Abstract
Septins are a highly conserved subfamily of GTPases that play an important role in the process of cytokinesis. To increase our understanding of the expression and localization of the different mammalian septins in human brain tumors, we used antibodies against septins 2, 3, 4, 5, 6, 7, 9, and 11 in immunofluorescence and Western blot analyses of astrocytomas and medulloblastomas. We then characterized the expression and subcellular distribution of the SEPT2 protein in aphidicolin-synchronized U373 MG astrocytoma cells by immunofluorescence and fluorescence-activated cell sorter analysis. To determine the role of SEPT2 in astrocytoma cytokinesis, we inducibly expressed a dominant-negative (DN) SEPT2 mutant in U373 MG astrocytoma cells. We show variable levels and expression patterns of the different septins in brain tissue, brain tumor specimens, and human brain tumor cell lines. SEPT2 was abundantly expressed in all brain tumor samples and cell lines studied. SEPT3 was expressed in medulloblastoma specimens and cell lines, but not in astrocytoma specimens or cell lines. SEPT2 expression was cell cycle-related, with maximal levels in G2-M. Immunocytochemical analysis showed endogenous levels of the different septins within the perinuclear and peripheral cytoplasmic regions. In mitosis, SEPT2 was concentrated at the cleavage furrow. By immunocytochemistry and flow cytometry, we show that a DN SEPT2 mutant inhibits the completion of cell division and results in the accumulation of multinucleated cells. These results suggest that septins are variably expressed in human brain tumors. Stable expression of the DN SEPT2 mutant leads to a G2-M cell cycle block in astrocytoma cells.
Keywords: Brain tumor, cytokinesis, astrocytoma, glioma, septins
Introduction
Septins are a highly conserved subfamily of GTPases that play an important role in the process of cytokinesis. The septins were first identified in the budding yeast, Saccharomyces cerevisiae, but have also been found in a variety of other fungi and animals [1–4]. Mutations in any of four yeast septin genes, Cdc3p, Cdc10p, Cdc11p, and Cdc12p, lead to deficiencies in hetero-oligomeric filaments that lie adjacent to the membrane at the mother-bud neck and give rise to abnormally elongated buds unable to complete cytokinesis [5]. The conserved requirement for septins in cell division was also revealed by the discovery that the Pnut gene in Drosophila melanogaster encoded a septin homologue. As in yeast, the three septins of Drosophila, Pnut, Sep1, and Sep2, also form 7-nm hetero-oligomeric filaments in vitro and these septin complexes accumulate at the cleavage furrow of mitotic cells [6]. Mutations in the Pnut gene result in the formation of multinucleated syncytia in imaginal discs during larval development due to failure to complete cytokinesis [1,7]. Similarly, mutations in the septins UNC-59 and UNC-61 of Caenorhabditis elegans result in some postembryonic defects in cell division [8]. Taken together, these data indicate that the septins play an important role in maintaining cytoskeletal structures necessary for the control of cell division, and are required for cytokinesis in flies [1,7], nematodes [8], and mammals [4].
To date, at least 10 mammalian septin homologues have been described. As the nomenclature for mammalian septins has recently been revised by Macara et al. [9], we shall refer to them by their symbols as approved by the Human Genome Organization Gene Nomenclature Committee. They are now referred to as SEPT1 to SEPT10 [4,10–21]. Most of these were identified through random sequencing strategies, subtractive screens, or as candidate genes within disease loci. All human septins have molecular masses in the range of 30 to 80 kDa and share considerable amino acid sequence identity. All except SEPT2 and SEPT7 have a GTP-binding domain near the N-terminus and a predicted coiled-coil motif near the carboxyl terminus [4]. The identification of septin homologues and molecules that functionally interact with septins in higher eukaryotes may provide clues to their functions in cytokinesis [1,4,6,22,23].
In our previous study, we showed that transient transfection of an antisense SEPT2 expression vector led to the failure of cytokinesis in U373 astrocytoma cells [10]. To further our understanding of the role of human septins in human brain tumors, here we characterize the expression and subcellular distribution of eight mammalian septins in a series of human brain tumor specimens and cell lines. Using a dominant-negative (DN) approach in stably transfected cell lines, we have now examined the role of persistent SEPT2 inhibition in cytokinesis and cell division. Our results suggest that the septins are variably expressed in different human brain tumor cell lines and tumor specimens, and within different subcellular compartments in the cell lines examined. Our observations also suggest a conserved requirement for SEPT2 in U373 astrocytoma cell division.
