the right. The tumor was supplied by the left vertebral artery. The left posterior inferior cerebellar artery (PICA) was enmeshed within the tumor.
Figure 1.
Contrast MRI shows an enhancing and extensive en plaque meningioma of the foramen magnum and posterior fossa. MRI, magnetic resonance image.
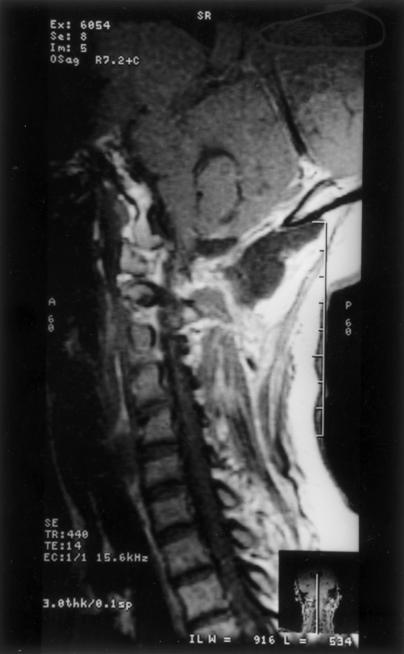
The extreme lateral approach was combined with a suboccipital craniectomy on the left side to approach the tumor. At surgery, portions of the posterior occipital condyle, occipital squama, and lateral mass of C1 were partially destroyed. The tumor was intradural and firm with highly vascular attachments to the dura, which it involved circumferentially. The posterior two thirds of the occipital condyle was removed. The left-sided vertebral artery was dissected, and the PICA was preserved with difficulty. The mass was debulked grossly to achieve a subtotal excision (Fig. 2). Postoperatively, the patient's gag reflex was impaired. She needed Ryle's tube feeding for about 2 months. At her 3-month follow-up examination, the patient's spasticity had improved by 80%. Her dysesthesia was relieved. Her strength had improved to 4 + /5, and she was ambulatory without support. Based on histopathological analysis, the mass was diagnosed as a fibrous en plaque meningioma. Radiotherapy is planned for treatment of the residual tumor in this patient.
Figure 2.
Postoperative MRI confirms almost total excision of the tumor. MRI, magnetic resonance image.
Case 2
A 10-year-old boy was admitted to our department after experiencing diplopia and inward deviation of the left eye for 1 year. He had difficulty walking. Nasal regurgitation, headache, and vomiting had been present for 3 weeks. On examination he exhibited paresis of the left third, sixth, and seventh cranial nerves (lower motor neuron type) and diminished bilateral gag reflex. He had left spastic hemiparesis (4 + /5 strength) and left-sided cerebellar signs. He could count fingers at 3 feet with his left eye.
Computed tomography (CT) of the head showed an isodense enhancing mass in the left cerebellopontine angle (CPA) cistern, base of the middle fossa, and prepontine cistern compressing the brain stem posteriorly. The lesion was hypointense on T1-weighted MRI and hyperintense on T2-weighted MRI and enhanced heterogeneously with contrast (Fig. 3). Four-vessel DSA showed that the basilar artery had shifted to the right. The left middle cerebral artery (MCA) was elevated. A clinicoradiological diagnosis of meningioma was considered.
Figure 3.
T2-weighted MRI shows a hyperintense mass occupying the left middle fossa base, left cerebellopontine angle cistern, and prepontine cistern. MRI, magnetic resonance image.
A two-stage resection of the tumor through an extended middle fossa approach and retromastoid suboccipital route was planned. During the first stage, the portion of the tumor involving the middle fossa was resected through an extended middle fossa (intradural, extradural, and transtentorial) approach. The posterior fossa component of the tumor, which was draped by the seventh, eighth, and lower cranial nerves posteriorly, could not be resected through the retromastoid route. No room was available from this posterior approach. The tumor was again approached through the first opening and excised in toto. The pinkish, vascular, nonsuctionable tumor extended along the petrous ridge to the cavernous sinus. It followed the tentorium into the posterior fossa, CPA cistern, and prepontine cistern. Postoperative CT confirmed total excision of the tumor (Fig. 4). Postoperatively, the patient developed seventh cranial nerve paresis and left cerebellar signs. Hemangiopericytoma was diagnosed by histopathological examination.
