Abstract
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is characterized by the patchy replacement of myocardium by fatty or fibrofatty tissue. These changes lead to structural abnormalities including right ventricular enlargement and wall motion abnormalities that can be detected by echocardiography, angiography, and cine MRI. ARVC/D is a genetically heterogeneous disorder, since it has been linked to several chromosomal loci. Myocarditis may also be a contributing etiological factor. Patients are typically diagnosed during adolescence or young adulthood. Presenting symptoms are generally related to ventricular arrhythmias. Concern for the risk of sudden cardiac death may lead to the implantation of an intracardiac defibrillator. An ongoing multicenter international registry should further our understanding of this disease.
Introduction
Arhythmogenic right ventricular cardiomypathy/dysplasia (ARVC/D) is a disorder in which the right ventricular myocardium is interspersed with fatty or fibrofatty tissue. This pathological process principally affects the right ventricle, but in advanced cases can lead to biventricular involvement. Linkage analysis has identified several loci on different chromosomes, indicating this disease is genetically heterogeneous. Specific mutations have been identified in the genes for desmoplakin [1], plakoglobin [2] and the ryanodine receptor gene [3]. The predominant presenting symptoms are due to ventricular arrhythmias, including palpitations, sustained ventricular tachycardia, or uncommonly, sudden cardiac death. Although ARVC/D is an unusual disorder, it has been described as the most common cause of sudden death in young athletes under the age of 35 in the Veneto area in Italy [4] . Heart failure is infrequent but may occur due to severe right or biventricular enlargement.
a) Structural Abnormalities and Pathophysiology
Anatomically, ARVC/D is characterized by replacement of myocardium with fatty and fibrous tissue that primarily affects the epicardium and the mid-myocardium, with relative sparing of the endocardium (Figure 1 and 2). This process most commonly affects the posterior and inferior areas of the right ventricular inflow tract adjacent to the tricuspid valve [5], but it also affects the anterior infundibulum and the apex, thus forming what is known as the "triangle of dysplasia" [6]. Loss of myocardium may result in aneurysm formation, commonly in the basal inferior wall, underneath the tricuspid valve [7]. Fibro-fatty replacement occurs in a segmental, patchy fashion and adjacent areas, particularly, the septum, are generally spared. Therefore, endomyocardial biopsies, which usually are performed from septal tissue, may be non-diagnostic. ARVC/D is distinguished from Uhls anomaly, a congenital disorder in which right ventricular myocardium is absent, resulting in a paper-thin right ventricular wall [8,9]. Recently, apoptotic cells have been reported in endomyocardial biopsy specimens [10-13]. It is unknown how the apoptotic pathway may be initiated in ARVC/D, but may relate to altered intracellular calcium concentration [1]. Inflammatory infiltrates consisting of lymphocytes may also be found on biopsy specimens, suggestive of a focal myocarditis, and may explain sporadic cases of ARVD/C.
Figure 1.
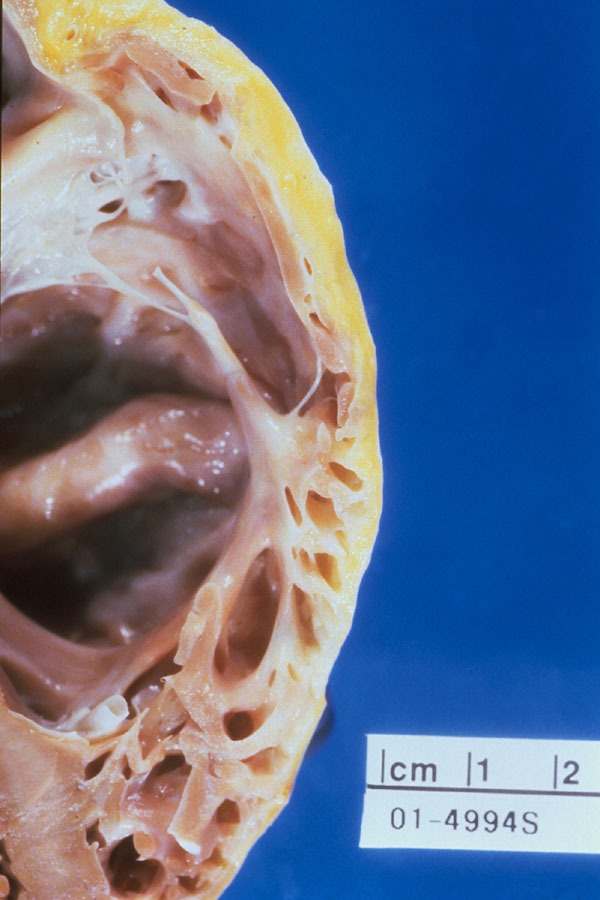
In ARVC/D there is progressive fibro-fatty replacement of the myocardium with thinning and enlargement of the RV wall. In this specimen there is gross fatty infiltration seen in a portion of the RV wall.
