Abstract
Fimbriae are thread-like polymers displayed in large amounts on the bacterial surface and used by many pathogens to attach to receptors on host tissue surfaces. Fimbriae contain disulfide bridges, contrary to many Escherichia coli surface proteins produced in bulk amounts. Here we investigate whether fimbriae expression can affect expression of other genes. Analysis of gene expression in two E.coli strains, differing in the fim locus, indicated the flu gene to be affected. The flu gene encodes the antigen 43 (Ag43) surface protein, specifically involved in bacterial aggregation, and microcolony and biofilm formation. Ag43 production is repressed by the global regulator OxyR, which monitors the cell’s thiol–disulfide status. Only the thiol form of OxyR represses Ag43 production. We demonstrate that production of several different disulfide-containing fimbriae results in the abolition of Ag43 production. No effect was observed in an oxyR mutant. We conclude that fimbriae expression per se constitutes a signal transduction mechanism that affects a number of unrelated genes via the thiol–disulfide status of OxyR. Thus, phase variation in fimbrial expression is coordinated with the expression of other disease- and colonization-related genes.
Keywords: antigen 43/fimbriae/oxidative stress/OxyR/disulfide formation
Introduction
The ability of bacteria to recognize and adhere to a specific surface is a fundamental aspect of microbial ecology and pathogenesis. Bacterial adhesins confer specific recognition and adhesion to a diverse spectrum of target molecules, ranging from components of tissue or cell surfaces to inorganic materials such as dental enamel (Klemm and Schembri, 2000). Type 1 fimbriae are thin, rigid, adhesive surface organelles found on many Escherichia coli strains and other members of the Enterobacteriaceae (Klemm and Krogfelt, 1994). On a fimbriated bacterium, several hundred of these organelles radiate peritrichously from the surface to a distance of ∼1 µm. Type 1 fimbriae exert their adhesive properties by virtue of the FimH adhesin, a minor organelle component located at the tip of the fimbriae (Choudhury et al., 1999). The FimH adhesin recognizes mannose-containing glycoproteins that are present on many mammalian host tissues, such as the surface of the urinary tract. This enables the bacteria to target, attach to and eventually to colonize the uroepithelium (Connell et al., 1996; Sauer et al., 2000). Type 1 fimbriation has been correlated with urovirulence in E.coli, the causative agent of >80% of urinary tract infections in humans.
Expression of type 1 fimbriae is phase variable due to a flip–flop-type control system based on an invertible DNA switch, located immediately upstream of the major sub unit gene. A promoter located in the switch drives the expression of the fim genes (Olsen and Klemm, 1994). On or off orientation of the switch results in a fimbriated or bald phenotype, respectively. Two tyrosine-class recombinases, FimB and FimE, catalyse the inversion of the switch (Klemm, 1986). Only the FimB recombinase efficiently promotes off-to-on switching, while FimE primarily catalyses on-to-off inversion (Klemm, 1986; Gally et al., 1996). A number of global regulators including integration host factor (IHF) (Blomfield et al., 1997), the leucine-responsive regulatory protein (Lrp) (Blomfield et al., 1993) and H-NS (Olsen and Klemm, 1994; Olsen et al., 1998; Schembri et al., 1998) also affect inversion of the fim switch.
Another prominent surface protein of E.coli subject to phase variation is the product of the flu gene (also termed agn), antigen 43 (Ag43) (Diderichsen, 1980; Henderson and Owen, 1999). The expression of flu is phase variable due to the concerted action of the Dam methylase (activation) and OxyR (repression) (Henderson and Owen, 1999; Haagmans and van der Woude, 2000). OxyR is involved in the cell’s oxidative stress response and also monitors the cellular thiol–disulfide status (Zheng et al., 1998; Åslund and Beckwith, 1999; Debarbieux and Beckwith, 1999; Storz and Imlay, 1999). OxyR can exist in both a reduced and an oxidized form in which two cysteine groups are oxidized to form a disulfide bridge. Unlike other genes in the OxyR regulon that are activated by the oxidized form of OxyR (Storz and Imlay, 1999), only the reduced form is able to repress flu efficiently (Henderson and Owen, 1999; Haagmans and van der Woude, 2000).