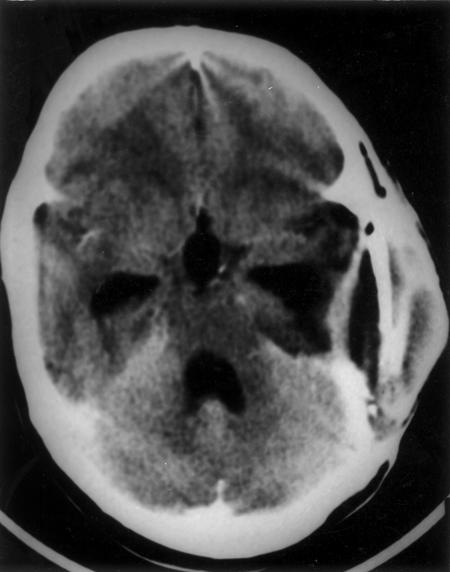
Figure 4.
Postoperative computed tomography scan shows postoperative changes.
At his 3-year follow-up examination, his seventh cranial nerve paresis had improved. He had no cerebellar signs. Given the known aggressive behavior of the tumor, the boy underwent postoperative irradiation.
Case 3
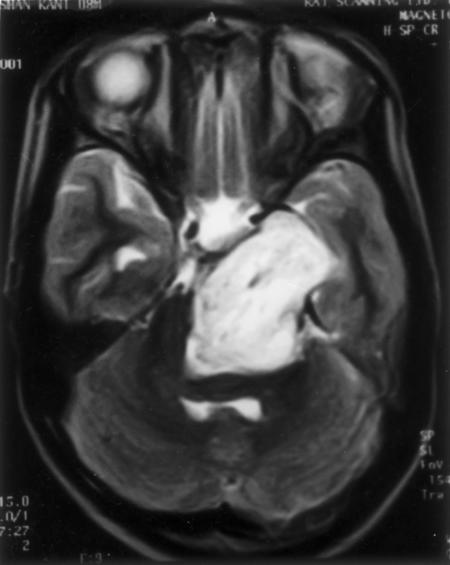
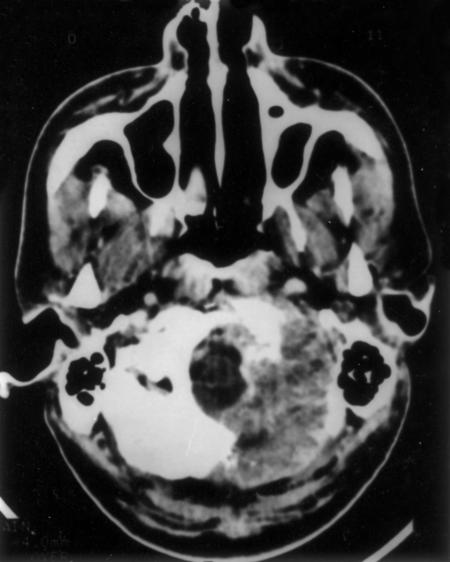
A 25-year-old man was admitted to our department with vertigo, pain, decreased hearing in his left ear, and unsteadiness while walking. His symptoms had been present 1 year. On examination he had mild sensorineural hearing loss in the left ear, left ninth and tenth cranial nerve paresis, and left cerebellar signs. CT of the head showed hyperostosis of the left occipital squama, mastoid, and petrous bone. His jugular foramen was normal. His left cerebellar hemisphere was compressed by hyperostotic bone. Compression of the fourth ventricle had caused obstructive hydrocephalus (Fig. 5).
Figure 5.