Figure 2.
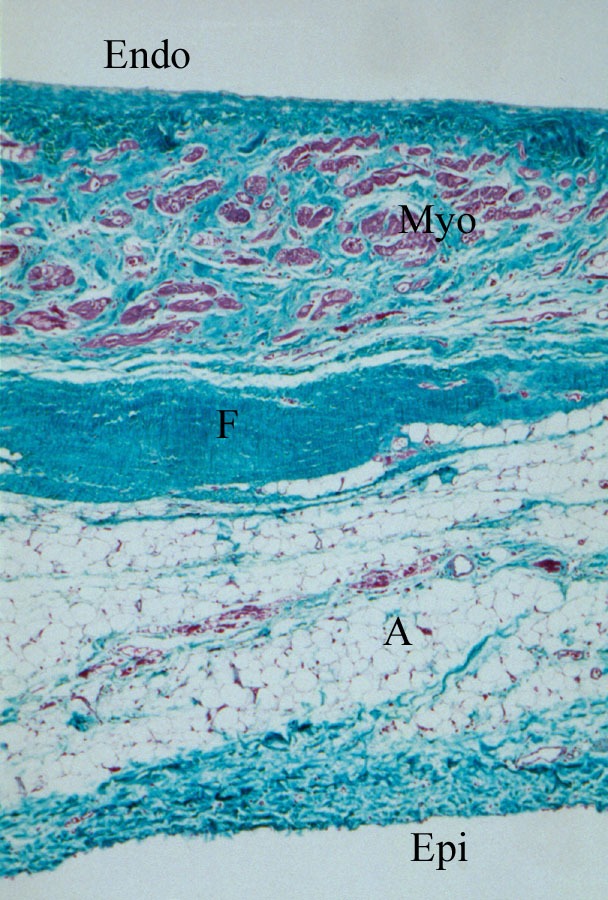
Trichrome staining shows areas of mature fibrosis (F) and adipose tissue (A) within the epicardial (Epi) and mid-myocardial zones, with remaining small clusters of myocytes (Myo) near the endocardial edge (Endo).
Diffuse hypokinesis or regional wall motion abnormalities of the right ventricle can be seen with contrast ventriculography, considered to be the most reliable test to assess structural abnormalities. RV angiography should be performed in four views: RAO 30, LAO 60, AP and lateral. Affected areas will show hypokinesis, akinesis, or dyskinesis. Wall motion abnormalities can also be observed by echocardiography but particular attention must be focussed on imaging the basal posterior and inferior walls near the tricuspid valve [5,14]. MRI is emerging as a useful non-invasive tool to identify morphologic abnormalities such as myocardial fat, right ventricular chamber size and shape, and wall motion abnormalities with newer cine techniques. However, with conventional MR technology, the identification of myocardial fat was not found to be a reliable predictor of the presence of ARVC/D [15].