Ag43 is a self-recognizing surface-displayed protein (∼50 000 copies per cell) that confers autoaggregation of bacteria by an intercellular handshake mechanism (Hasman et al., 1999). Expression of Ag43 causes a characteristic frizzy colony morphology type on solid media and settling of cells from standing liquid cultures (Hasman et al., 2000). Recently, Ag43 was demonstrated to be involved in bacterial biofilm formation (Danese et al., 2000; Kjærgaard et al., 2000; O’Toole et al., 2000). Interestingly, Ag43-mediated autoaggregation is abolished by the presence of type 1 fimbriae on the cells (Hasman et al., 1999). Apparently, the presence of these ∼1-µm-long, rigid organelles on the cell surface impedes the close cell contact required for the Ag43–Ag43 handshake function; thus, Fim is phenotypically dominant to Ag43.
Adhering to a particular tissue or cell surface by fimbriae-mediated attachment heralds a significant event in the life cycle of a bacterium. It is therefore implicit that expression of such target recognition organelles is of fundamental importance in a bacterium’s interplay with the environment and might influence the expression of other genes required in the new habitat. Furthermore, a type 1 fimbriated cell has up to 500 fimbriae on the surface, i.e. 0.5 million structural proteins, representing ∼8% of the total cellular protein and a significant resource drain for the cell. With this in mind, we have investigated whether the fimbriate state of a bacterium influences the expression of other non-related genes by inter-system cross-talk.
Results
Deletion of the fim gene cluster affects transcription of flu
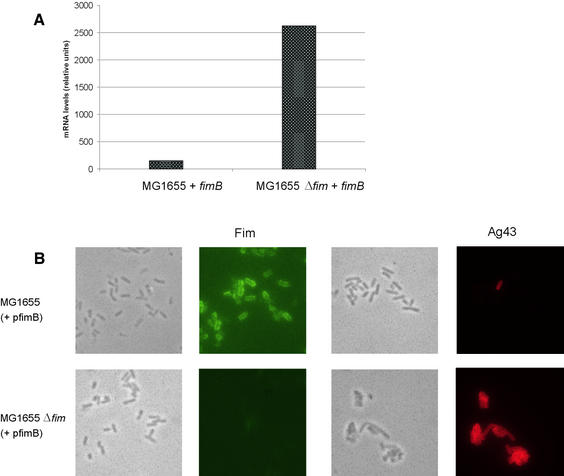
The advent of genome-wide DNA microarrays for analysis of gene expression and, specifically, a DNA chip based on the genome of the E.coli K-12 reference strain MG1655 (Selinger et al., 2000) provided an attractive way of analysing expression of genes in E.coli that might be affected by the fimbriation status of the cell. An isogenic pair of strains consisting of E.coli strain MG1655 and its Δfim sibling were transformed with a plasmid, pPKL9 (pfimB), encoding the fimB recombinase, to ensure optimal expression of the fim gene cluster. PCR monitoring of the fim switch in MG1655(pfimB) revealed it to be in the on orientation (it is absent in MG1655Δfim; data not shown). Phenotypically, the two strains represent the extremes in fimbrial phase variation, being either fimbriated or bald, respectively. Total mRNA was isolated from each of the two strains and investigated by DNA microarray analysis. Comparison of the transcript signals of strains MG1655(pfimB) and MG1655Δfim(pfimB) revealed that the transcription of the flu gene, diametrically positioned to the fim locus on the E.coli chromosome, was increased ∼20-fold when fim was absent (Figure 1A). We previously found that Ag43-mediated autoaggregation is abolished by the presence of type 1 fimbriae on the cells; thus, Fim is phenotypically dominant to Ag43 (Hasman et al., 1999). However, the dramatic change in transcription of the flu gene observed in the DNA microarray experiments suggested a possible interaction between fim and flu at the transcriptional level. Furthermore, when we re- examined our MG1655(pfimB) and MG1655Δfim(pfimB) strains by fluorescence microscopy employing either Fim- or Ag43-specific antisera, Ag43 expression was only observed in the MG1655Δfim(pfimB) strain (Figure 1B).
Fig. 1. (A) Comparison of flu gene transcription levels in E.coli strains MG1655(pfimB) and MG1655Δfim(pfimB) (a derivative in which the fim genes have been deleted). Quantification of mRNA levels was performed by direct scanning of DNA chips. (B) Phase contrast and immunofluorescence microscopy of E.coli strains MG1655(pfimB) and MG1655Δfim(pfimB) employing anti-Fim and anti-Ag43 serum, respectively.