Materials and Methods
Cell Lines and Cell Culture Conditions
The permanent human glioma cell lines U138, U251, SF-126, SF-188, and SF-539 were obtained from Dolores Dougherty (The Brain Tumor Research Center, University of California San Francisco, San Francisco, CA); U343 astrocytoma, U373 MG (malignant glioma), and U87 MG were a generous gift of Bengt Westermark (Uppsala, Sweden). The Daoy medulloblastoma cell line was obtained from the ATCC (Manassis, VA). The PFSK supratentorial PNET cell line was a gift from Dr. Dan Fults, University of Utah (Salt Lake City, UT). All cell lines have been previously well characterized [24–27]. Primary cultures of human fibroblasts were initiated from human foreskin specimens. Permission to use these materials was granted by the Research Ethics Board (REB) at The Hospital for Sick Children (Toronto, Ontario, Canada). All cell lines and cultures were grown in monolayer and maintained in alpha minimal essential medium (α-MEM) or Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and a 1% antibiotic/antifungal mixture (Cellgro, Mediatech, Molecular Research Laboratories, Herndon, VA) in a humidified atmosphere of 5% CO2 in air at 37°C. Inducible expression of wild-type and mutant-type SEPT2-green fluorescent protein (GFP) fusion protein in the U373 MG cell line was accomplished using the tetracycline-repressor gene expression system as previously described [28]. Briefly, stably transfected U373 MG cells (U373 Tet cells) were maintained in culture containing 300 µg/ml hygromycin and 500 µg/ml geneticin (G418 sulfate; Gibco-BRL, Gaithersburg, MD). Wild-type and DN mutant SEPT2-GFP fusion protein expression was induced by 48 hours of treatment with 4 µg/ml tetracycline.
Antibodies and Regents
Antibodies to septins 2, 3, 4, 5, 6, 7, 9, and a novel septin represented by clones FLJ10849 and AL110300 and hereafter called Septin 11, were generated by immunizing rabbits with synthetic peptides generated against unique regions of the N-termini of these proteins. These sequences were not shared with any other polypeptides in the GenBank database. Antibodies to Septins 2, 4, 5, and 9 have been described elsewhere [29–33] and were provided by William Trimble (Program in Cell Biology, The Hospital for Sick Children). All antibodies used in this study are specific for the corresponding septins with no cross-reactivity to other septins or any other proteins. Western and immunocytochemical signals are completely abrogated by preincubation of the antibody with an excess of the corresponding peptide. Details of the antibody generation will be published elsewhere.
In order to detect the expression of GFP and its fusion protein, an anti-α-GFP polyclonal antibody (Clontech, Palo Alto, CA) was used at 1:250 for Western blot analysis. A goat anti-rabbit IgG HRP conjugate (Bio-Rad, Hercules, CA) was used as a secondary antibody in Western blot at 1:8000 dilution. Phalloidin-TRITC (Sigma, St. Louis, MO), which binds only to polymeric actin, was used at a concentration of 50 µg/ml in phosphate-buffered saline (PBS) for immunocytochemistry. Affinity-purified fluorescein isothiocyanate (FITC)-labeled goat antibodies against rabbit IgG that had been absorbed with rabbit serum were purchased from Chemicon International (Temecula, CA) and were used at 1:75 for immunocytochemistry. Aphidicolin (Wako, Osaka, Japan) was used for cell synchronization. Low concentrations of aphidicolin (1.25 µg/ml) can inhibit the growth of eukaryotic cells by selectively inhibiting DNA synthesis without interfering with mitochondrial DNA synthesis, RNA, protein and nucleic acid precursors synthesis, or other major metabolic pathways [34].
Western Blot
Human brain tumor samples were obtained at the time of surgery from patients at The Hospital for Sick Children. There were seven posterior fossa medulloblastoma specimens derived from children ages 3 to 14; a pilocytic low-grade astrocytoma from the parietal lobe of a 7-year-old male; an anaplastic astrocytoma from the thalamus of a 12-year-old female; and a glioblastoma multiforme from the frontal lobe of a 10-year-old male. Nonneoplastic frontal and temporal lobe specimens were obtained in two children during the course of epilepsy surgery to excise the epileptic focus. Nonneoplastic cerebellum from an 8-year-old male was obtained as marginal tissue surrounding an arteriovenous malformation during the resection of this lesion. Approval to use these specimens was given by the REB, The Hospital for Sick Children. Specimens were snap-frozen in liquid nitrogen. Proteins were extracted from tissue samples using 0.5% NP-40 lysis buffer and the lysate was loaded for immunoblotting analysis as described below.
Cytosolic soluble fractions and insoluble membrane fractions were extracted according to the procedure previously described [10]. Briefly, cells were lysed in extraction buffer [120 mM NaC1, 0.5% Nonidet P-40 (NP-40), 50 mM Tris-HC1 (pH 8.0)] for 30 minutes on ice. The extracts were cleared by centrifugation at 14,000 rpm for 15 minutes at 4°C. Extracted soluble cytosolic proteins of 20 µg were separated by electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to electrotransfer to polyvinylidene difluoride membranes (Immobilon P; Millipore, Bedford, MA) by semidry electrotransfer (0.8 mA/cm2). Blots were rehydrated and blocked with 5% skim milk in PBS-0.1% Tween-20 at room temperature for 1 hour. Primary and secondary antibody incubations were performed in blocking solution at room temperature for 1 hour. Horseradish peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad) were used at 1:8000 dilution and detected with the enhanced chemoluminescence (ECL) system (Amersham, Oakville, Ontario, Canada). Quantitative analysis of protein levels on immunoblots was performed by densitometry.