b) Electrocardiographic Abnormalities
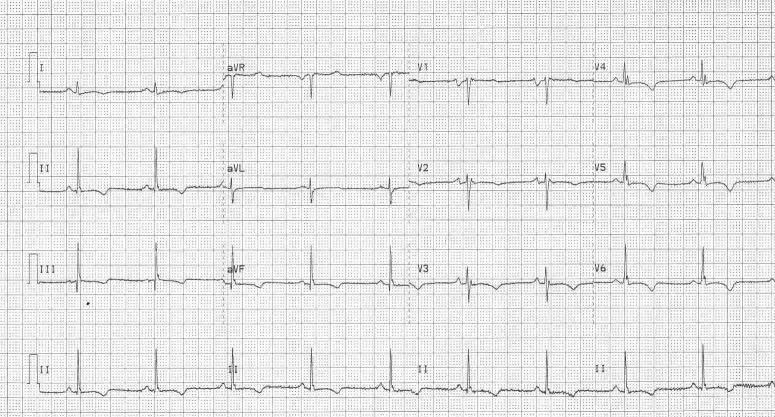
Electrocardiographic changes include inverted T waves in the right precordial leads beyond V1 (Figure 3) in the absence of right bundle branch block [16,17]. Right ventricular late potentials in the form of epsilon waves may be found on the routine 12 lead ECG. If not present overtly, they may be identified by recording the ECG at double standard speed (50mm/sec) and amplitude (20mm/mV) and utilizing a 40 Hz filter [5] (Figure 4) . Evidence of right ventricular parietal block is a QRS duration that is longer in the right (leads V1, V2 and V3) than in the left (V4, V5, V6) precordial leads. Suggested parameters to verify this include a localized QRS duration greater than 110ms in the right precordial leads, or a maximum QRS duration in leads V1, V2 or V3 or more than 25ms above the QRS duration in lead V6 in the presence of incomplete or complete right bundle branch block [18]. The signal averaged electrocardiogram is often abnormal and supports the diagnosis of ARVC/D, but if normal, does not exclude the diagnosis. The sensitivity of the signal averaged electrocardiogram can be improved by using a 25 Hz high pass filter and paying particular attention to the Z lead [19].
Figure 3.
This ECG from a patient with ARVC/D shows T wave inversion throughout the precordial leads.
Figure 4.
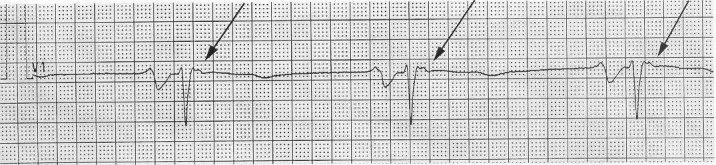
Epsilon waves, representing right ventricular late potentials, can be brought out by recording the ECG at double speed (50mm/s), double amplitude (20mm/mV), and a 40 Hz filter. Here, epsilon waves are evident as small, notched deflections just after the QRS in lead V1 (arrows).
c) Arrhythmias
The ventricular arrhythmias generally arise from the right ventricle, and therefore have a left branch block morphology. Right ventricular outflow tract tachycardia (RVOT), must be excluded as this diagnosis carries a benign prognosis and is not hereditary5 . RVOT tachycardia has a left bundle branch block morphology. The QRS points to the right and inferior (positive in leads II, III, AVF and negative in AVL); this morphology can also be seen in ventricular tachycardia due to ARVC/D. Patients with ARVC/D may also experience supraventricular arrhythmias [20] including atrial flutter [21-23].
d) Genetics
Thirty to fifty percent of patients with ARVC/D will have evidence of familial disease [25,26]. The variable clinical expression and course is believed to be at least in part due to genetic heterogeneity. Inheritance is autosomal dominant, with the exception of Naxos disease, which is autosomal recessive. Linkage analysis in families with ARVD has revealed several loci for this disorder on chromosomes 1, 2, 3, 10, 14, and 17 [26-31] (see Table 1). The mutation for ARVD8 has been identified in a gene for desmoplakin on chromosome 6, a component of desmosomes that are cellular adhesion proteins responsible for cellular binding [1]. This is the first gene identified to cause a major form of the disease, with autosomal dominant inheritance. The locus on chromosome 1 leads to a form of ARVC/D that is notable for exertional polymorphic, instead of monomorphic, ventricular tachycardia [3,32] and has been further identified to encode a cardiac ryanodine receptor gene that is responsible for calcium release from the sarcoplasmic reticulum. This genetic mutation has also been implicated in familial catecholaminergic polymorphic ventricular tachycardia [33,34]. Naxos disease discovered on the Greek island, Naxos, is characterized by palmoplantar keratosis and woolly hair in addition to RV dysplasia. Naxos disease has been mapped to chromosome 17 involving a mutation in the gene that encodes plakoglobin, a component of desmosomes and adherens junctions responsible for maintaining tight adhesions between cells [2]. Another mutation in the gene for desmoplakin that also causes an autosomal recessive syndrome with woolly hair and a dilated left ventricle has been reported in patients from Ecuador [35]. Therefore, ARVD can result from a variety of genetic mechanisms, and in particular, appears to be a disorder of proteins involved in cellular adhesion and calcium release.
Table 1.
