Abstract
The Major Facilitator Superfamily lactose transport protein (LacS) undergoes reversible self-association in the detergent-solubilized state, and is present in the membrane as a dimer. We determined the functional unit for proton motive force (Δp)-driven lactose uptake and lactose/methyl-β-d-galactopyranoside equilibrium exchange in a proteoliposomal system in which a single cysteine mutant, LacS-C67, defective in Δp-driven uptake, was co-reconstituted with fully functional cysteine-less protein, LacS-cl. From the quadratic relationship between the uptake activity and the ratio of LacS-C67/LacS-cl, we conclude that the dimeric state of LacS is required for Δp-driven uptake. N-ethylmaleimide (NEM) treatment of proteoliposomes abolished the LacS-C67 exchange activity but left the LacS-cl unaffected. After NEM treatment, the exchange activity decreased linearly with increasing ratios of LacS-C67/LacS-cl, suggesting that the monomeric state of LacS is sufficient for this mode of transport. We propose that the two subunits of LacS are functionally coupled in the step associated with conformational reorientation of the empty binding site, a step unique for Δp-driven uptake.
Keywords: cooperative/dimer/membrane protein/quaternary structure/sugar transport
Introduction
Secondary transporters, including uniporters, symporters and antiporters, catalyze transport of solutes across the membrane in dependence of the electrochemical gradients of the translocated solutes. The largest family of this class of transporters is the Major Facilitator Superfamily (MFS), including over 1000 members from all three kingdoms of living organisms and with specificities ranging from sugars or compatible solutes to drugs (Pao et al., 1998; Saier, 2000). Within the MFS, the lactose transport protein LacS from Streptococcus thermophilus belongs to the galactoside-pentoside-hexuronide (GPH):cation symporter family (Poolman et al., 1996).
LacS catalyzes the transport of galactose and a wide range of C1-substituted galactosides in different modes of transport, e.g. proton motive force (Δp)-driven sugar/proton symport, giving rise to accumulation of substrate, and carrier-mediated equilibration of sugars via exchange transport (Poolman et al., 1995; Veenhoff and Poolman, 1999). As, in growing cultures, lactose uptake results in the stoichiometric excretion of galactose, lactose/galactose exchange has been proposed to be the physiologically most relevant reaction. The activity of LacS is regulated as a function of the energy state of the cell and the availability of substrate in the medium, by phosphorylation of the cytoplasmic IIA domain (Gunnewijk et al., 1999; Gunnewijk and Poolman, 2000a,b). This IIA domain is not present in all members of the GPH family and is not required for translocation activity of LacS (Poolman et al., 1995).
Most carriers from the MFS and all members of the GPH family have a predicted fold of 12 putative transmembrane-spanning (TMS) α-helices. In the absence of high-resolution structural data, structure–function relationships are based largely on site-directed mutants, selection of second-site suppressors, cross-linking and spectroscopic measurements. This has led to detailed topological information, the identification of binding sites or catalytically important residues, and helix packing models (Sahin-Tóth et al., 2000; Veenhoff et al., 2000). The quaternary or oligomeric structure, however, has been addressed for only a few proteins from the MFS, and for even fewer has the functional unit been convincingly defined. Often, the structural and functional unit of secondary transporters is thought to be a 10–14 TMS α-helical bundle (Maloney, 1990; Paulsen et al., 1998). Examples are the monomeric 12 TMS α-helical lactose carrier LacY and the sugar-phosphate antiporter UhpT from Escherichia coli (Ambudkar et al., 1990; Sahin-Tóth et al., 1994), the dimeric six TMS α-helical mitochondrial transport proteins (Schroers et al., 1998), and probably also the 14 TMS α-helical Na+/glucose cotransporter SGLT1 from the intestinal brush border membranes (Eskandari et al., 1998). Secondary transport proteins with 12 or 14 TMS α-helices that are proposed to exist in the membrane as oligomeric structures are the secretory Na+-K+-2Cl– cotransporter NKCC1 (Moore-Hoon and Turner, 2000), the tetracycline cation/proton antiporter TetA (McMurry and Levy, 1995; Yin et al., 2000), the erythrocyte anion transport system Band 3 (Casey and Reithmeier, 1991; Wang et al., 1994), the erythrocyte glucose transport protein GLUT1 (Hebert and Carruthers, 1992; Zottola et al., 1995), the renal Na+/H+ exchanger NHE1 (Fafournoux et al., 1994), and the NhaA Na+/H+ antiporter from E.coli (Williams, 2000; Gerchman et al., 2001).
The quaternary structure of LacS has been determined in the detergent-solubilized state using analytical ultracentrifugation, and in the membrane-reconstituted state using freeze–fracture electron microscopy analysis and saturation-transfer electron spin resonance spectroscopy (Friesen et al., 2000; Spooner et al., 2000). These studies have shown that dodecyl-maltoside solubilized LacS undergoes reversible self-association and that the membrane-embedded protein is dimeric. In this study, we determine the functional unit of LacS in proteoliposomes, in which Δp-driven uptake and/or exchange transport in one of the two co-reconstituted species is selectively inactivated by mutation or chemical modification.