Immunocytochemistry and Confocal Microscopy
U373 MG cells were grown on eight-well chamber slides (Nunc, Naperville, IL) and then fixed in 4% paraformaldehyde in phosphate buffer (pH 7.2) for 40 minutes. Fixed cells were washed with PBS and permeabilized with 0.02% Triton X-100 in PBS for 2 minutes and blocked with 0.5% bovine serum albumin in PBS for 1 hour. The cells were incubated with the respective anti-septin polyclonal antibody (1:1000) for 1 hour at room temperature. FITC-conjugated secondary anti-rabbit IgG antibody (Pierce, Rockford, IL) was then used at 1:100 dilution. Phalloidin-TRITC for actin staining was used at a concentration of 50 µg/ml in PBS for 30 minutes. 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) was used at the concentration of 1 µg/ml in mounting medium for nuclear staining. Cell imaging was performed using a Leica epifluoresence microscope equipped with Cool Snap camera and software or a Leica confocal laser scanning microscope.
Cell Cycle Synchronization and Flow Cytometry Analysis
Cell synchronization was achieved using aphidicolin. Briefly, aphidicolin (1.25 µg/ml) was added to the growth media 24 hours after plating 5 x 105 U373 MG cells in a 10-cm2 tissue culture plate. After 15 hours, the cells were washed three times with growth media and were allowed to progress through the cell cycle for 9 hours. This process was repeated twice. Cells were harvested at 6-hour intervals through one complete cell cycle. Cell synchronization was confirmed by flow cytometry. For flow cytometric analysis, cells were trypsinized, washed in PBS, and resuspended in ice-cold 80% ethanol. The fixed cells were resuspended in 250 µl of propidium iodide (PI) solution (500 µg/ml PI in 3.8 M sodium citrate at pH 7.0; Sigma), and 250 µl of RNase A (10 mg/ml prepared in 10 mM Tris-HCl at pH 7.5; Sigma) was added to each sample and incubated in the dark at 37°C for 30 minutes. The stained cells were filtered through the lids of Falcon polystyrene round-bottom tubes with cell strainer caps. DNA content was analyzed on a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA). Percent cell cycle phase was determined using Cell Fit software (Becton Dickinson). Data were collected from at least 10,000 cells.
Construction of SEPT2-GFP Fusion Gene Expression Plasmid
The full-length cDNA encoding human SEPT2 in pGEX-2T (718 bp) was used as previously described (wild-type SEPT2) [10]. The DN SEPT2 mutant was obtained by converting serine, the 51st amino acid of human SEPT2, to asparagine (S51N SEPT2 mutant). In order to obtain the SEPT2-GFP fusion gene (1.1 kb), we inserted the wild-type and mutant SEPT2 fragments into the SmaI and HindIII sites of the pEGFP-N1 vector (BD Clontech, Palo Alto, CA). The SEPT2-GFP fusion gene was cloned into the inducible pTRE2hyg expression vector (BD Clontech). Proper orientation was determined by restriction digest. Protein expression was determined by Western blot using the SEPT2 antibody. The S51N SEPT2-GFP fusion gene in the pTRE2-hyg vector was then used to investigate the role of SEPT2 on U373 MG astrocytoma cells.
Transfection of SEPT2-GFP Fusion Gene into U373-rtTA Cells
U373 MG cells were initially transfected with the pUHD 172-1 neoplasmid encoding the reverse transactivator (rtTA) coding sequence downstream of the hCMV promoter/enhancer and neomycin resistance gene transfection reagent [35,36]. All cell line transfections were performed using Fugene 6 transfection reagent according to the manufacturer's instructions (Roche Diagnostics, Basel, Switzerland). Stable transfectants were selected and the expression of the rtTA was determined using the VP16 antibody (Clontech) in a Western blot analysis. A high VP16-expressing cell clone was selected and used to transfect the inducible SEPT2-GFP or S51N-GFP mutant. The inducibility of the SEPT2 or S51N protein expression was determined by Western blot analysis after treatment of the cells with 4 µg/ml tetracycline for 48 hours. The effects of wild-type and mutant SEPT2 fusion protein expression on the distribution of actin filaments and cell morphology were determined by immunofluorescence microscopy as described above.
To confirm the inhibitory function of S51N mutant SEPT2 on cytokinesis, DNA content was measured by fluorescence-activated cell sorter (FACS) analysis in parental U373 MG cells, transfected uninduced (Tet-off) cell clones, and transfected tetracycline-induced cell clones for both wild-type and S51N mutant SEPT2-expressing cells. The FACS analysis gate was set to select GFP-positive cells with a green fluorescence signal for Tet-on state cells. Tet-on cells are harvested 7 days after treatment with tetracycline. The DNA histograms from each assay contained data from over 10,000 cells.
Results
Septins Are Variably Expressed in Human Brain Tumor Cell Lines and Specimens
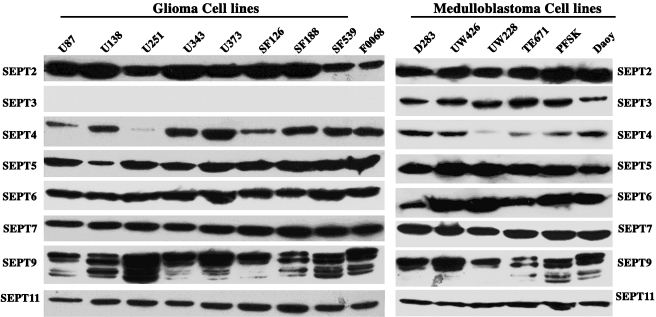
Septin expression was detected predominantly in the cytosolic fraction. There were no visible differences between cytosolic septin expressions among the different cell lines, with the exception of SEPT3. Interestingly, SEPT3 was highly expressed in cell lines and tumor samples of neuronal origin such as medulloblastoma and supratentorial PNET origin such as PFSK, but was not expressed in astrocytoma cell lines (Figure 1). The anti-SEPT2 antibody identified an immunoreactive band in all astrocytoma and medulloblastoma cell lines examined.