(A, B) Hyperostotic paraganglioma involving the occipital squama, mastoid, and petrous bone compresses the left cerebellum.
Left suboccipital craniectomy and almost total excision of the involved bone were achieved. A grayish, soft, and highly vascular bony mass at the region of the FM had expanded the diploic region. The middle and inner ears were not involved. The sigmoid sinus was thrombosed. There was no intradural extension of the mass. A biopsy of the excised mass was diagnosed as paraganglioma. Postoperatively, the patient's vertigo was relieved. Given the diagnosis, further MRI was obtained to determine if the tumor had extended to the jugular foramen. Fortunately, it was not involved. At his 6-month follow-up examination, he had no pain and gait instability.
Case 4
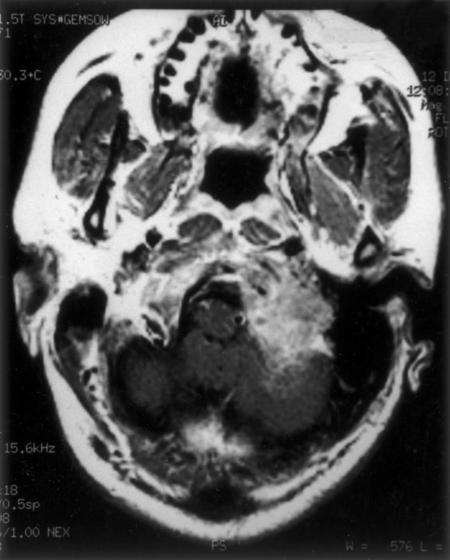
A 37-year-old man had experienced a holocranial headache for 9 months and difficulty with swallowing, hoarseness of voice, and nasal regurgitation for 5 months. On examination, he had left ninth, tenth, eleventh, and twelfth cranial nerve palsy along with left cerebellar signs. The working diagnosis of jugular foramen tumor or lower cranial nerve schwannoma was made. CT of the head showed a large, hyperdense, heterogeneously enhancing mass at the base of the left posterior fossa and at the lateral rim of the FM. The mass had eroded the clivus, occipital squama, and occipital condyle and extended to the left jugular foramen (Fig. 6). On MRI, the extent of tumor was the same. The lesion was hypointense on T-weighted images, hyperintense on T2-weighted images, and enhanced heterogeneously with contrast (Fig. 7). The transverse and sigmoid sinuses were normal, and there was no vascular abnormality on DSA.
Figure 6.
Contrast-enhanced computed tomography shows an enhancing mass at the base of the left posterior fossa destroying the lower clivus, occipital squama, and lateral mass of C1.
Figure 7.
MRI shows large enhancing soft tissue mass in the region of the left jugular foramen and lateral to the foramen magnum. MRI, magnetic resonance image.
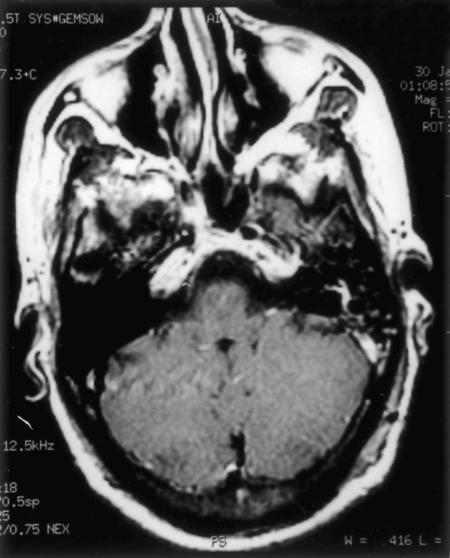
The tumor was approached through an extreme lateral approach, and gross total excision was achieved (Fig. 8). The tumor had eroded the posterior portion of the left occipital condyle, occipital squama, and posterior arch of C1. The firm, highly vascular soft tissue mass was located extradurally. There was no intradural extension. Two thirds of the occipital condyle was drilled and the vertebral artery was mobilized. The mass was adherent to the dura and extended to the posterior portion of the jugular foramen. Postoperatively, his recovery was uneventful.