Results
Negative dominance to study oligomeric function
The question regarding the functional unit of the LacS transport system was addressed by measuring the activity of heterodimers consisting of one active and one inactive LacS molecule. Providing that homo- and heterodimers are formed randomly upon mixing of active and inactive species, the activity of the heterodimer can be deduced from the total activity measured. If the minimal functional unit is the monomer, one expects heterodimers to be 50% active compared with homodimers of active species. In other words, the activity will decrease linearly with increasing ratio of inactive over active species. If, on the other hand, the dimer is the minimal functional unit then only homodimers of the active species will be active and the total activity will decrease quadratically with an increasing ratio of inactive over active species.
LacS is readily purified and highly stable in detergent solution. Reconstitution into artificial lipid bilayers yields proteoliposomes in which LacS is incorporated in a unidirectional orientation (Knol et al., 1996, 1998). The functional unit of LacS was determined for both lactose/methyl-β-d-galactopyranoside (TMG) exchange and Δp-driven lactose uptake. The two LacS species used were the cysteine-less LacS(C320A) and a single cysteine mutant LacS(C320A, E67C), hereafter referred to as LacS-cl and LacS-C67, respectively.
Site-directed manipulation of exchange activity by LacS-C67 and LacS-cl
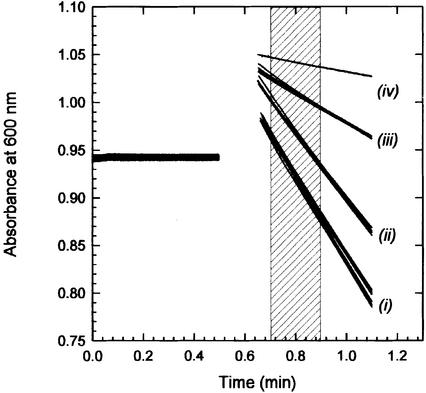
Lactose/TMG exchange was followed with a spectroscopic assay that monitors the reduction of the electron acceptor 2,6-dichloroindophenol (Cl2Ind) upon oxidation of transported lactose by internal PQQ-dependent soluble glucose dehydrogenase (sGDH) (Heuberger and Poolman, 2000). Since TMG is not a substrate of sGDH, this lactose analog was used as a counter substrate in the exchange reaction by pre-loading the proteoliposomes with TMG. Figure 1 shows the lactose/TMG exchange activity of three different batches of proteoliposomes, each measured in duplicate, demonstrating the excellent reproducibility of the measurements. The transport rate was estimated from the decrease in absorbance between 0.2 and 0.4 min after the start of the reaction (shaded area). The decrease in absorbance upon addition of glucose is due to a small fraction of sGDH associated with the external surface of the proteoliposomes (lines iv). Since glucose is not a substrate of LacS, the measurements are readily corrected for this background activity. LacS-cl (lines i) and LacS-C67 (lines ii) catalyze lactose/TMG exchange at 1157 ± 36 and 901 ± 34 nmol/min/mg of LacS, respectively. After site-directed cysteine modification with N-ethyl maleimide (NEM), the activity of LacS-C67 was 302 ± 22 nmol/min/mg (lines iii). The exact mechanism of inactivation is unknown, but as residue 67 is part of the substrate translocation pathway, we propose that site-directed modification with the relatively small alkylating reagent obstructs binding and/or transport without modifying the overall structure (Poolman et al., 1996; Veenhoff et al., 2000). As cysteine-less LacS remained unaffected by NEM treatment (Figure 1, lines i), the fraction of LacS-C67 in proteoliposomes containing both LacS-C67 and LacS-cl can specifically be inactivated.
Fig. 1. TMG/lactose exchange by LacS-C67, NEM-labeled LacS-C67 and LacS-cl. Transport of lactose was followed in TMG-loaded proteoliposomes by measuring the decrease in A600 as a result of reduction of Cl2Ind, following oxidation of transported lactose by internal sGDH. TMG was used as a counter substrate in the exchange reaction as this lactose analog is not oxidized by sGDH. The exchange reaction was started at timepoint 0.5 min by dilution of 10 µl of proteoliposomes into 500 µl of 50 mM KPi pH 7.0, with 50 µM Cl2Ind plus 5 mM lactose (lines i, ii and iii) or glucose (lines iv). Per mutant the uptake was measured for three different proteoliposome batches, each batch was assayed twice. Lines ‘i’ represent the uptake of lactose in proteoliposomes containing LacS-cl, before and after labeling with NEM; lines ‘ii’ and ‘iii’ are proteoliposomes containing LacS-C67 and NEM-labeled LacS-C67, respectively. The uptake rates were determined from the linear decrease in absorbance between 0.7 and 0.9 min (shaded area).
LacS-cl/LacS-C67 heterodimer formation
Complete modification of LacS-C67 with NEM was confirmed by subsequent labeling with 2-(4′-maleimidyl anilino)naphthalene-6-sulfonic acid (Mal-Ans), a maleimide derivative that becomes fluorescent upon reaction with thiols. The increase in fluorescence after NEM labeling of LacS-C67 was very low and comparable to that of cysteine-less LacS (Table I). Using sedimentation velocity centrifugation analysis, the weight average sedimentation coefficients of purified n-dodecyl-β-d- maltoside (DDM)-solubilized LacS-C67 and NEM-labeled LacS-C67 were determined for the protein concentration range of 0.02–1.5 mg/ml (Figure 2; Table I). The association constants were 7.2 ml/mg and 8.5 ml/mg for LacS-C67 and NEM-labeled LacS-C67, respectively, which is within the error of the value for wild-type LacS (5.4 ± 3.6 ml/mg) (Friesen et al., 2000). We conclude that in solution LacS-C67, LacS-C67-NEM and LacS-cl undergo self-association with similar association constants. Upon mixing of LacS-C67 and LacS-cl, homo- and heterodimers will thus be formed equally efficiently. The relatively high KA for dimerization of the protein in the presence of DDM favors the rapid equilibration of the different dimeric LacS species. To form proteoliposomes with randomly distributed homo- and heterodimers, the different species of detergent-solubilized and purified protein were mixed and incubated for 2 h before the reconstitution.