Figure 1.
Expression of human septins in human brain tumor cell lines and primary human fibroblasts. There were no visible differences between the expression of septins among the different cell lines, except SEPT3. SEPT3 was not expressed in astrocytoma cell lines and primary human fibroblasts (F0068), but was expressed by all medulloblastoma and supratentorial PNET cell lines. At least four isoforms of SEPT9 were observed in most of the cell lines.
The molecular masses of the septins examined ranged from 35 to 65 kDa, which is in agreement with their predicted sizes (Figure 2). There were at least four isoforms of SEPT9 in astrocytoma and medulloblastoma cell lines. The molecular masses of SEPT9 isoforms ranged from 40 to 65 kDa.
Figure 2.
Molecular mass determinations of the septins in human brain tumors. The molecular masses of the various septins range from 40 to 55 kDa. SEPT3 was evaluated in a medulloblastoma cell line.
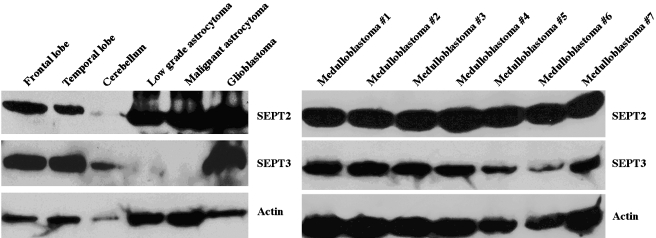
In solid brain tumor specimens, SEPT2 protein was detected in various brain tumors including low-grade astrocytomas, anaplastic astrocytomas, glioblastomas, and medulloblastomas (Figure 3). Nonneoplastic brain tissues also demonstrated SEPT2 expression. However, the expression of SEPT2 in the frontal and temporal lobes and the cerebellum was less than that observed in the primary astrocytic tumors by densitometry. In addition, the expression of SEPT2 in glioblastoma was greater than that in low-grade astrocytoma. SEPT3 was expressed in all medulloblastoma samples, and in nonneoplastic brain tissue. It was not expressed in the astrocytic tumors except for one glioblastoma multiforme (Figure 3). Western blot analysis in additional 11 glioblastoma multiforme samples, however, could not reveal SEPT3 expression (data not shown).
Figure 3.
Expression of SEPT2 and SEPT3 in brain tissues and brain tumor samples. SEPT2 is variably expressed in SDS-soluble fractions of normal brain tissues (frontal lobe, temporal lobe, and cerebellum), three astrocytomas of different histopathological grade, and seven medulloblastoma samples. SEPT2 expression in astrocytomas is greater than that in normal brain tissues. SEPT2 expression in glioblastoma is greater than that in low-grade astrocytoma. SEPT3 is also expressed in all samples except low-grade and malignant astrocytomas.
Subcellular Localization of Septins
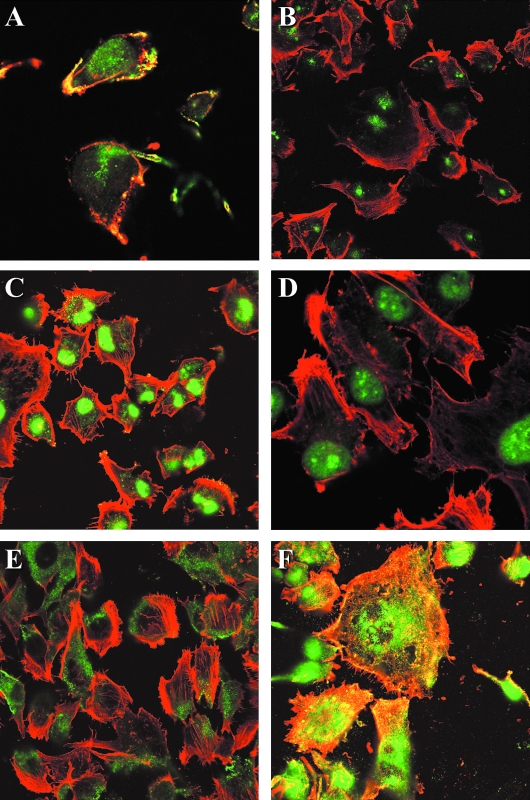
Double immunofluorescence analysis for all septins revealed a rather variable subcellular expression of the respective septins. Although SEPT2 was immunoreactive with short, linear or granular structures adjacent to actin filaments within the cytoplasm and very prominent at the plasma membrane in U373 cells (Figure 4A), SEPT3, SEPT4, and SEPT6 were predominantly expressed in a perinuclear fashion (Figure 4, B–D, respectively). SEPT7 showed strong cytoplasmic staining excluding the nuclear region (Figure 4E). SEPT3, SEPT4, SEPT6, and SEPT7 demonstrated a mutually exclusive immunolocalization with actin. The expression of SEPT9 (data not shown) and SEPT11 (Figure 4F) was perinuclear and cytoplasmic with some degree of overlap with actin. The immunofluorescence staining for SEPT5 was weak around the nucleus (data not shown).