Production of disulfide-containing fimbriae affects Ag43 phenotype and implicates OxyR
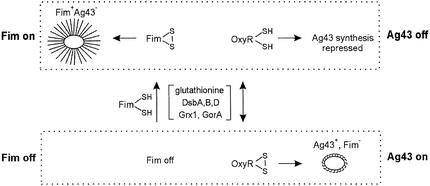
The expression of flu is phase variable due to the concerted action of the Dam methylase (activation) and OxyR (repression) (Henderson and Owen, 1999; Haagmans and van der Woude, 2000). OxyR is involved in the cell’s oxidative stress response and also monitors the cellular thiol–disulfide status (Zheng et al., 1998; Åslund and Beckwith, 1999; Debarbieux and Beckwith, 1999; Storz and Imlay, 1999). OxyR can exist in both a reduced and an oxidized form in which two cysteine groups are oxidized to form a disulfide bridge. Unlike other genes in the OxyR regulon that are activated by the oxidized form of OxyR (Storz and Imlay, 1999), only the reduced form is able to repress flu efficiently (Henderson and Owen, 1999; Haagmans and van der Woude, 2000). OxyR can be invoked to mediate signal transduction between fim and flu according to the following model. The nascent structural components of type 1 fimbriae all contain multiple cysteine (-SH) residues. When fimbriae are being made, several hundred thousand copies of the structural components are transported to the surface with the concomitant formation of S–S disulfide bridges; in effect, a net oxidation that must be accompanied by a similar reduction in order to maintain the cellular status quo (Raina and Missiakas, 1997). Thus, fimbrial biosynthesis and the accompanying S–S bridge formation might be invoked to influence the oxidation level of OxyR and drive it toward the reduced state, which is an active repressor of the flu gene. Fimbriae formation, accompanied by massive disulfide bridge form ation, might therefore constitute a signal that represses the expression of the flu gene via the thiol–disulfide status of OxyR (Figure 2).
Fig. 2. Model describing the coordination of the phase-variable Fim and Ag43 phenotypes via OxyR-relayed thiol–disulfide signal transduction. In the fimbrial phase on state, disulfide bond formation of fimbrial subunit proteins takes place in the periplasm catalysed by DsbA, -B and -C (Raina and Missiakas, 1997; Åslund and Beckwith, 1999; Debarbieux and Beckwith, 1999). The ultimate oxidative potential is linked to the cytoplasmic pool of glutathione via the cytoplasmic membrane-located DsbB (Raina and Missiakas, 1997; Debarbieux and Beckwith, 1999). The thiol–disulfide status of glutathione is monitored by OxyR (Zheng et al., 1998; Storz and Imlay, 1999), which under these conditions is driven to the reduced state and acts as an active repressor of the flu gene. Other auxiliary enzymes are indicated. In the fimbrial phase off state, OxyR can exist as both a reduced and oxidized form, with the result that repression of Ag43 synthesis is relieved.
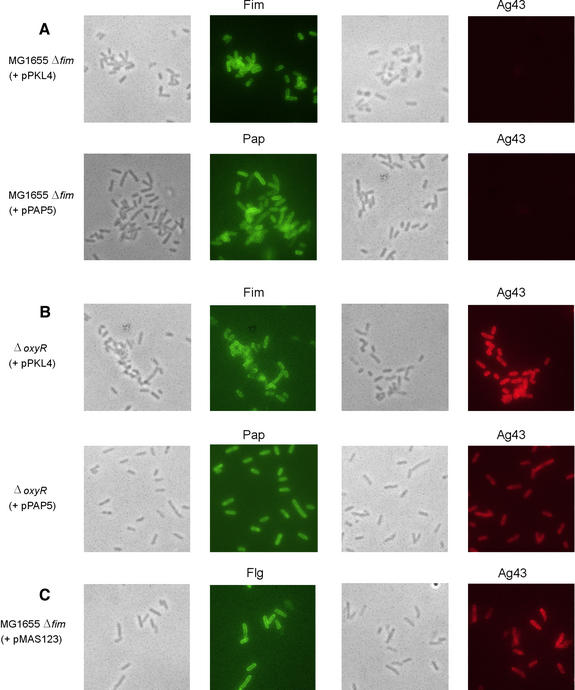
In order to investigate this issue further, Ag43 expression as a function of fimbriation was probed by immunofluorescence microscopy (Figure 3). A dramatic difference in Ag43 expression was observed when MG1655Δfim was transformed with plasmids pPKL4 (encoding the fim gene cluster) and pPAP5 (encoding the pap gene cluster responsible for P fimbriae production), respectively. Virtually none of the cells of the two fimbriae-producing recombinants expressed Ag43, whereas the MG1655Δfim control did. The architecture of P fimbriae and type 1 fimbriae is similar, i.e. both organelles are polymers composed of disulfide bridge-containing proteins. The fact that production of P fimbriae causes abolition of Ag43 production is consistent with our model that production of these organelles communicates with the flu gene via the thiol–disulfide state of OxyR. Production of a third disulfide-containing fimbrial system, F1C, was also shown to have the same negative effect on Ag43 production (data not shown).