Figure 8.
Postoperative MRI of same patient as seen in Figure 7 shows only postoperative changes. MRI, magnetic resonance image.
Histopathological analysis of the tumor showed metastastic adenocarcinoma. A thorough screening of the body with ultrasound showed an enlargement of the left kidney. A biopsy proved to be renal cell carcinoma. He was referred to the urology and radiotherapy departments for further management. At his 3-months follow-up examination, he was asymptomatic.
Case 5
A year before presentation, a 67-year-old man had been operated on for fibroma of the abdominal wall. For 6 months he had been experiencing pain on the left side of his face, diplopia, and tinnitus and decreased hearing in the left ear. He demonstrated hypesthesia in the left V1, V2, and V3 distributions of the left trigeminal nerve; left sixth through tenth cranial nerve paresis; and left cerebellar signs. A CT of the head showed an extensive mass involving the left side of the middle and posterior fossae. The mass was eroding the sphenoid body, clivus, and petrous bone on the left and was filling the left CPA cistern and middle fossa. The tumor was compressing the left cerebellar peduncle and brain stem. The mass was isointense on T1- and T2-weighted MRIs and enhanced heterogeneously with contrast. The left carotid artery was enmeshed within the mass.
A transnasal trans-sphenoidal biopsy and decompression of the sphenoid mass were performed to identify the tumor. An extended middle fossa, extradural and intradural approach was used to achieve subtotal decompression (90%) of the mass. The grayish, soft, suctionable, and moderately vascular mass filled the sphenoid sinus and posterior cavernous sinus, occupying the region of the destroyed clivus and petrous bone. Both intradural and extradural decompression were performed with removal of the petrous apex and the destroyed portion of the clivus. The CPA extension of the tumor was also removed. Within 4 hours of surgery, the patient underwent a reoperation to treat a hematoma at the operative site. Postoperatively his lower cranial nerve palsy improved and he was able to ambulate. The biopsy specimen was diagnosed as metastasis from undifferentiated adenocarcinoma. Screening for the primary site remained negative. He was offered radiotherapy after surgery. The patient died 1.5 years later from the extensive disease.
DISCUSSION
The clinical presentation of meningiomas involving the FM is variable. Sometimes the symptoms are disproportionate to the signs. These patients usually present with neck pain, paresthesias, and motor weakness, depending on the exact location of the tumor at the FM. The lower cranial nerves are unlikely to be involved.5,6 Our patient (Case 1), however, had weakness and incapacitating paresthesias in all limbs and the trunk. This case did not show the manifestations of lower cranial nerve paresis despite the extensive presence of tumor in the left lateral cerebellomedullary cistern.
En plaque meningiomas manifest as slow-growing tumors of limited thickness with a flat or slightly nodular shape.7 They tend to form a thin layer that closely adheres to a large area of the inner table of the skull and leads to bony hyperostosis. Hyperostosis is significantly greater than found with other meningiomas. In our patient (Case 1), the occipital squama was involved, and tumor was found all around the FM. The significantly thickened dura had merged with the tumor. The dura also was adherent to the hyperostotic occipital squama. The thick, bulky tumor was present circumferentially along the entire medulla and upper spinal cord (to C2). The left cranial nerves and the PICA were enmeshed with the tumor. The extensive involvement and slow progression of these lesions lead to a high rate of morbidity associated with their surgical resection. Many surgeons believe that surgical resection cannot be justified.7,8 However, studies have found favorable outcomes after surgical excision of these tumors,9 as was also achieved in our case.