Figure 4.
Subcellular localization of different septins in U373 astrocytoma cells. (A) U373 cells stained for SEPT2 show a perinuclear as well as membrane-bound immunoreactivity colocalizing with the actin cytoskeleton. By way of contrast, immunoflourescence staining for SEPT3 (B), SEPT4 (C), and SEPT6 (D) was largely confined to the perinuclear regions without significant overlap with actin staining. Immunoreactivity to SEPT7 (E) was detected only in the cytoplasm and was mutually exclusive to the distribution of actin. Immunostains for SEPT11 (F) showed some colocalization with actin but remained predominantly nuclear or perinuclear in distribution. Immunofluorescence microscopy of (A), (D), and (F), x 250; (B), (C), and (E), x 150 (green = immunoreactivity for the respective septins stained with antiseptin antibody FITC; red = actin stained by Phalloidine-TRITC; yellow = colocalization of actin and the respective septin).
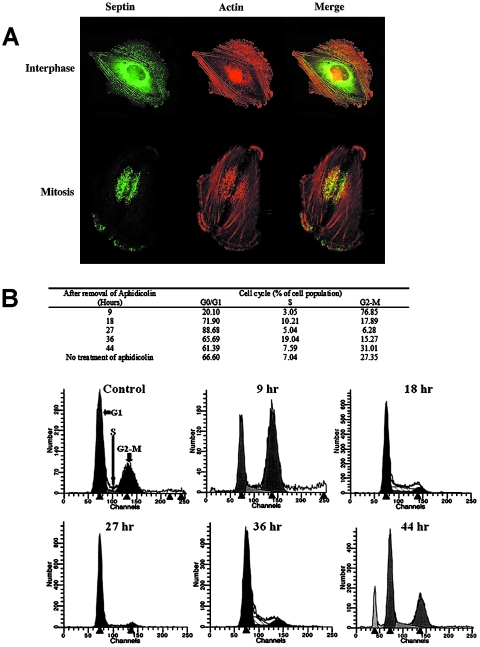
SEPT2 was variably expressed during cell synchronization with aphidicolin in SF-188 glioma cells (Figure 5A). In interphase cells, endogenous SEPT2 was immunolocalized to granular and short filamentous structures that overlap with actin filaments. The perinuclear region was densely immunoreactive to SEPT2 (Figure 5A, upper). In mitosis, SEPT2-containing fibers were in close apposition to the actin-based contractile ring and around the cleavage furrow between two daughter cells (Figure 5A, lower).
Figure 5.
(A) Subcellular localization of endogenous SEPT2 in synchronized SF-188 MG cells. In interphase cells (upper), most of the endogenous SEPT2 were found in short, linear structures localized close to actin filaments. However, SEPT2 immunoreactivity also existed amorphously in a perinuclear distribution. In mitosis (lower), the SEPT2-containing filaments were in close apposition to the actin-based contractile ring, and amorphous SEPT2 proteins were strongly concentrated around the cleavage furrow between two daughter cells (green = SEPT2; red = actin; yellow = colocalization of actin and SEPT2). Confocal immunofluorescence microscopy, all x 250. (B) Cell synchronization of U373 cells using aphidicolin. Flow cytometry showed that aphidicolin caused over 75% of the cells to arrest in G2-M. Most of the cells arrested in G2-M phase by aphidicolin were then able to enter G1 phase 18 hours after removal of aphidicolin.
Septin Expression Is Maximal at G2-M Phase of Synchronized U373 MG Astrocytoma Cells
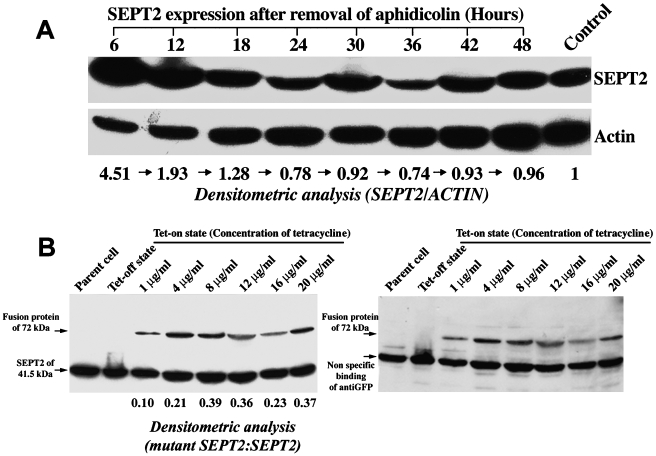
By flow cytometry, aphidicolin arrested over 75% of U373 MG astrocytoma cells in the G2-M phase of the cell cycle, with each cell containing two kinetoplasts. Most cells arrested by aphidicolin were able to enter G1 phase 18 hours after removal of aphidicolin (Figure 5B). The effects of cell synchronization on SEPT2 expression at various time points throughout the cell cycle are shown in Figure 6A. SEPT2 expression was maximal at G2-M phase in synchronized U373 MG cells. By Western blot analysis, SEPT2 expression decreased 18 to 36 hours after removal of aphidicolin as cells progressed through the cell cycle. At 42 to 48 hours after the removal of aphidicolin, the expression of SEPT2 returned to basal (steady state) levels similar to untreated control cells.