Fig. 3. Phase contrast and immunofluorescence microscopy of E.coli Δfim (A) or ΔoxyR (B) strains expressing type 1 (pPKL4) or P (pPAP5) fimbriae employing anti-Fim, -P or -Ag43 serum, respectively. (C) Phase contrast and immunofluorescence microscopy of E.coli strain MG1655Δfim(pMAS123) induced for overexpression of flagella employing anti-flagella and anti-Ag43 serum, respectively.
Fimbriation does not affect Ag43 production in an oxyR background
To examine directly the role of OxyR in communicating the cellular fimbriae status to flu, we monitored the effect of fimbriae production by immunofluorescence microscopy with the same set of fimbriae-encoding plasmids in an oxyR mutant. Here no effect of fimbriation on Ag43 production was observed, arguably due to disrupted communication between the systems in the absence of OxyR (Figure 3). It should also be noted that the expres sion control systems of type 1, P and F1C fimbriae are totally different and unlikely to play any role in the observed cross-talk with flu.
Production of flagella does not affect Ag43 phenotype
Production of flagella is equivalent to fimbriae production in that a similar amount of protein is exported to the bacterial surface. However, the major flagellar component, FliC, does not contain any cysteine residues and flagella production would therefore not be expected to influence the cellular thiol–disulfide status monitored by OxyR. Flagella production in Enterobacteriaceae is controlled ultimately by the flhDC master control locus (Macnab, 1992). Indeed, when we introduced a plasmid, pMAS123, encoding the flhDC locus into MG1655Δfim, a high level of flagellation was observed by immunofluorescence microscopy, whereas Ag43 production was unaffected (Figure 3). This suggested that only production of disulfide-containing organelles such as type 1 and related fimbriae could affect the cellular thiol–disulfide status, whereas large-scale production of surface structures basically devoid of cysteine residues, such as flagella, had no influence. Furthermore, it is comforting to note that Ag43, in spite of its large size (∼110 kDa), does not contain any cysteine residues (Henderson and Owen, 1999) and expression and export of Ag43 would not affect the cellular thiol–disulfide status. In fact, relatively few E.coli proteins that are exported in bulk amounts contain cysteine residues. Indeed the two major outer membrane porins of E.coli, OmpC and OmpF, do not contain any cysteines, and would therefore not affect the OxyR-based signal transduction system described here; type 1 and related fimbriae appear unique in this respect.