Table I. Association constants for LacS dimerization, integrity of the proteoliposomes and efficiency of NEM labeling.
| LacS-wt | LacS-cl | LacS-C67 | LacS-C67-NEM | |
|---|---|---|---|---|
| Association constants (ml/mg) | 5.4 ± 3.6a | ndb | 7.2 | 8.5 |
| [14C]lactose efflux from proteoliposomes (% loss in 40 s)c | nd | <2% | <2% | <2% |
| ΔpH in proteoliposomes (in pH units) | ||||
| at time zero | nd | 2.4 | 2.4 | nd |
| after 40 s | nd | 2.35 | 2.33 | nd |
| Mal-Ans labeling rate | ||||
| fluorescence units/min | nd | 0.02 | 0.86 | 0.02 |
| total fluorescence developed | nd | <8% | 100% | <5% |
aFrom Friesen et al. (2000).
bNot determined.
cEfflux of [14C]lactose was measured in the absence and presence of a Δp under conditions identical to the exchange and Δp-driven uptake experiments.
Fig. 2. Dimerization of LacS-C67 and NEM-labeled LacS-C67. Weight average sedimentation coefficients of DDM-solubilized LacS-C67 (squares) and NEM-labeled LacS-C67 (circles) as a function of protein concentration were determined by analytical ultracentrifugation. The lines are least square fits assuming a monomer to dimer mode of association; residuals are plotted as the observed minus the calculated values.
A monomer is the functional unit for galactoside exchange
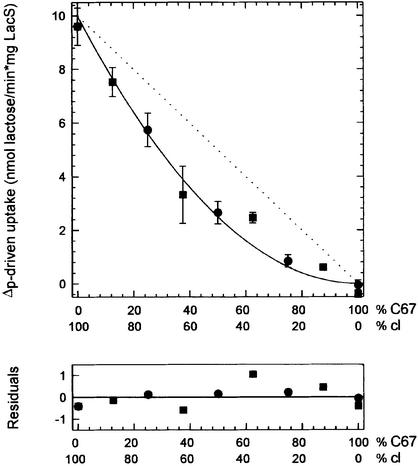
Membrane reconstitution with different ratios of LacS-C67 and LacS-cl yielded proteoliposomes in which the lactose/TMG exchange activity decreased linearly when the fraction LacS-C67 was increased from 0 to 100% (Figure 3). After labeling the same proteoliposomes with NEM, the activity dropped linearly with increasing amount of LacS-C67. Each point represents the average activity of duplicate measurements with three different batches of proteoliposomes, for which the standard error of the measurements is indicated. The circles and squares are from independent sets of experiments. The dotted line indicates the activity that would have been observed if homodimers of active LacS species were required for exchange, assuming that the distribution of LacS-C67 and LacS-cl over the population heterodimers and homodimers was random. As the data clearly fit to a linear curve, they strongly suggest that LacS-cl in heterodimers transports as efficiently as the molecules present in LacS-cl/LacS-cl homodimers.
Fig. 3. TMG/lactose equilibrium exchange in proteoliposomes with different LacS-C67/LacS-cl ratios. Lactose/TMG exchange transport activity was measured as described in the legend to Figure 1. Each point is the average of three different proteoliposome batches and each sample was measured twice; the standard errors of the measurements are indicated. Circles and squares correspond to independent sets of experiments. Open and closed symbols represent the activity measured before and after treatment with NEM, respectively. The data could be fitted with linear regression; the residuals are plotted as the observed minus the calculated values. The dotted line represents the predicted activity when both LacS species randomly form dimers but only the LacS-cl homodimer would be active.
Dimeric LacS is the functional unit for Δp-driven uptake
LacS-C67 is unable to accumulate lactose in response to a Δp as a result of the glutamate to cysteine mutation in helix II, a region that is involved in coupling sugar and proton transport in this family of transporters (Poolman et al., 1996; Veenhoff et al., 2000). The transport activity of three different batches of LacS-cl proteoliposomes showed a 10-fold accumulation of lactose inside the proteoliposomes in 40 s, corresponding to an activity of 10 nmol/min/mg LacS, whereas the LacS-C67 proteoliposomes showed no accumulation, and only equilibration of external and internal sugar was observed (Figure 4). Since failure to accumulate solutes against a concentration gradient can be due to sugar translocation without concomitant proton translocation (ES leak pathway; for details see Lolkema and Poolman, 1995) or carrier-mediated proton leakage (EH leak), a number of control experiments were performed first. If the C67 mutation introduces a high proton leak, the artificially generated pH gradient, and thus the driving force for uptake, would decrease with increasing LacS-C67/LacS-cl. The pH gradient in LacS-C67 and LacS-cl-containing proteoliposomes was determined by monitoring the fluorescence of the pH indicator pyranine. The ΔpH generated was ∼2.4 pH units in both LacS-C67 and LacS-cl proteoliposomes, and constant over the time period (40 s) used for the activity measurements (Table I). This shows that the failure of LacS-C67 to accumulate lactose inside the proteoliposomes is not due to a lowered ΔpH. One can thus safely assume that the Δp-driven uptake in proteoliposomes with heterodimer complexes (below) is not affected by variations in the Δp. Since the net uptake rate is determined by Δp-driven uptake and lactose efflux down the concentration gradient, it was also important to establish whether or not LacS-cl and LacS-C67 exhibit similar efflux activities. The rates of exit of lactose from LacS-C67 or LacS-cl proteoliposomes were comparable and insignificant on the time scale (40 s) of the uptake measurements (Table I).