The various approaches to FM meningiomas include the midline subocciptal approach, the far-lateral suboccipital approach, and an extreme transcondylar approach. Given the extent of tumor and its cerebellomedullary extension in Case 1, we combined an extreme lateral approach with a left suboccipital craniectomy to remove about 80% of the tumor. This extensive skull base procedure was chosen for two reasons. First, the lateral and anterior extensions of the tumor could have not been removed through a conventional midline subocccipital approach. Second, obtaining a small amount of tissue for biopsy alone through a conventional approach could not have provided decompression of the cranial nerves and brain stem. Some tumor had to be left, especially where it was enmeshed with PICA. On the left side, the extradural vertebral artery could be dissected from the tumor up to its dural entrance. The medulla, spinal cord, and lower cranial nerves were decompressed with difficulty. Hence, an extreme lateral approach offers enough room to access anteriorly placed tumors associated with posterior extensions.
Hemangiopericytomas are controversial tumors in terms of their classification. First described in 1942,10 they were assumed to arise from pericytes of Zimmerman, contractile cells found along capillary walls. Many workers believe that these tumors are a variant of meningiomas.11 Others think that they arise from pericytes12 and are different from meningiomas because their incidence in males and females is almost equal. Their histolopathological features resemble those of peripheral hemangiopericytomas. If considered as a variant of meningiomas (angioblastic meningiomas), hemangiopericytomas account for 3 to 4% of meningiomas13 and fewer than 1% of all central nervous system tumors.
Hemangiopericytomas tend to occur in the posterior fossa along the FM.14 In our patient (Case 2), the location was highly unusual. Preoperatively, we did not include the possibility of a hemangiopericytoma in the differential diagnosis. Most workers have found that patients are symptomatic for less than a year15 before diagnosis, as occurred in our patient.
These extremely vascular tumors are challenging to treat. This problem is compounded if the patient is in the pediatric age group. The overall blood volume of a child is less than that of an adult. Therefore, the surgeon must be careful when interrupting the blood supply to the tumor before resection is attempted.
The mean survival of these patients is 84 months, much less than that associated with typical meningiomas.16 These tumors have a high rate of recurrence: 67% after 5 years and 76% after 10 years.16 With a follow-up of 36 months after total excision, our patient has not yet had a recurrence. Tumors of the middle fossa, posterior fossa, and tentorium may be more lethal than their supratentorial counterparts. However, many workers have found that patients benefit the most after their first operation. Subsequent operations are less successful. Hence, every effort must be made to achieve complete excision at the first surgery.16
In our patient we approached the posterior fossa extension of the tumor through a retromastoid suboccipital craniectomy, because we did not want to use an extensive skull base approach. We also planned to remove the tumor in two stages to minimize blood loss and morbidity related to a long operative time in a child. However, the tumor could not be removed because it was located too anteriorly and involved with the cranial nerves. Consequently, we approached the tumor through an extended subtemporal middle fossa approach (both intradural and extradural and by resecting the tentorium) to achieve gross total resection in two sittings.
Paragangliomas originate from adrenal or extra-adrenal paraganglian cells, which are a part of the neuroendocrine system and are usually associated with sympathetic ganglia. In our patient (Case 3), the tumor involved the occipital squama, part of the petrous bone, and the mastoid portion of the temporal bone. Surprisingly, there was no bony erosion and no soft tissue mass, but massive hyperostosis of the occipital squama was encountered. After biopsy, a glomus tumor was suspected, so the jugular fossa was rescreened and was found to be normal. Paragangliomas of the jugulotympanic region are irregularly shaped, homogeneous, soft tissue masses centered on the jugular foramen and associated with an enlarged jugular foramen. In contrast to the findings in our case, they have a moth-eaten appearance.
Paragangliomas are relatively common in this location, but the presentation of a paraganglioma as a pure bony mass has only been reported once.17 In this case, the left cerebellar hemisphere was pushed and compressed medially. The patient had a mild retrocochlear sensorineural hearing loss and vertigo. Involvement of the left ninth and tenth cranial nerves probably reflected pressure on the nerves from hyperostotic bone. The anatomical location was unusual because the tumor did not arise from the jugular fossa or from the tympanic plexus of the middle ear. Rather, it probably originated from some vessel or nerve branch in the occipital bone. The possibility of its originating from the aberrant migration of chief glomus cells cannot be discounted. The treatment of choice for jugulotympanic paragangliomas is surgical resection.