ARVC/D Types
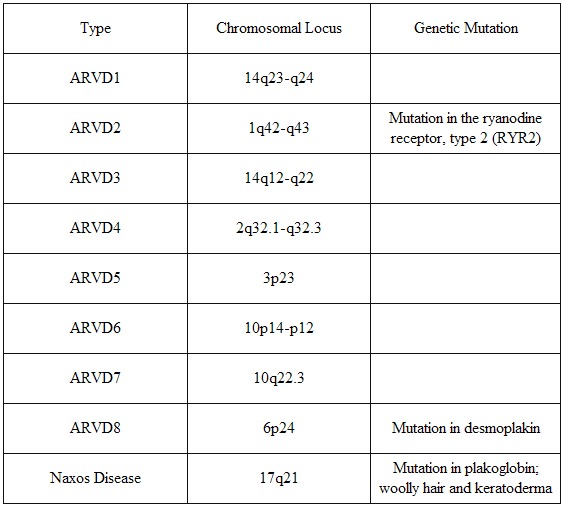
ARVD types 1 through 8 are inherited as an autosomal dominant condition, while in Naxos disease the inheritance is autosomal recessive.
Not all cases of ARVD are inherited since mutations may occur sporadically. Nonetheless, because of the genetic basis for this disorder, it is recommended that first degree relatives of ARVD patients be screened with an ECG and echocardiogram [5]. Other non-invasive tests to identify affected family members include signal-averaged ECG, Holter monitor and exercise stress testing.
Other Contributory Factors
A large number of ARVC/D patients are noted to be athletes, and sudden cardiac death is more likely during exertion [4]. Athletic activity, possibly through stretch of myocardial fibers or elevated catecholamines may accelerate the degeneration of the ventricular myocardium and leads to ventricular arrhythmias. Also in support of the importance of other etiologic factors are the reports of Coxsackie virus B3 biopsy specimens [36], and recently, enterovirus and adenovirus by polymerase chain reaction in 7 of 12 specimens from sporadic cases [37]. This raises the possibility that the myocardium in ARVD is particularly vulnerable to inflammatory processes. A superimposed myocarditis could result in lymphocytic infiltrates and fibrofatty replacement of the myocardium [38,39]. It is not known whether the detection of viral DNA or RNA indicates that right ventricular cardiomyopathy is a result of viral myocarditis or this cardiomyopathy predisposes the heart to viral infection. This process may only affect the RV, but can affect both ventricles as well. Patients with left ventricular involvement tend to be older and more symptomatic from both arrhythmias and heart failure, consistent with the view that the disease is progressive [40].
Since there is no single "gold standard" diagnostic test to reliably verify this condition, a combination of diagnostic criteria have been established by the Task Force of the Working Group on Myocardial and Pericardial Disease [41]. Major criteria include demonstration of severe wall motion abnormalities, fibrofatty replacement by biopsy, epsilon waves and family history of histologically confirmed disease. Minor criteria include milder alterations of ventricular function, inverted T waves in the right precordial leads, late potentials, ventricular tachycardia with left bundle branch morphology or frequent premature ventricular complexes (>1000 PVCs in a 24 hour period), or family history of suspected or clinically diagnosed ARVC/D. A diagnosis can then be made if at least two major, 1 major and 2 minor, or 4 minor criteria from different categories are fulfilled Disease [41].
A prospective study of the course of disease in 37 affected families with 365 relatives in Italy showed that 41% could be diagnosed with certainty to have ARVC/D while in another 11% the diagnosis was uncertain Disease [42]. Patients were diagnosed mostly during adolescence and early adulthood. After a mean follow-up of 8.5 years, an additional 15 subjects developed structural abnormalities or ventricular arrhythmias. In an evaluation of relatives of 67 ARVC/D patients in the United Kingdom, 28% of patients had relatives that satisfied the Task Force criteria, but another 20% had relatives with only minor ECG, echo or Holter abnormalities suggestive of early or mild disease Disease [43]. Thus familial disease may have been present in up to 48% of index cases, which is plausible for a disease with autosomal dominant inheritance. It has been suggested by these authors that the standard diagnostic criteria should be modified to identify ARVC/D in relatives of patients with confirmed disease if they satisfy any one of the following abnormalities: i) T-wave inversion in the precordial leads ii) abnormal SAECG iii) ventricular tachycardia with left bundle branch morphology during ECG, Holter monitoring or exercise testing iv) greater than 200 PVCs in a 24 hour period or v) any mild echocardiographic abnormalities such as RV dilatation with preserved LV function Disease [42] .