Figure 6.
(A) SEPT2 expression in synchronized U373 MG astrocytoma cells. SEPT2 expression is maximal at the G2-M phase of synchronized U373 MG cells (6–12 hours after removal of aphidicolin). By western blot analysis, SEPT2 expression diminishes in the time period 24 to 36 hours after removal of aphidicolin. SEPT2 expression then returned to basal (steady state) levels 42 to 48 hours after removal of aphidicolin. (B) Inducible expression of wild-type and mutant SEPT2 in U373 MG cells. The inducibility of the wild-type or S51N mutant SEPT2-GFP fusion protein was determined by Western blot analysis using anti-SEPT2 antibodies (left) and anti-α-GFP antibody (right). Both antibodies detected the expected 72-kDa fusion protein 48 hours after induction with 1 to 20 µg/ml tetracycline.
Expression of a DN SEPT2 Mutant Inhibits Cytokinesis
Western blot analysis of transfected U373 MG astrocytoma cells induced for 48 hours with tetracycline revealed a 72-kDA fusion protein with the anti-SEPT2 antibody (Figure 6B, left panel). When the membrane was reprobed with an anti-GFP antibody, the same 72-kDa protein was identified (Figure 6B, right panel). The ratio of ectopic mutant to normal SEPT2 was measured by densitometry of the above Western blots, where it was found that the ratio for mutant to normal expression ranged from 0.1 to 0.39, depending on the degree of induction by tetracycline.
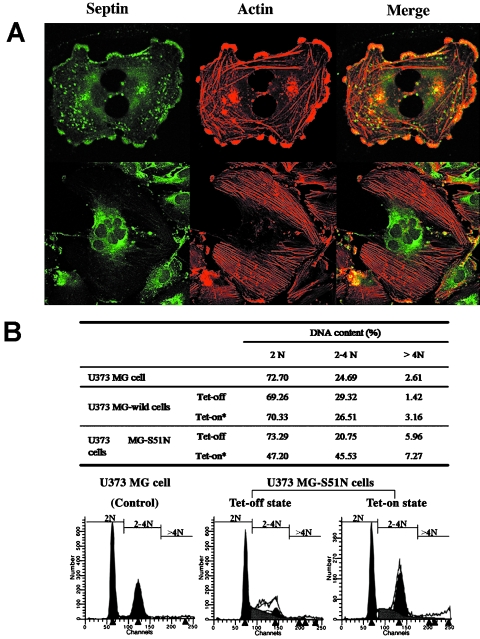
Successfully transfected cells could be readily identified by virtue of the GFP tag by direct inspection with the fluorescent microscope. As expected, the wild-type SEPT2-GFP fusion protein was identified in short filamentous filamentous structures and in a perinuclear distribution in a pattern very similar to endogenous SEPT2 (data not shown). There were no major differences in immunolocalization patterns of actin and cellular morphology between parental U373 MG cells and cells transfected with the wild-type SEPT2-GFP fusion protein. By way of contrast, the mutant SEPT2-GFP fusion protein appeared as large aggregates predominantly in the perinuclear cytoplasm, or localized sporadically throughout the cytoplasm but distinct from actin filaments, which became prominent at the peripheral cytoplasm. In dividing cells, expression of the mutant SEPT2 protein led to the formation of numerous bizarre, multinucleated cells (Figure 7A). By Western blot analysis, the expression of the wild-type or mutant SEPT2-GFP fusion proteins did not interfere with the expression levels of actin (data not shown).
Figure 7.
(A) Immunocytochemistry of mutant SEPT2 expressing U373 MG cells. S51N mutant SEPT2 cells undergo a distinct morphological change characterized by incompletion of cytokinesis, formation of long cytoplasmic connections between dividing cells (upper), and multinucleated cells (lower) (green = fusion protein by virtue of the GFP tag under confocal microscopy; red = actin stained by Phalloidine-TRITC; yellow = merge). Confocal microscopy, x 250. (B) DNA histograms after induction of DN SEPT2 mutant. DNA content is compared in the parental cell line, Tet-off state, and Tet-on state from U373 MG wild-type cells and U373 MG-S51N cells. There is no difference in DNA content among the parental cell line, the uninduced U373-S51N cells, and the uninduced and induced U373 wild-type cells. In contrast, in the Tet-on state, there is a significant increase in 4 N and >4 N phase cells in U373-S51N cells.
FACS analysis of DNA content was compared in the parental cell line, uninduced state, and induced state from U373 MG wild-type cells and U373 MG-S51N cells (Figure 7B). There was no difference in DNA content among the parental cell line, the uninduced U373-S51N cells, and the uninduced and induced U373 MG wild-type cells. In contrast, tetracycline induction of U373-S51N cells led to a significant increase in >2 N phase cells.
