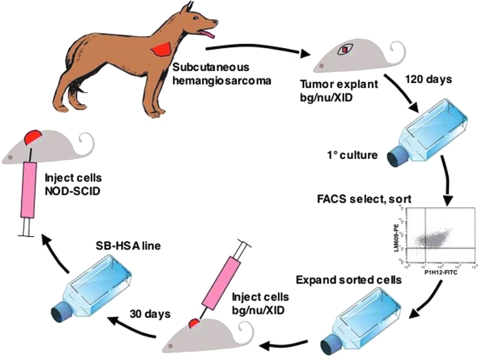
Figure 1.
Establishment of a canine hemangiosarcoma (HSA) xenograft model. A biopsy was taken from a subcutaneous HSA of the right shoulder of a 9-year-old German shepherd dog. Several small pieces of the HSA biopsy were engrafted subcutaneously in the dorsal cervical area of a bg/nu/XID mouse and the skin wound was sutured closed. The mouse was monitored for 120 days, at which time a 1-cm-diameter tumor was present and the animal was sacrificed. The tumor was excised and a portion was snap-frozen with liquid nitrogen in OCT compound for immunohistochemical staining and the remainder was used for establishing the primary canine HSA cell line. After 14 passages, the cells were sorted and cells labeling positive for expression of αv3 integrin (with a canine-selective antibody) and CD146 (P1H12) were collected and expanded. About 5 x 106 cells were injected subcutaneously into the dorsum of a second bg/nu/XID mouse and allowed to grow for 30 days until a 1-cm3 tumor had developed. The tumor was then excised. A portion was snap-frozen in OCT compound for immunohistochemical staining, and the remaining tumor was used to establish a single-cell suspension that was then expanded. These cells, called the SB-HSA cell line developed from the second passage in a bg/nu/XID mouse, were used for the remainder of the experiments to induce tumors in NOD-SCID mice.