Fig. 4. Δp-driven uptake by LacS-C67 and LacS-cl. The accumulation of [14C]lactose in LacS-C67 (open symbols) and LacS-cl (closed symbols) proteoliposomes in response to an artificial Δp was measured in three batches of proteoliposomes for each of the mutants (triangles, squares and circles).
In the proteoliposomes with different LacS-cl/LacS-C67 ratios, the Δp-driven lactose accumulation dropped quadratically from 10 to 0 nmol/min/mg of LacS (Figure 5). The data of independent sets of experiments are shown as circles and squares, and each point represents the average activity of three different batches of proteoliposomes; the standard error of the measurements is indicated. The dependency of the activity of the LacS-cl/LacS-C67 ratio indicates that the LacS-C67/LacS-C67 homodimers and the LacS-cl/LacS-C67 heterodimers do not facilitate Δp-driven uptake. We conclude that for Δp-driven uptake two functional monomers must be associated in a dimer in order to generate an active functional unit. NEM treatment of the proteoliposomes did not alter the measured activities (not shown), indicating that cooperativity is unaffected by modification of LacS-C67 with NEM.
Fig. 5. Δp-driven uptake in proteoliposomes with different LacS-C67/LacS-cl ratios. Δp-driven [14C]lactose transport activity was measured as described in the legend of Figure 4. Each point is the average of three different proteoliposome batches, and the standard errors are indicated. Circles and squares correspond to independent sets of experiments. The data could be fitted with a quadratic function, corresponding to a situation in which the heterodimers and LacS-C67 homodimers are inactive; residuals are plotted as the observed minus the calculated values.
Discussion
Compared with soluble proteins, membrane proteins reside in an environment where processes like protein localization, orientational restriction and volume exclusion enhance self-association of the molecules (Grasberger et al., 1986). To determine the functional unit of a membrane protein is not simple as the oligomeric state in the membrane can not easily be manipulated or related to a particular activity. In this study we show that the dimeric state of LacS, a lactose transport protein from the GPH family of Major Facilitators, is the functional unit for lactose/H+ symport (Δp-driven uptake), whereas the individual subunits of dimeric LacS are sufficient for galactoside exchange.
Purification and subsequent membrane reconstitution of LacS-cl and LacS-C67 at different ratios yielded proteoliposomes in which lactose/TMG exchange and Δp-driven lactose accumulation could be measured accurately and reproducibly (Figures 1 and 4). Random homo- and heterodimer formation between LacS-cl and LacS-C67 or NEM-modified LacS-C67 is expected, since analytical ultracentrifugation experiments showed that the association constants for dimer formation of the different species is the same as for wild-type protein (Figure 2). Hetero dimer formation upon co-reconstitution of LacS-cl and LacS-C67 was confirmed by the dominant-negative effect of LacS-C67 in the Δp-driven transport reaction (Figure 5). Surprisingly, NEM-inactivated LacS-C67 did not result in a dominant-negative effect when the exchange mode of transport was assayed (Figure 3). Although we can not fully exclude the possibility that heterodimers are 50% active because the effect of inactivation of LacS-C67 is somehow partly compensated by the activity of LacS-cl, we favor the possibility that the lack of a dominant-negative effect is due to the lack of cooperativity within the dimer. We propose that one monomer of LacS is the functional unit for exchange transport and thus capable of forming the sugar binding site and translocation pathway. The apparent cooperativity observed in Δp-driven uptake is thus more likely to be a kinetic effect rather than a structural requirement for the formation of binding sites and/or a translocation pathway.