Thiol–disulfide signal transduction can be driven by simple reducing agents
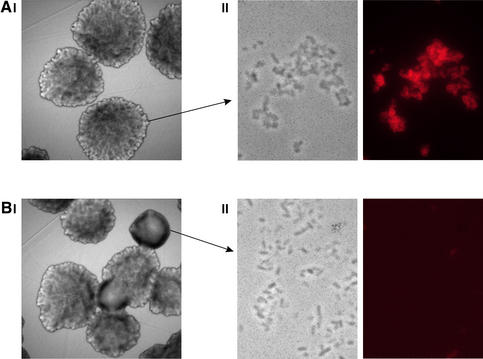
In our model, fimbrial biosynthesis and the accompanying disulfide bridge formation influence the cellular thiol– disulfide status and drive it toward a more reduced state with a simultaneous OxyRox→OxyRred shift causing down-regulation of the flu gene. In line with this, we speculated whether simple reducing agents could influence the cellular thiol–disulfide status in a similar way and influence Ag43 production. As previously mentioned, expression of Ag43 is phase variable; Ag43 production results in a characteristic frizzy colony morphology (Hasman et al., 2000). MG1655Δfim(pfimB) can give rise to two different colony morphology forms: form 1 (Ag43+) with a frizzy surface and form 3 (Ag43–) with a smooth surface. We speculated that it would be possible to influence Ag43 expression by altering the cellular thiol– disulfide status by adding a simple reducing agent such as dithiothreitol (DTT) to the cells. This would, albeit crudely, mimic production of disulfide-containing fimbriae with an OxyRox→OxyRred shift and might cause detectable down-regulation of the flu gene. Aliquots of cells from a form 1 colony were plated out on either normal LB plates or LB plates containing DTT. Progeny on normal LB plates were virtually all of form 1; however, ∼10% (104/953) of the colonies on DTT plates were form 3 (Figure 4). Form 1 and form 3 colonies were verified to consist almost entirely of Ag43+ and Ag43– cells, respectively (Figure 4). This result suggests that Ag43 production can indeed be influenced by manipulation of the redox potential of the growth medium. Also, it lends strong credence to our model invoking biosyn thesis of disulfide-containing surface structures such as fimbriae to constitute a signal that alters the cellular thiol–disulfide status, thereby affecting OxyR and, in turn, Ag43 production.
Fig. 4. Colony morphology and surface expression of Ag43 by MG1655Δfim(pfimB) in the absence (A) and presence (B) of the reducing agent DTT. (I) Phase contrast microscopy of colonies. (II) Phase contrast and immunofluorescence microscopy of cells employing anti-Ag43 serum. Exposure of cells from Ag43-positive (form 1) colonies to DTT resulted in ∼10 times the number of Ag43-negative (form 3) colonies.
Discussion
Escherichia coli is the most frequent cause of urinary tract infection (UTI), and most isolates are able to express several different adhesins that mediate specific binding to the uroepithelium with different receptor specificities. The most common fimbriae on UTI strains are type 1 and P fimbriae, and >80% of the strains are able to express either or both fimbrial types (Nowicki et al., 1984; Gander and Thomas, 1987); ∼8% are able to express F1C fimbriae (Pere et al., 1987). Expression of type 1, P and F1C fimbriae is phase variable, although the underlying regulatory systems are completely different; more specifically, type 1 fimbriae expression is based on DNA inversion, whereas phase variation of P fimbriae depends on a combination of Dam methylation and the PapB and PapI regulators (Forsman et al., 1989; Braaten et al., 1994). F1C expression is similar to that of P fimbriae. Interestingly, expression of P and type 1 fimbriae seems to be coordinated through inter-system cross-talk via PapB in which P fimbriation dominates (Xia et al., 2000). The expression of the Ag43 self-recognizing adhesin is also phase variable through the concerted action of Dam methylase and OxyR. The present results add another dimension of expressional coordination and molecular cross-talk among these phase-variable adhesins.
Our data indicate that production of disulfide-containing fimbriae in E.coli constitutes an en bloc signal that causes repression of the Ag43-encoding flu gene via a novel pathway invoking the thiol–disulfide status of OxyR: in effect, a one-directional signalling system where fimbriation dominates. From a cellular economy perspective, this makes sense because fimbrial display has been shown to abrogate the Ag43-mediated cell aggregating function (Hasman et al., 1999). Arguably, there is no reason to produce copious amounts of Ag43 when the associated cell–cell aggregating phenotype is blocked. Production of fimbriae is phase variable and affected by various environmental cues (Klemm, 1994). Fimbriae confer specific adhesion to receptors on mammalian tissue surfaces, and many strains are able to express several different types of fimbriae. On the other hand, Ag43 expression is involved intimately in bacterial aggregation, and microcolony and biofilm formation (Danese et al., 2000; Kjærgaard et al., 2000; O’Toole et al., 2000). Bacterial biofilms constitute an efficient protective state against host defences (Costerton et al., 1999).