Treatment/Management
Therapy with beta blockers Disease [44], sotalol [44] or amiodarone [45] may be effective in suppressing ventricular arrhythmias and possibly in preventing sudden cardiac death. Implantation of an ICD may also be indicated to prevent sudden death. Catheter ablation of ventricular tachycardia may be useful in patients with refractory symptoms despite antiarrhythmic therapy. However, ventricular arrhythmias may recur from other areas. Patients should also be advised not to perform vigorous exercise or engage in competitive sports. Surgical disarticulation of the right ventricular free wall from its attachments to the left ventricle and septum can prevent the electrical propagation of ventricular arrythmias from the right to the left ventricle [46,47]. This was an effective means to prevent sudden death prior to the availability of the ICD, but resulted in severe right ventricular failure. Management of heart failure includes standard medical therapy with consideration of heart transplantation if severe ventricular, especially biventricular, dysfunction is present.
It is important to emphasize that risk factors for sudden death are not well characterized. A history of syncope, right or left ventricular abnormalities seen on radionuclide angiography and QRS dispersion greater than 40ms are independent predictors of sudden death [48]. However, the absence of these factors cannot provide absolute assurance that a patient or relative is free of risk. The diverse phenotypic expression of ARVC/D is still being appreciated as new genetic loci are discovered. Furthermore, the risk of sudden death is probably variable among different types of ARVC/D. In Newfoundland, Canada, ARVD5 was reported to cause sudden death in 44% of affected males, while females had a more benign course with no sudden deaths. Thus, determining prognosis is complicated by the genetic heterogeneity of this disorder.
It is unclear how to advise family members of individuals that have died suddenly with ARVD. The eight-year actuarial probability of developing ventricular arrhythmias in asymptomatic family members with normal echocardiograms was found to be 3%, with no sudden deaths [50]. The potential risks associated with the implantation and long-term management of an intracardiac defibrillator in family members with suspected disease but minimal right ventricular dysfunction may far outweigh its potential benefits. Counseling of family members needs to be individualized.
The Multidisciplinary Study of Right Ventricular Dysplasia is a multicenter study funded by the National Institutes of Health and National Heart, Lung, and Blood Institutes that is enrolling patients with a recent diagnosis of ARVC/D into a North American registry. A large registry of patients is needed to answer important questions related to diagnosis of the disorder, in particular in family members, as well as clinical management of affected patients and risk stratification for sudden death. This study will also facilitate genetic research to identify other specific mutations and their role in the pathogenesis of this disease [51,52].
Acknowledgments
We thank Dr. Richard Sobonya for preparation of the gross and microscopic pathology images.
Footnotes
This paper has been published in the First International Symposium on Arrhythmogenic Right Ventricular Dysplasia
References
- Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. American Journal of Human Genetics. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKoy G, Protonotarios N, Crosby JM, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- Tiso C, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventircular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- Corrado D, Thiene G, Nava A, et al. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Medicine. 1990;89:588–596. doi: 10.1016/0002-9343(90)90176-e. [DOI] [PubMed] [Google Scholar]
- Marcus FI. Arrhythmogenic right ventricular dysplasia. Cardiac Electrophys Rev. 1999;3:205–206. [Google Scholar]
- Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- Thiene G, Basso C, Danieli G, et al. Arrhythmogenic right ventricular cardiomyopathy. A still underrecognized clinic entity. Trends Cardiovasc Med. 1997;7:84–90. doi: 10.1016/S1050-1738(97)00011-X. [DOI] [PubMed] [Google Scholar]