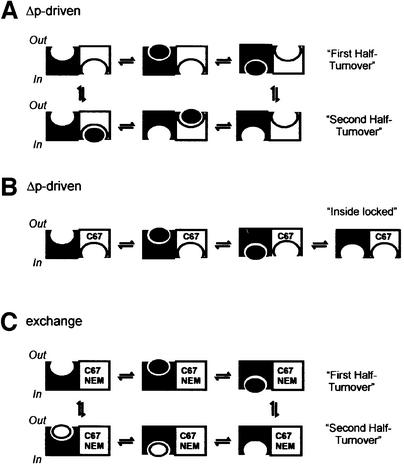
Lactose/TMG exchange and lactose/H+ symport are described by different catalytic cycles which involve a number of common steps (for details see Poolman et al., 1995). One cycle of Δp-driven uptake comprises a fast conformational change of the binding site from outside-facing to inside-facing, that is, the reorientation of the ternary enzyme (carrier)–lactose–proton complex (ESHout→ ESHin), and one slow conformational change involving the reorientation of the unloaded carrier (Ein→Eout). In catalyzing exchange transport, the carrier always changes orientation of the binding sites via the ternary complexes (ESHout→ESHin and ESHin→ESHout) (Poolman et al., 1995). We propose that it is the reorientation of the empty carrier, Ein→Eout, that is dependent on oligomeric structure, whereas reorientation of the ternary complex is not, or is only marginally. One could envisage that binding and reorientation of the ternary complex of one monomer in the dimer is coupled to the reorientation of the unloaded carrier in the other half of the dimeric molecule (Figure 6A). At any time, the substrate binding sites within the dimer would face opposite sides of the membrane. Since in Δp-driven uptake the substrate is donated to the outside-facing binding site only, it requires two half-turnovers involving both subunits before the system has returned to the initial configuration (Figure 6A). The Cys67 mutation would slow down the isomerization of the empty binding site or abolish the acceleration of this step by the membrane potential. The effect of the mutation would be not only confined to LacS-C67 but also communicated via the dimer interface to the LacS-cl subunit. As a result, after one half-turnover of LacS-cl, the heterodimer would be locked in a conformation with the two binding sites facing inward (Figure 6B). Cys67, either modified or not with NEM, would not prevent the liganded binding site isomerizing, and consequently two half-turnovers of exchange transport via one subunit would bring the system back to the initial configuration (Figure 6C).
Fig. 6. Schematic presentation of Δp-driven uptake by LacS-cl homodimers (A) and LacS-cl/LacS-C67 heterodimers (B), and exchange by LacS-cl/LacS-C67-NEM heterodimers (C). For simplicity, only the sugar ligand is shown; black and white ovals represent ligands from external and internal compartments, respectively. A dimer of LacS is shown as a white and black subunit. In the LacS-cl homodimer, catalyzing Δp-driven lactose uptake, reorientation of the liganded binding site from outside-facing to inside-facing is coupled to the opposite reorientation of the empty binding site in the other subunit (A). In a heterodimer with inactive LacS-C67, LacS-cl is unable to isomerize back to the outside-facing conformation without internal ligand (B). If, on the other hand, sugar ligand is present on the inside as well as outside (exchange mode of transport), the active subunit is capable of reorienting its binding sites in both directions (C).
Functional aspects of oligomerization have only been addressed for a few other members of the MFS. Mostly in vivo negative dominance or complementation studies are used to demonstrate oligomeric function, but often the data are ambiguous or inconsistent. Nevertheless, the minimal functional unit of the lactose carrier LacY (Sahin-Tóth et al., 1994) and the sugar-phosphate antiporter UhpT from E.coli (Ambudkar et al., 1990) is thought to be the monomer, whereas the human erythrocyte glucose transporter GLUT1 (Zottola et al., 1995) and the tetracycline cation/proton antiporter TetA (Rubin and Levy, 1990) are proposed to function as oligomers. Especially for the human erythrocyte glucose transporter GLUT1, the observations are consistent with a minimal functional unit larger than a monomer; two dimers facing opposite sides of the membrane are proposed to act cooperatively in the tetramer (Zottola et al., 1995). As observed for LacS, cooperativity is not evident in the exchange mode of transport, and it has been suggested that the reorientation of the empty carrier is coupled to the reorientation of a fully loaded carrier.
In conclusion, we show that the lactose transport protein of S.thermophilus, consisting of 12 TMS α-helices per monomer, catalyzes Δp-driven uptake only as dimer, whereas the functional unit for exchange transport is the monomer. In order to increase our understanding of the action mechanism of the members of the MFS, consideration of the functional unit for the different modes of transport seems a topic that requires further attention.
Materials and methods
Materials
d-(glucose-1-)[14C]lactose (2.11 teslabecquerel/mol) was obtained from the Radiochemical Center, Amersham Pharmacia Biotech; Ni-nitrilotriacetic acid resin was from Qiagen, Inc.; Bio-Beads SM-2 were from Bio-Rad; Triton X-100 was from Amersham Pharmacia Biotech; and DDM was from Anatrace Inc. Total E.coli lipids and egg yolk l-α-phosphatidylcholine were obtained from Avanti Polar Lipids and Sigma, respectively. NEM was purchased from Sigma. sGDH was a generous gift from Prof. J.A.Duine from the Technical University of Delft. All other materials were reagent grade and obtained from commercial sources.
Strains, plasmids and growth conditions
pGKhis(C320A, E67C) was constructed by exchange of the AatII–PstI fragment from pSKE8his(C310A, E67C) (Veenhoff et al., 2000) to pGKhis (Knol et al., 1996). LacS(C320A, E67C) and LacS(C320A) were overexpressed in S.thermophilus ST11ΔlacS, using the plasmids pGKhis(C320A, E67C) and pGKhis(C320A) as described (Knol et al., 1996).