Discussion
In this study, we have examined the expression of eight mammalian septins (SEPT2, SEPT3, SEPT4, SEPT5, SEPT6, SEPT7, SEPT9, and SEPT11) in normal brain tissues, a variety of astrocytoma and medulloblastoma cell lines, and several solid brain tumor specimens. We have confirmed the predicted molecular masses of the various septins, which range from 35 to 65 kDa. By confocal double immunofluorescence microscopy, SEPT2, SEPT9, and SEPT11 were immunolocalized along with the actin microfilamentous system. Using aphidicolin to arrest cells in G2-M phase, we show that SEPT2 is a cell cycle-regulated protein essential for the process of cytokinesis in human astrocytoma cells and localizes to the actin-based contractile ring during cytokinesis. Expression of a DN mutant SEPT2 gene inhibits cytokinesis and results in multinucleated cells. Taken together, these observations suggest a conserved requirement for SEPT2 in U373 astrocytoma cell division.
Despite considerable expression of septins in human tissues, the precise function of the septin gene family remains unclear. The most consistent function of septins is an orthologous function in cytokinesis [1,4,6,37,38]. Investigations in yeast suggest that septins are putative cytoskeletal structures that play a role in cell division and are localized exclusively to the bud neck throughout the entire cell cycle, from bud emergence to cytokinesis. To date, only one mammalian septin, SEPT2, has been shown to play a role in cytokinesis [4].
Xue et al. [19] reported that SEPT3 is a brain-specific septin that may be regulated by type I cGMP-dependent protein kinase (PKG) in neurons. SEPT3 mRNA (5.0 kb) is highly expressed in the brain and undetectable in 12 other tissues [19]. These results suggest that the SEPT3 is primarily a neuronal protein. In our study, we showed SEPT3 expression in human brain tissues from frontal and temporal lobes and cerebellum, and in medulloblastoma specimens and cell lines. However, we were unable to show any significant expression of SEPT3 in astrocytoma cell lines or specimens by Western blot analysis. Only 1 of 12 glioblastoma specimens examined expressed SEPT3. As for the other septins, SEPT3, SEPT4, and SEPT6 showed a strong perinuclear localization, whereas SEPT7 was exclusively found in the cytoplasm sparing the nuclear region. SEPT9 and SEPT11 were diffusely distributed and demonstrated, to some degree, colocalization with actin-based structures. Precisely what these different patterns of septin immunolocalization mean in astrocytoma cells awaits further study.
We have previously shown a relative increase in SEPT2 transcript levels from late G1 to G2-M phases and an increase in SEPT2 protein levels from S to G2-M phases in lovastatin- and nocodazole-synchronized U373 MG cells [10]. In the present study, we used aphidicolin to synchronize U373 MG cells in G2-M. By performing immunofluorescence analysis of SEPT2 and actin in synchronized U373 MG cells, we show that SEPT2 is found in granular and short filamentous structures in interphase cells and colocalizes with actin filaments. In contrast to actin, however, SEPT2 is also found amorphously in a perinuclear distribution at this time. In mitosis, SEPT2 immunoreactivity is found in close apposition to the actin-based contractile ring. These data and the Western blot time course study in U373 MG cells released from aphidicolin support the notion that SEPT2 may be expressed in a cell cycle-dependent fashion.
Using a technique complementary to antisense SEPT2 expression [10], we transfected U373 MG astrocytoma cells with a DN mutant of SEPT2. This mutant was created by converting amino acid 51 (serine) to asparagine [39]. In contrast to our previous report [10], the DN approach used here leads to the selection of stable cell clones that can be induced to express mutant SEPT2 by virtue of the Tet-on system. By immunocytochemistry, we show that mutant SEPT2 expression leads to distinct cytological changes characterized by incomplete cytokinesis, formation of long cytoplasmic connections between dividing cells, and numerous multinucleated cells. By FACS analysis, expression of the mutant SEPT2 fusion protein led to a significant increase in the number of multinucleated cells when compared to controls. Our inability to block cytokinesis completely in all glioma cells may relate to the differences in expression levels between the endogenous SEPT2 protein and the ectopic mutant protein. The higher levels of the endogenous SEPT2 protein may have prevented the mutant protein from squelching all of the normal protein functions. We recognize the limitations of the expression of ectopic proteins in artificial cell systems such as this. However, these results are similar to those reported in S. cerevisiae and Drosophila when septins in these species are mutated.
The relationship between SEPT2 and actin is important for properly conducted and timed cytokinesis. Actin-based contractile rings have been implicated in the cell division of multiple eukaryotic organisms [40–42]. By immunocytochemistry, we showed the colocalization of SEPT2 and actin bundles in interphase cells, with strong consolidation around actin-based contracting ring in mitotic cells of glioma cells. These findings are similar to those reported previously in other mammalian cells [4,10]. Lippincott and Li [43,44] used time-lapsed microscopy to show that the septin structure does not constrict during cytokinesis, unlike the actomyosin ring. This goes along with our findings for all other septins, which were not seen in relation to the contractile ring. Further studies are necessary to determine whether the actin-based contractile ring serves as a scaffold for septin assembly, or whether a preformed septin scaffold recruits actin-based contractile rings as in budding yeast [45].