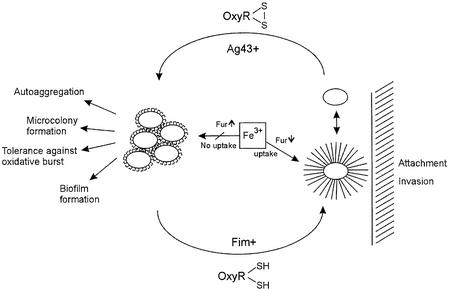
The on↔off phase-variable expression of type 1 fimbriae, P fimbriae and Ag43 might appear at first sight as random phenomena but are now known to be influenced by various environmental cues. Here we report that these processes are highly coordinated with a hierarchic structure: P fimbriation is dominant to type 1 fimbriation and fimbriation in general is dominant to Ag43 expression. The fimbrial phase on allows bacterial attachment to various epithelial targets, and the differential expression of type 1 and P fimbriae through inter-system cross-talk allows differential target and niche selection. The fimbrial phase off allows expression of Ag43, which enhances microcolony and biofilm formation. Finally, the Ag43 phase off allows bacterial dispersal and might lead to colonization of additional sites, thus completing the cycle (Figure 5).
Fig. 5. Model describing the thiol–disulfide-induced niche-specific adaptation of E.coli in host environments. The expression of fimbriae and Ag43 leads to diametrically opposed virulence-associated cellular processes.
It has not escaped our attention that the fimbriate status of the cell (Fim+↔Fim–) can influence a wide range of cellular parameters via the outlined OxyR-mediated signal transduction mechanism, allowing rapid adaptation to new environmental niches (Figure 5). In this respect, it is noteworthy that the oxidized (disulfide) form of OxyR is known to activate the expression of several genes involved in the oxidative stress response (Zheng et al., 1998; Storz and Imlay, 1999). Leukocytes are known to produce oxidative bursts upon bacterial attachment; however, OxyR-activated gene products such as the KatG catalase are ideal to counter this oxidative threat. Furthermore, the oxidized form of OxyR activates global regulators such as fur (Zheng et al., 1999). Free iron is limited in mammalian body fluids, but bacteria have evolved efficient systems for iron uptake (Guerinot, 1994). Fur is the global cellular repressor of ferric ion uptake from the environment (Crosa, 1997). A fimbriated phenotype, promoting the reduced state of OxyR, would be concomitant with reduced activity of the fur gene and derepression of Fe3+ uptake systems. Interestingly, the present fimbriae–OxyR signal transduction mechanism might be invoked to explain the reported enigmatic influence of P fimbriation on iron uptake in E.coli (Zhang and Normark, 1996). Disulfide-containing fimbriae are produced by a wide range of bacteria and OxyR is ubiquitous in bacteria. The present findings might therefore have implications for our general understanding of bacterial signal transduction in environments such as the human body.
Materials and methods
Bacterial strains and plasmids
Escherichia coli K-12 reference strain MG1655 was used in this work. A Δfim derivative of MG1655 was constructed using the temperature-sensitive plasmid pMAS115 (Kjærgaard et al., 2000). Plasmid pfimB (alias pPKL9) contains the fimB recombinase gene under transcriptional control of the tetracycline gene promoter (Klemm, 1986) and was used to ensure ‘on’ orientation of the fim switch. Plasmids pPKL4 (type 1 fimbriae), pPAP5 (P fimbriae) and pPKL143 (F1C fimbriae) have been described previously (Lindberg et al., 1984; Klemm, 1986; Klemm et al., 1994). Plasmid pMAS123 contains the E.coli flhDC flagellar master control genes placed under transcriptional control of the inducible arabinose promoter in the expression vector pBADHisA.
Global gene expression analysis
Cells from glycerol stocks were grown overnight in LB broth at 37°C with shaking. These cultures were then diluted 1:100 and grown under the same conditions to an OD600 of 0.9. Cells were harvested and RNA was extracted using Qiagen RNeasy columns. Enrichment of mRNA and microarray analysis were performed according to the Affymetrix Expression Analysis Technical Manual. The E.coli GeneChip microarrays were purchased from Affymetrix, Santa Clara, CA. Microarray experiments demonstrating the negative effect of type 1 fimbriation on Ag43 expression and the overexpression of Ag43 in the absence of type 1 fimbriae were demonstrated in two independent experiments and essentially the same results were obtained.
Fluorescence microscopy
Cells were grown as described above. In the case of induction for flagella production, cells were grown to an OD600 of 0.4 and then induced for 4 h with 0.2% arabinose. Antibodies against type 1 fimbriae, P fimbriae, F1C fimbriae, Ag43 and flagella were employed to assess surface presentation of corresponding organelles/proteins by immunofluorescence microscopy. As secondary antibody, a fluorescein isothiocyanate- or tetramethyl rhodamine isothiocyanate-labelled anti-rabbit or mouse serum was used. Cell fixation, immunolabelling and microscopy were performed as previously described (Hasman et al., 1999).