When possible, total tumor resection should be attempted. Ideally, it should be achieved without increasing neurological deficits. The role of radiotherapy is controversial. In patients with limited neurological deficits and extensive tumors involving the cancellous bone or petrous apex around the carotid artery, an attempt at total tumor resection may increase neurological deficits. In such cases, subtotal resection with postoperative radiotherapy is probably the treatment of choice. In our cases, we could only achieve subtotal excision through the conventional suboccipital approach to avoid increasing deficits.
Metastases are the most common tumor involving the posterior fossa in adults and elderly patients.18 The cerebellum is the primary site of involvement; the meninges and brain stem can also be involved.19 Metastatic lesions in the brain parenchyma manifest by neurological dysfunction and increased intracranial tension. Those at the skull base become symptomatic by compression of cranial nerves. Intraparenchymal metastases are usually subcortical and grow rapidly. They manifest with extensive edema, even if small. Neurological deterioration occurs quickly. In contrast, skull base involvement is usually confined to the bones and progresses relatively slowly. In these cases, the primary manifestation is cranial nerve involvement. Renal cell carcinoma is the fourth most common source of brain metastasis (after lung, breast, and malignant melanoma).20 The secondary tumors from renal cell carcinoma tend to be highly vascular. In one of our patients (Case 4), an extradural metastatic adenocarcinoma had metastasized from the kidney. It involved the occipital condyle, jugular foramen, and occipital squama, and was a diagnostic enigma before surgery.
Radiotherapy is typically considered the preferred treatment for brain metastasis. However, surgical removal of the tumor mass is the most effective palliation, especially for tumors derived from radioresistant disease like melanomas and carcinomas of the kidney and colon.21 The poor results associated with the surgical excision of metastases in the early days of treatment left many pioneer neurosurgeons unenthusiastic about this form of treatment. However, contemporary neurosurgical techniques, approaches, and perioperative care have the potential to increase the benefit of surgical resection. For certain patients, this form of treatment has become routine.
In both of our patients (Cases 4 and 5) with metastatic adenocarcinomas, radiotherapy or radiosurgery was not considered. In Case 4, the diagnosis was unknown. Even with a preoperative biopsy in Case 5, we could not have achieved good results because metastatic adenocarcinomas are know to be radioresistant. Furthermore, these patients would have been deprived of the benefits of cytoreduction.
In both patients, surgery was performed to decompress the neuraxis to relieve their symptoms and to obtain an accurate diagnosis after surgical decompression. Given the radioresistant nature of adenocarcinomas, surgery was an appropriate treatment modality. One patient (Case 5) had a relatively favorable outcome, surviving for 1.5 years. The other patient (Case 4) is being followed (3 months).
CONCLUSION
If used for surgical excision of tumors located at the posterior skull base, good decompression can be achieved with the skull base approaches and their combinations. Surgery seems to offer better palliation than radiotherapy in radioresistant metastases. Rare tumors of the skull base can be a diagnostic enigma before surgery.
REFERENCES
- Osborn A. Brain tumors and tumor like processes. Diagnostic Neuroradiology. Mosby. 1994:401–529. [Google Scholar]
- Sampson J H, Rossitch E, Jr, Young J N, et al. Solitary eosinophilic granuloma invading the clivus of an adult. Neurosurgery. 1992;31:755–758. doi: 10.1227/00006123-199210000-00022. [DOI] [PubMed] [Google Scholar]
- Weber A L. Magnetic resonance imaging and computed tomography of the internal auditory canal and cerebellopontine angle. Isr J Med Sci. 1992;28:173–182. [PubMed] [Google Scholar]
- Samii M, Bini W. In: Sekhar LN, Janecka IR eds, editor. Surgery of Cranial Base Tumours. New York, NY: Raven Press; 1993. Surgical strategy for jugular foramen tumours. pp. 379–387.