Purification and reconstitution
Purification of the His-tagged LacS(C320A) and LacS(C320A, E67C) for reconstitution purposes was carried out by a Ni-NTA chromatography step in DDM as described by Knol et al. (1996), with some modifications (Friesen et al., 2000). The incubation of the solubilisate with the column material was prolonged to 1 h. Peak fractions containing >1 mg of LacS/ml were used for membrane reconstitution without prior concentration. Absorption spectra from 240–340 nm were taken on a Varian Cary 100 Bio UV-visible spectrophotometer to estimate protein concentration and possible aggregation; the extinction coefficient of LacS is 76 320 M–1cm–1. Protein samples were adjusted to 0.75 mg/ml with elution buffer. Samples of 200 µl of purified protein, consisting of different ratios of LacS(C320A) and LacS(C320A, E67C), were prepared and allowed to equilibrate for 2 h at room temperature. Membrane reconstitution at a protein to lipid ratio (w/w) of 1:53 was performed essentially as described by Knol et al. (1996), but the following modifications were made to improve the reproducibility. Two millilitres of Triton X-100-destabilized liposomes of 4 mg/ml of purified lipids [E.coli lipids and egg yolk l-α-phosphatidyl choline in a 3:1 (w/w) ratio] were added to 200 µl of purified LacS, and the mixture was incubated under mild shaking at room temperature for 30 min. To remove the detergent, 33 mg of Biobeads were added and the incubation was continued under mild shaking at room temperature for 30 min. After another addition of 33 mg of Biobeads, the samples were transferred to mild shaking at 4°C for the remaining time of the reconstitution procedure. After 1 h of incubation another 40 mg of Biobeads were added and the mixture was incubated overnight. Finally, 60 mg of Biobeads were added and the proteoliposomes were incubated for another 2 h. The proteoliposomes were collected by centrifugation (280 000 g, 25 min at 4°C) and prepared for the transport experiments. When LacS was purified for analysis in the analytical ultracentrifuge, a second purification step involving Q-Sepharose fast flow chromatography was performed, and the protein was concentrated to 1.8–2 mg/ml using Microcon-100 filters with a cut-off of 100 kDa as described (Friesen et al., 2000). Protein samples were dialyzed overnight against 1000 vols of 100 mM potassium phosphate (KPi) pH 7.0, with 2 mM K-EDTA plus 0.05% DDM (w/v).
Analytical ultracentrifugation
Sedimentation velocity experiments were performed in a Beckman Optima XL-1 analytical ultracentrifuge using AN-50 Ti eight place rotor with two-channel charcoal-filled centerpieces at 20°C and 38 000 r.p.m., as described (Friesen et al., 2000). Data were collected at 280 or 230 nm in a continuous mode with radial step size of 0.005 cm at 6 min time intervals. Observed sedimentation coefficients were determined from the midpoint of the sedimentation boundaries as described by Arisaka and Van Holde (1979).
Transport activity measurements
Δp-driven lactose uptake. Following the membrane reconstitution procedure, the proteoliposomes were resuspended in 100 mM potassium acetate, 20 mM KPi pH 6.5 plus 2 mM MgSO4, and frozen in liquid nitrogen. After slowly thawing at room temperature, the samples were extruded through a 400 nm filter, washed and resuspended in the same buffer into samples of 100 mg exactly. The samples were adjusted to 100 mg rather than to a specific volume, as weight determination of these viscous proteoliposome samples is more accurate. Aliquots of 5 µl were diluted into 200 µl of 120 mM Na-PIPES pH 6.5, 2 mM MgSO4 plus 0.5 µM valinomycin and 7 µM [14C]lactose. The reaction was stopped by rapid dilution–filtration as described (Knol et al., 1996).
TMG/lactose exchange. Proteoliposomes were prepared essentially as described by Heuberger and Poolman (2000), except that samples were incubated at 50°C in the presence of EDTA to inactivate external sGDH; this step has no influence on the LacS activity (E.H.M.L.Heuberger, personal communication). In short, after the membrane reconstitution, the proteoliposomes were resuspended in 400 µl of 50 mM KPi pH 7.0, 1 mM MgSO4 plus 65 µg/ml sGDH, and frozen in liquid nitrogen. After slowly thawing at room temperature, the samples were extruded through a 400 nm filter, washed in 50 mM KPi pH 8.0, 2 mM K-EDTA and resuspended in 400 µl of the same buffer. After 30 min of incubation at 50°C, the samples were washed twice with 50 mM KPi pH 7.0, once with 50 mM KPi pH 7.0, 2 mM MgSO4 plus 10 mM TMG, and resuspended in the same buffer to exactly 100 mg. The exchange reaction was started by dilution of 10 µl of proteoliposomes into 500 µl of 50 mM KPi pH 7.0, 50 µM Cl2Ind plus 5 mM lactose. The decrease in absorbance at 600 nm of Cl2Ind was measured on a Varian Cary 100 Bio UV-visible spectrophotometer at 25°C. The background decrease in absorbance due to external sGDH was subtracted from all measurements, taking into account that at 5 mM concentration the background hydrolysis of lactose is 15% lower than that of glucose due to differences in Km and Vmax (Heuberger and Poolman, 2000). The extinction coefficient of Cl2Ind (20 600 M–1cm–1) was used to convert the absorbance decrease into the amount of lactose hydrolyzed. The actual rates of lactose uptake were obtained by multiplication of the amount of lactose hydrolysis by a factor of 1.66, because in solution the ratio of α- over β-anomer is 2:3, and only the β-anomer is converted by sGDH, whereas both are transported by LacS.
Efflux down the concentration gradient. Proteoliposomes were prepared as described for Δp-driven uptake either in 100 mM potassium acetate, 20 mM KPi pH 6.5, 2 mM MgSO4 plus 0.6 mM [14C]lactose, or in 50 mM KPi pH 7.0, 2 mM MgSO4 plus 5 mM [14C]lactose. Efflux was measured at 30°C by dilution of 6 µl of the potassium acetate-preloaded proteo liposomes into 200 µl of 120 mM Na-PIPES pH 6.5, 2 mM MgSO4 plus 0.5 µM valinomycin, or 2 µl of KPi-preloaded proteoliposomes into 200 µl of 50 mM KPi pH 7.0.