DDT induction of Ag43 phase variation
Ag43-expressing (form 1) colonies of MG1655Δfim(pfimB) were resuspended in phosphate-buffered saline (PBS) and diluted to a concentration of 102 c.f.u./ml. The cells were incubated in the presence of 100 mM DTT at room temperature for 30 min and then plated onto LB agar plates containing DTT. These plates were prepared by spreading 100 µl of a 1 M DTT solution onto the surface of the plate just prior to the addition of bacteria. Plates were then incubated overnight at 37°C. As a control, Ag43-expressing colonies were subjected to exactly the same treatment but in the absence of DTT. Colony morphology was viewed with a Carl Zeiss Axioplan epifluorescence microscope and digital images were captured with a 12-bit cooled slow-scan charge-coupled device camera (KAF 1400 chip; photometrics; Tuscon, AZ) controlled by the PMIS software (Photometrics).
Acknowledgments
Acknowledgements
We thank Stanley Brown and Trinad Chakraborty for their constructive comments. This work was supported by the Danish Medical and Natural Sciences Research Councils (grants 9802358 and 51-00-0291).
References
- Åslund F. and Beckwith,J. (1999) Bridge over troubled waters: sensing stress by disulfide bond formation. Cell, 96, 751–753. [DOI] [PubMed] [Google Scholar]
- Blomfield I.C., Calie,P.J., Eberhardt,K.J., McClain,M.S. and Eisenstein,B.I. (1993) Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J. Bacteriol., 175, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield I.C., Kulasekara,D.H. and Eisenstein,B.I. (1997) Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli. Mol. Microbiol., 23, 705–717. [DOI] [PubMed] [Google Scholar]
- Braaten B.A., Nou,X., Kaltenbach,L.S. and Low,D. (1994) Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in Escherichia coli. Cell, 76, 577–588. [DOI] [PubMed] [Google Scholar]
- Choudhury D., Thompson,A., Stojanoff,V., Langermann,S., Pinkner,J., Hultgren,S.J. and Knight,S.D. (1999) X-ray structure of the FimC–FimH chaperone–adhesin complex from uropathogenic Escherichia coli. Science, 285, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Connell H., Agace,W., Hedlund,M., Klemm,P., Schembri,M. and Svanborg,C. (1996) Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl Acad. Sci. USA, 93, 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J.W., Stewart,P.S. and Greenberg,E.P. (1999) Bacterial biofilms: a common cause of persistent infections. Science, 284, 2137–2142. [DOI] [PubMed] [Google Scholar]
- Crosa J.H. (1997) Signal transduction and transcriptional and post transcriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev., 61, 319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese P.N., Pratt,L.A. and Kolter,R. (2000) The outer membrane protein, antigen 43, mediates cell-to-cell binding within Escherichia coli biofilms. Mol. Microbiol., 37, 424–432. [DOI] [PubMed] [Google Scholar]
- Debarbieux L. and Beckwith,J. (1999) Electron avenue: pathways of disulfide bond formation and isomerization. Cell, 99, 117–119. [DOI] [PubMed] [Google Scholar]
- Diderichsen B. (1980) flu a metastable gene controlling the surface properties of Escherichia coli. J. Bacteriol., 141, 858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman K., Göransson,M. and Uhlin,B.E. (1989) Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E.coli pili biogenesis. EMBO J., 8, 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally D.L., Leathart,J. and Blomfield,I.C. (1996) Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol., 21, 725–738. [DOI] [PubMed] [Google Scholar]
- Gander R.M. and Thomas,V.L. (1987) Distribution of type 1 and P fimbriae on uropathogenic Escherichia coli O6. Infect. Immun., 55, 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M.L. (1994) Microbial iron transport. Annu. Rev. Microbiol., 48, 743–772. [DOI] [PubMed] [Google Scholar]
- Haagmans W. and van der Woude,M. (2000) Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol., 35, 877–887. [DOI] [PubMed] [Google Scholar]
- Hasman H., Chakraborty,T. and Klemm,P. (1999) Antigen 43-mediated autoaggregation is blocked by fimbriation. J. Bacteriol., 181, 4834–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasman H., Schembri,M.A. and Klemm,P. (2000) Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol., 182, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I.R. and Owen,P. (1999) The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported proteins and is regulated by a novel mechanism involving Dam and OxyR. J. Bacteriol., 181, 2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjærgaard K., Schembri,M.A., Ramos,C., Molin,S. and Klemm,P. (2000) Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol., 2, 695–702. [DOI] [PubMed] [Google Scholar]
- Klemm P. (1986) Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J., 5, 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. (1994) Fimbriae: Adhesion, Genetics, Biogenesis and Vaccines. CRC Press, Boca Raton, FL.