- Martinez R, Vaquero J, Areitio E, Bravo G. Meningiomas of the posterior fossa. Surg Neurol. 1983;19:237–243. doi: 10.1016/s0090-3019(83)80007-x. [DOI] [PubMed] [Google Scholar]
- Meyer F. Benign tumours of the foramen magnum. J Neurosurg. 1984;61:136–142. doi: 10.3171/jns.1984.61.1.0136. [DOI] [PubMed] [Google Scholar]
- Castellano F, Guidetti B, Olivecrona H. Pterional meningioma en plaque. J Neurosurg. 1952;9:188–196. doi: 10.3171/jns.1952.9.2.0188. [DOI] [PubMed] [Google Scholar]
- Abbott K H, Glass B. Pterional meningioma en plaque: report of a case of thirty six years' duration. J Neurosurg. 1955;12:50–52. doi: 10.3171/jns.1955.12.1.0050. [DOI] [PubMed] [Google Scholar]
- Derome P J, Guiot G. Bone problems in meningiomas invading the base of the skull. Clin Neurosurg. 1978;25:435–451. doi: 10.1093/neurosurgery/25.cn_suppl_1.435. [DOI] [PubMed] [Google Scholar]
- Stout A, Murray M. Hemangiopericytoma: a vascular tumour from Zimmerman's pericytes. Am Surg. 1942;116:26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horten B C, Urich H, Rubinstein L J, Montague S R. The angioblastic meningioma: a reappraisal of the nosological problem. Light-, electron-microscopic, tissue, and organ culture observations. J Neurol Sci. 1977;31:387–410. doi: 10.1016/0022-510x(77)90217-9. [DOI] [PubMed] [Google Scholar]
- Kochanek S, Schroder R, Firsching R. Hemangiopericytoma of meninges. I. Histopathological variability and differential diagnosis. Zentralbl neurochir. 1986;47:183–190. [PubMed] [Google Scholar]
- Pena C E. Meningioma and intracranial hemangiopericytoma. A comparative electron microscopic study. Acta Neuropathol (Berl) 1977;39:69–74. doi: 10.1007/BF00690387. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Servo A, Haltia M, Wahlstrom T, Voltonen S. Intracranial hemangiopericytoma: radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol. 1985;23:227–236. doi: 10.1016/0090-3019(85)90087-4. [DOI] [PubMed] [Google Scholar]
- Guthrie B L, Ebersold M J, Scheithauer B W, Shaw E G. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- Chan R C, Thompson G B. Morbidity, mortality and quality of life following surgery for intracranial meningiomas. J Neurosurg. 1984;60:52–60. doi: 10.3171/jns.1984.60.1.0052. [DOI] [PubMed] [Google Scholar]
- Kumar R, Malik V, Tyagi I, Pal L, Singh S N. Hyperostotic paraganglioma of occipitotemporal bone. Neurosurg Rev. 2004;27:46–49. doi: 10.1007/s10143-003-0278-5. [DOI] [PubMed] [Google Scholar]
- Potts D J, Abbott G F, Von Sneidern J V. National Cancer Institute Study: evaluation of computed tomography in the diagnosis of intracranial neoplasms. III. Metastatic tumors. Radiology. 1980;136:657–664. doi: 10.1148/radiology.136.3.7403544. [DOI] [PubMed] [Google Scholar]
- Naheedy M H, Kido D K, O'Reilly G V. Computed tomographic evaluation of subdural and epidural metastases. J Comput Assist Tomogr. 1980;4:311–315. doi: 10.1097/00004728-198006000-00004. [DOI] [PubMed] [Google Scholar]
- Delattre J Y, Krol G, Thaler H T, Posner J B. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- Galicich J, Arbit E. In: Youmans J, Neurological Surgery. 3rd, editor. Philadelphia, PA: WB Saunders; 1990. Metastatic brain tumours. p. 3204.