Cysteine modification
For analytical ultrcentrifugation. A 10-times molar excess of NEM was added to purified and concentrated LacS, and the sample was incubated for 20 min at room temperature before overnight dialysis. To confirm that the labeling with NEM was quantitative, the dialyzed protein was diluted into 50 mM KPi pH 8.0, 100 mM NaCl, 0.05% (w/v) DDM, to a concentration of 0.6 µM of LacS, and, after 5 min of equilibration, 8 µM of Mal-Ans was added and the development of fluorescence was followed. The labeling was measured at 30°C on an SLM Aminco SPF-500™ spectrofluorometer at excitation and emission wavelengths of 328 and 416 nm, respectively, and slit widths of 4 nm.
For activity measurements. Proteoliposomes were labeled with a final concentration of 0.7 mM NEM at room temperature for 1 h. Kinetic analysis of the decrease of LacS-C67 exchange activity in proteoliposomes indicated that the labeling was complete in ∼10 min, and the residual activity remained stable for days.
ΔpH measurement
The magnitude and stability of the ΔpH generated in proteoliposomes was measured with the pH-sensitive fluorophore pyranine. Proteoliposomes were prepared as described for measurement of Δp-driven lactose uptake, except that freeze, thaw and extrusion steps were performed in the presence of 20 mM KPi pH 6.5, 100 mM potassium acetate, 2 mM MgSO4 plus 0.5 mM pyranine. External pyranine was removed by extensive washing of the proteoliposomes. The internal pH was followed by measuring the fluorescence (excitation at 461 ± 4 nm, emission at 511 ± 4 nm) after dilution of 10 µl proteoliposomes into 400 µl Na-PIPES pH 6.5, 2 mM MgSO4 plus 0.5 µM valinomycin. A correlation between the fluorescence signal and the pH was made after complete dissipation of the pH gradient by 0.5 µM nigericin and subsequent titration with NaOH in parallel with pH measurements.
Acknowledgments
Acknowledgements
We thank R.H.E.Friesen and W.N.Konings for helpful discussions and G.T.Robillard for critical reading of the manuscript. This work was supported by funding from the European Community (Grants BI0-4-CT-960129 and -960439) and a ‘Jonge chemici’ grant from the Netherlands Organization for Scientific Research (NWO) under the auspices of the Council for Chemical Sciences.
References
- Ambudkar S.V., Anantharam,V. and Maloney,P.C. (1990) UhpT, the sugar phosphate antiporter of Escherichia coli, functions as a monomer. J. Biol. Chem., 265, 12287–12292. [PubMed] [Google Scholar]
- Arisaka F. and Van Holde,K.E. (1979) Allosteric properties and the association equilibria of hemocyanin from Callianassa californiensis. J. Mol. Biol., 134, 41–73. [DOI] [PubMed] [Google Scholar]
- Casey J.R. and Reithmeier,R.A.F. (1991) Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J. Biol. Chem., 266, 15726–15737. [PubMed] [Google Scholar]
- Eskandari S., Wright,E.M., Kreman,M., Starace,D.M. and Zampighi,G.A. (1998) Structural analysis of cloned plasma membrane proteins by freeze–fracture electron microscopy. Proc. Natl Acad. Sci. USA, 95, 11235–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafournoux P., Noël,J. and Pouysségur,J. (1994) Evidence that the Na+H+ exchanger isoforms NHE1 and NHE3 exist as stable dimers in membranes with a high degree of specificity for homodimers. J. Biol. Chem., 269, 2589–2596. [PubMed] [Google Scholar]
- Friesen R.H.E., Knol,J. and Poolman,B. (2000) Quaternary structure of the lactose transport protein of Streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem., 275, 33527–33535. [DOI] [PubMed] [Google Scholar]
- Gerchman Y., Rimon,A., Venturi,M. and Padan,E. (2001) Oligomeriz ation of NhaA, the Na+/H+ antiporter of Escherichia coli in the membrane and its functional and structural consequences. Biochemistry, 40, 3403–3412. [DOI] [PubMed] [Google Scholar]
- Grasberger B., Minton,A.P., DeLisi,C. and Metzger,H. (1986) Inter action between proteins localized in membranes. Proc. Natl Acad. Sci. USA, 83, 6258–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnewijk M.G.W. and Poolman,B. (2000a) HPr(His∼P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem., 275, 34080–34085. [DOI] [PubMed] [Google Scholar]
- Gunnewijk M.G.W. and Poolman,B. (2000b) Phosphorylation state of HPr determines the level of expression and the extent of phosphorylation of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem., 275, 34073–34079. [DOI] [PubMed] [Google Scholar]
- Gunnewijk M.G.W., Postma,P.W. and Poolman,B. (1999) Phosphoryl ation and functional properties of the IIA domain of the lactose transport protein of Streptococcus thermophilus. J. Bacteriol., 181, 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D.N. and Carruthers,A. (1992) Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J. Biol. Chem., 267, 23829–23838. [PubMed] [Google Scholar]