- Klemm P. and Krogfelt,K.A. (1994) Type 1 fimbriae of Escherichia coli. In Klemm,P. (ed.), Fimbriae: Adhesion, Genetics, Biogenesis and Vaccines. CRC Press, Boca Raton, FL, pp. 9–26
- Klemm P. and Schembri,M.A. (2000) Bacterial adhesins: structure and function. Int. J. Med. Microbiol., 290, 27–35. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen,G., Kreft,B., Marre,R. and Bergmans,H. (1994) Reciprocal exchange of minor components of type 1 and F1C fimbriae results in hybrid organelles with changed receptor specificity. J. Bacteriol., 176, 2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Lund,B. and Normark,S. (1984) Genes of pyelo nephritogenic E.coli required for digalactoside-specific agglutination of human cells. EMBO J., 3, 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R.M. (1992) Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet., 26, 131–158. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Rhen,M., Vaisanen-Rhen,V., Pere,A. and Korhonen,T.K. (1984) Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J. Bacteriol., 160, 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen P.B. and Klemm,P. (1994) Localization of promoters in the fim gene cluster and the effect of H-NS on the transcription of fimB and fimE. FEMS Microbiol. Lett., 116, 95–100. [DOI] [PubMed] [Google Scholar]
- Olsen P.B., Schembri,M.A., Gally,D.L. and Klemm,P. (1998) Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol. Lett., 162, 17–23. [DOI] [PubMed] [Google Scholar]
- O’Toole G., Kaplan,H.B. and Kolter,R. (2000) Biofilm formation as microbial development. Annu. Rev. Microbiol., 54, 49–79. [DOI] [PubMed] [Google Scholar]
- Pere A., Nowicki,B., Saxen,H., Siitonen,A. and Korhonen,T.K. (1987) Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis., 156, 567–574. [DOI] [PubMed] [Google Scholar]
- Raina S. and Missiakas,D. (1997) Making and breaking of disulfide bonds. Annu. Rev. Microbiol., 51, 179–202. [DOI] [PubMed] [Google Scholar]
- Sauer F.G., Mulvey,M.A., Schilling,J.D., Martinez,J.J. and Hultgren,S.J. (2000) Bacterial pili: molecular mechanisms of pathogenesis. Curr. Opin. Microbiol., 3, 65–72. [DOI] [PubMed] [Google Scholar]
- Schembri M.A., Olsen,P.B. and Klemm,P. (1998) Orientation-dependent enhancement by H-NS of the activity of the type 1 fimbrial phase switch promoter in Escherichia coli. Mol. Gen. Genet., 259, 336–344. [DOI] [PubMed] [Google Scholar]
- Selinger D.W., Cheung,K.J., Mei,R., Johansson,E.M., Richmond,C.S., Blattner,F.R., Lockhart,D.J. and Church,G.M. (2000) RNA expression analysis using a 30 base pair resolution Escherichia coli genome array. Nature Biotechnol., 18, 1262–1268. [DOI] [PubMed] [Google Scholar]
- Storz G. and Imlay,J.A. (1999) Oxidative stress. Curr. Opin. Microbiol., 2, 188–194. [DOI] [PubMed] [Google Scholar]
- Xia Y., Gally,D., Forsman-Semb,K. and Uhlin,B.E. (2000) Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J., 19, 1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.P. and Normark,S. (1996) Induction of gene expression in Escherichia coli after pilus-mediated attachment. Science, 273, 1234–1236. [DOI] [PubMed] [Google Scholar]
- Zheng M., Årslund,F. and Storz,G. (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science, 279, 1718–1721. [DOI] [PubMed] [Google Scholar]
- Zheng M., Doan,B., Schneider,T.D. and Storz,G. (1999) OxyR and SoxRS regulation of fur. J. Bacteriol., 181, 4639–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]