- Gerlis LM, Schmidt-Ott SC, Ho Sy, et al. Dysplastic conditions of the right ventircular myocardium: Uhl's anomaly versus arrhythmogenic right ventircualr dysplasia. Br Heart J. 1993;69:142–150. doi: 10.1136/hrt.69.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine G, Fontaliran F, Frank R. Arrhythmogenic right ventricular cardiomyopathies. Clinical forms and main differential diagnoses. Circulation. 1998;97:1532–1535. doi: 10.1161/01.cir.97.16.1532. [DOI] [PubMed] [Google Scholar]
- Danieli GA, Rampazzo A. Genetics of arrhythmogenic right ventricular cardiomyopathy. Curr Opin in Card. 2002;17:218–221. doi: 10.1097/00001573-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Kavantzas NG, Lazaris AC, Agapitos EV, et al. Histological assessment of apoptotic cell death in cardiomyopathies. Pathology. 2000;32:176–180. [PubMed] [Google Scholar]
- Runge MS, Stouffer GA, Sheahan RG. Morphological patterns of death by myocytes in arrhythmogenic right ventricular dysplasia. Am J Med Sci. 2000;320:310–319. doi: 10.1097/00000441-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Nagata M, Hiroe M, Ishiyama S, et al. Apoptotic cell death in arrhythmogenic right ventircular cardiomoypathpy: a comparative study with idiopathic susptained ventricular tachycardia. Jpn Heart J. 2000;41:733–741. doi: 10.1536/jhj.41.733. [DOI] [PubMed] [Google Scholar]
- Villanova C. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: echocardiographic features and criteria. Padua Italy: Universita Degli Studi Di Padova; 1998. Dec, [Google Scholar]
- Bluemke D, Krupinski EA, Ovitt T, et al. Imaging of arrhythmogenic right ventircular cardiomyopathy: morphologic findings and interobserver reliability. Cardiology. 2003 doi: 10.1159/000070672. In press. [DOI] [PubMed] [Google Scholar]
- Frank R, Fontaine G, Vedel J, et al. Electrocardiologie de quatre cas de dysplasia ventriculaire droite arythmogene. Arch Mal Coeur Vaiss. 1978;71:963–972. [PubMed] [Google Scholar]
- Nava A, Canciani B, Buja GF, et al. Electrovectorcardiographic study of negative T waves on precordial leads in arrhythmogenic right ventricular dysplasia: relationship with right ventricular volumes. J Electrocardiol. 1988;21:239–245. doi: 10.1016/0022-0736(88)90098-2. [DOI] [PubMed] [Google Scholar]
- Fontaine G, Fontaliran F, Hebert JL, et al. Arrhythmogenic right ventircular dysplasia. Annu Rev Med. 1999;50:17–35. doi: 10.1146/annurev.med.50.1.17. [DOI] [PubMed] [Google Scholar]
- Nasir K, Rutberg J, Tandri H, et al. Utility of SAECG in arrhythmogenic right ventricle dysplasia. ANE. 2003;8:112–120. doi: 10.1046/j.1542-474X.2003.08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonet JL, Castro-Miranda R, Iwa T, et al. Frequency of supraventricular tachyarrhythmias in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 1991;67:1153. doi: 10.1016/0002-9149(91)90886-p. [DOI] [PubMed] [Google Scholar]
- Nakazato Y, Nakata Y, Tokano T, et al. A case of arrhythmogenic right ventricular dysplasia with atrial flutter; Japanese Heart Journal; 1994. pp. 689–694. [DOI] [PubMed] [Google Scholar]
- Caglar N, Pamir G, Kural T, et al. Right ventricular cardiomypathy similar to Uhl's anomaly with atrial flutter and complete AV block. International J Cardiol. 1993;38:199–201. doi: 10.1016/0167-5273(93)90183-h. [DOI] [PubMed] [Google Scholar]
- Lui CY, Marcus FI, Sobonya RE, et al. Arrhythmogenic right ventricular dysplasia masquerading as peripartum cardiomyopathy with atrial flutter, advanced atrioventricular block and embolic stroke. Cardiology. 2002;97:49–50. doi: 10.1159/000047419. [DOI] [PubMed] [Google Scholar]
- Nava A, Bauce B, Basso C, et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventircular cardiomyopathy. J Am Coll Card. 2000;36:2226–2233. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- Hamid MS, Norman M, Quaraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Card. 2002;40:1445–1450. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- Bauce B, Nava A, Rampazzo A, et al. Familial effort polymorphic ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy map to chromosome 1q42-43. Am J Cardiol. 2000;85:573–579. doi: 10.1016/s0002-9149(99)00814-0. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Danieli GA, et al. The gene for arrhytmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet. 1994;3:959–962. doi: 10.1093/hmg/3.6.959. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Miorin M, et al. ARVD4 a new locus for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 2 long arm. Genomics. 1997;45:259–263. doi: 10.1006/geno.1997.4927. [DOI] [PubMed] [Google Scholar]