- Heuberger E.H.M.L. and Poolman,B. (2000) A spectroscopic assay for the analysis of carbohydrate transport reactions. Eur. J. Biochem., 267, 228–234. [DOI] [PubMed] [Google Scholar]
- Knol J., Veenhoff,L.M., Liang,W.J., Henderson,P.J.F., Leblanc,G. and Poolman,B. (1996) Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem., 271, 15358–15366. [DOI] [PubMed] [Google Scholar]
- Knol J., Sjollema,K. and Poolman,B. (1998) Detergent-mediated reconstitution of membrane proteins. Biochemistry, 37, 16410–16415. [DOI] [PubMed] [Google Scholar]
- Lolkema J.S. and Poolman,B. (1995) Uncoupling in secondary transport proteins. A mechanistic explanation for mutants of lac permease with an uncoupled phenotype. J. Biol. Chem., 270, 12670–12676. [DOI] [PubMed] [Google Scholar]
- Maloney P.C. (1990) A consensus structure for membrane transport. Res. Microbiol., 141, 374–383. [DOI] [PubMed] [Google Scholar]
- McMurry L.M. and Levy,S.B. (1995) The NH2-terminal half of the Tn10-specified tetracycline efflux protein TetA contains a dimerization domain. J. Biol. Chem., 270, 22752–22757. [DOI] [PubMed] [Google Scholar]
- Moore-Hoon M.L. and Turner,R.J. (2000) The structural unit of the secretory Na+-K+-2Cl– cotransporter (NKCC1) is a homodimer. Biochemistry, 39, 3718–3724. [DOI] [PubMed] [Google Scholar]
- Pao S.S., Paulsen,I.T. and Saier,M.H.,Jr (1998) Major facilitator superfamily. Microbiol. Mol. Biol. Rev., 62, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen I.T., Sliwinski,M.K. and Saier,M.H.,Jr (1998) Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol., 277, 573–592. [DOI] [PubMed] [Google Scholar]
- Poolman B., Knol,J. and Lolkema,J.S. (1995) Kinetic analysis of lactose and proton coupling in Glu379 mutants of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem., 270, 12995–13003. [DOI] [PubMed] [Google Scholar]
- Poolman B., Knol,J., Mollet,B., Nieuwenhuis,B. and Sulter,G. (1995) Regulation of bacterial sugar-H+ symport by phosphoenolpyruvate-dependent enzyme I/HPr-mediated phosphorylation. Proc. Natl Acad. Sci. USA, 92, 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Knol,J., van der Does,C., Henderson,P.J.F., Liang,W.J., Leblanc,G., Pourcher,T. and Mus-Veteau,I. (1996) Cation and sugar selectivity determinants in a novel family of transport proteins. Mol. Microbiol., 19, 911–922. [DOI] [PubMed] [Google Scholar]
- Rubin R.A. and Levy,S.B. (1990) Interdomain hybrid Tet proteins confer tetracycline resistance only when they are derived from closely related members of the tet gene family. J. Bacteriol., 172, 2303–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Tóth M., Lawrence,M.C. and Kaback,H.R. (1994) Properties of permease dimer, a fusion protein containing two lactose permease molecules from Escherichia coli. Proc. Natl Acad. Sci. USA, 91, 5421–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Tóth M., Karlin,A. and Kaback,H.R. (2000) Unraveling the mechanism of the lactose permease of Escherichia coli. Proc. Natl Acad. Sci. USA, 97, 10729–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M.H. Jr (2000) Families of transmembrane sugar transport proteins. Mol. Microbiol., 35, 699–710. [DOI] [PubMed] [Google Scholar]
- Schroers A., Burkovski,A., Wohlrab,H. and Krämer,R. (1998) The phosphate carrier from yeast mitochondria. Dimerization is a prerequisite for function. J. Biol. Chem., 273, 14269–14276. [DOI] [PubMed] [Google Scholar]
- Spooner P.J., Friesen,R.H.E., Knol,J., Poolman,B. and Watts,A. (2000) Rotational mobility and orientational stability of a transport protein in lipid membranes. Biophys. J., 79, 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhoff L.M. and Poolman,B. (1999) Substrate recognition at the cytoplasmic and extracellular binding site of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem., 274, 33244–33250. [DOI] [PubMed] [Google Scholar]
- Veenhoff L.M., Geertsma,E.R., Knol,J. and Poolman,B. (2000) Close approximation of putative α-helices II, IV, VII, X and XI in the translocation pathway of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem., 275, 23834–23840. [DOI] [PubMed] [Google Scholar]
- Wang D.N., Sarabia,V.E., Reithmeier,R.A.F. and Kühlbrandt,W. (1994) Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO J., 13, 3230–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.A. (2000) Three-dimensional structure of the ion-coupled transport protein NhaA. Nature, 403, 112–115. [DOI] [PubMed] [Google Scholar]
- Yin C.C., Aldema-Ramos,M.L., Borges-Walmsley,M.I., Taylor,R.W., Walmsley,A.R., Levy,S.B. and Bullough,P.A. (2000) The quarternary molecular architecture of TetA, a secondary tetracycline transporter from Escherichia coli. Mol. Microbiol., 38, 482–492. [DOI] [PubMed] [Google Scholar]
- Zottola R.J., Cloherty,E.K., Coderre,P.E., Hansen,A., Hebert,D.N. and Carruthers,A. (1995) Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry, 34, 9734–9747. [DOI] [PubMed] [Google Scholar]