- Severini GM, Krajinovic M, Pinamonti B, et al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics. 1996;31:193–200. doi: 10.1006/geno.1996.0031. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Li D, Karibe A. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation. 1998;98:2791–2795. doi: 10.1161/01.cir.98.25.2791. [DOI] [PubMed] [Google Scholar]
- Coonar AS, Protonotarios N, Tsatsopoulou A, et al. Gene for arrythmogenic right ventricular cardiomyopathy with diffuse non epidermolytic palmoplantar keratoderma and wooly hair (Naxos disease) maps to 17q21. Circulation. 1998;97:2049–2058. doi: 10.1161/01.cir.97.20.2049. [DOI] [PubMed] [Google Scholar]
- Bauce B, Nava A, Rampazzo A, et al. Familial effort polymorphic ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy map to chromosome 1q42-43. Am J Cardiol. 2000;85:573–579. doi: 10.1016/s0002-9149(99)00814-0. [DOI] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piipo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic vetnricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Guerta L, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, wooly hair and keratoderma. Hum Mol Genetics. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- Grumbach IM, Heim A, Vonhof S, et al. Coxsackievirus genome in myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Cardiology. 1998;89:241–245. doi: 10.1159/000006794. [DOI] [PubMed] [Google Scholar]
- Bowles NE, Ni J, Marcus F. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventircular dysplasia/cardiomyopathy. J Am Coll Card. 2002;39:892–895. doi: 10.1016/s0735-1097(02)01688-1. [DOI] [PubMed] [Google Scholar]
- Fontaine G, Fontaliran F, Rosas Andrade F, et al. The arrhythmogenic right ventricle. Dysplasia versus cardiomyopathy. Heart Vessels. 1995;10:227–235. doi: 10.1007/BF01744901. [DOI] [PubMed] [Google Scholar]
- Thiene G, Corrado D, Nava A, et al. Right ventricular cardiomypathy: is there evidence of an inflammatory aetiology? Eur Heart J. 1991;12:22–25. doi: 10.1093/eurheartj/12.suppl_d.22. [DOI] [PubMed] [Google Scholar]
- Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomypathy/dysplasia: A multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava A, Bauce B, Basso C, et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventircular cardiomyopathy. J Am Coll Card. 2000;36:2226–2230. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- Hamid MS, Norman MS, Quaraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Card. 2002;40:1445–1450. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- Wichter T, Hindricks G, Lerch H, et al. Regional myocardial sympathetic dysinnervation in arrhythmogenic right ventricular cardiomyopathy: an analysis using 123I metaiodobenzylguanidine scintigraphy. Circulation. 1994;89:667–683. doi: 10.1161/01.cir.89.2.667. [DOI] [PubMed] [Google Scholar]
- Marcus FI, Fontaine G. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: a review. Pacing Clin Electrophysiol. 1995;18:1298–1314. doi: 10.1111/j.1540-8159.1995.tb06971.x. [DOI] [PubMed] [Google Scholar]
- Guiraudon GM, Klein GJ, Gulamhusein SS, et al. Total disconnection of the right ventricular free wall: surgical treatment of right ventricular tachycardia associated with right ventricular dysplasia; Circulation; 1983. pp. 463–470. [DOI] [PubMed] [Google Scholar]
- Doig JC, Nimkhedkar K, Bourke JP, et al. Acute and chronic hemodynamic impact of total right ventricular disarticulation. PACE. 1991;14:1971–1975. doi: 10.1111/j.1540-8159.1991.tb02800.x. [DOI] [PubMed] [Google Scholar]
- Turrini P, Corrado D, Basso C, et al. Noninvasive risk stratification in arrhythmogenic right ventricular cardiomyopathy. ANE. 2003;8:161–169. doi: 10.1046/j.1542-474X.2003.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicks E, Hodgkinson K, Conners S, et al. Initial clinical manifestations of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Gen. 2000;67:114a. [Google Scholar]
- Scognamiglio R, Rahimtooia S, Thiene G, et al. Concealed phase of familiar arrhythmogenic right ventricular cardiomyopathy (ARVD). Early recognition and long-term follow-up. J Am Coll Card. 1997;29:194A. [Google Scholar]
- Corrado D, Fontaine G, Marcus FI, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. European Society of Cardiology and the Scientific Council on Cardiomyopathies of the World Heart Federation. Journal of Cardiovascular Electrophysiology. 2000;11:827–832. doi: 10.1111/j.1540-8167.2000.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Gear K, Marcus F. Arrhythmogenic right ventirculardysplasia/cardiomyopathy. Circulation. 2003;107:e31–e33. doi: 10.1161/01.cir.0000053943.38763.70. [DOI] [PubMed] [Google Scholar]