In addition to their roles in cytokinesis, septins may subserve other cellular functions. All mammalian septins contain the same coiled-coil domain structure; however, they exhibit even greater variations in the length and primary structure of their N- and C-termini. The functions of the septins may relate to the organization of specialized domains within the cells. The availability of a septin domain suggests that individual septins could participate in protein-protein interactions [2]. Previous studies of SEPT2 and SEPT4 have shown them to be present near the plasma membrane in a filamentous form colocalizing with the actin cytoskeleton [4,29]. We found SEPT4 only in a perinuclear distribution. Recently, Surka et al. [32] reported that SEPT9 colocalized with SEPT2, actin-based filaments, and microtubules in interphase cells. The molecular similarity between the septins suggests a certain redundancy in their functions; however, previous studies have shown restricted expression of at least some septins. For example, SEPT5 is expressed primarily in the brain. SEPT3, as stated above, is found in cells and tissues of neuronal origin. Interestingly, SEPT2, which is considered to be closely related to cell division, is broadly expressed in most human organs that are predominantly nonmitotic [4,46,47]. We recognize that our study is limited by the availability of septin antibodies, and that antibody specificity remains an important issue [48]. Still, none of the septin antibodies cross-reacts with other septins, and all were developed from unique sequences as entered in GenBank.
Studies on septins in yeast may provide us with clues to their functions in mammalian cells. Yeast septin mutants exhibit not only a multinuclear phenotype as a result of defective cytokinesis, but also abnormalities in bud site selection, cell polarity, and partitioning membrane domains [2,49–52]. On the other hand, Drosophila septin, Sep1, accumulates at the leading edge of migrating epithelial cells [6]. Biochemical interactions of the mammalian SEPT4 and SEPT5 complex with the sec6/sec8 complex [11] and with syntaxin-1 [31] suggest possible involvement of the membrane fusion machinery in mammalian cytokinesis, as has been implicated in nematodes and plants [52–54]. The mammalian septins may be involved in other cellular processes including apoptosis [20], leukemogenesis and carcinogenesis [55–57], and neurodegeneration [15,58]. Although information is fragmentary at this time, septins represent a diverse and important family of proteins in dividing and postmitotic cells. Our preliminary experiments suggest that as a septin, SEPT2 may be a valuable new target for strategies aimed at inhibiting astrocytoma cell division. Future studies will focus on determining if the inhibition of the various septins (alone and in combination) using various strategies, such as antisense oligonucleotide, Tet-on inducible expression, or pTAT delivery, can serve as a rational approach to blocking the growth of astrocytoma cells.
Acknowledgements
James Rutka is a Scientist of the Canadian Institutes of Health Research. We thank William Trimble for the gifts of septin antibodies, and for helpful discussions regarding the experiments in this study.
Footnotes
Dong-Seok Kim was supported by a postdoctoral fellowship program from the Korea Science and Engineering Foundation (KOSEF). This work was supported, in part, by grants from the Canadian Institutes of Health Research, the Laurie Berman Fund for Brain Tumor Research, the Wiley Fund, and Brainchild (to J.T.R.). Aurelia Peraud was funded by the Deutsche Forschungsgemeinschaft (Pe 758/2-1).
References
- 1.Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 2.Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- 3.Longtine MS, Fares H, Pringle JR. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 5.Sanders SL, Field CM. Cell division. Septins in common? Curr Biol. 1994;4:907–910. doi: 10.1016/s0960-9822(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 6.Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol Biol Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam JC, Pringle JR, Pfeifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123–3135. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113(Pt 21):3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- 9.Macara IG, Baldarelli R, Field CM, Glotzer M, Hayashi Y, Hsu SC, Kennedy MB, Kinoshita M, Longtine M, Low C, Maltais LJ, McKenzie L, Mitchison TJ, Nishikawa T, Noda M, Petty EM, Peifer M, Pringle JR, Robinson PJ, Roth D, Russell SE, Stuhlmann H, Tanaka M, Tanaka T, Trimble WS, Ware J, Zeleznik-Le NJ, Zieger B. Mammalian septins nomenclature. Mol Biol Cell. 2002;13:4111–4113. doi: 10.1091/mbc.E02-07-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai K, Kurimoto M, Tsugu A, Hubbard SL, Trimble WS, Rutka JT. Expression of Nedd5, a mammalian septin, in human brain tumors. J Neuro-Oncol. 2002;57:169–177. doi: 10.1023/a:1015721801075. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 12.Borkhardt A, Teigler-Schlegel A, Fuchs U, Keller C, Konig M, Harbott J, Haas OA. An ins(X;11)(q24;q23) fuses the MLL and the Septin 6/KIAA0128 gene in an infant with AML-M2. Genes Chromosomes Cancer. 2001;32:82–88. doi: 10.1002/gcc.1169. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Gao YK, Chen JY. KIAA0202, a human septin family member, interacting with hPFTAIRE1. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2002;34:520–525. [PubMed] [Google Scholar]
- 14.Blaser S, Jersch K, Hainmann I, Wunderle D, Zgaga-Griesz A, Busse A, Zieger B. Human septin-septin interaction: CDCrel-1 partners with KIAA0202. FEBS Lett. 2002;519:169–172. doi: 10.1016/s0014-5793(02)02749-7. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita A, Kinoshita M, Akiyama H, Tomimoto H, Akiguchi I, Kumar S, Noda M, Kimura J. Identification of septins in neurofibrillary tangles in Alzheimer's disease. Am J Pathol. 1998;153:1551–1560. doi: 10.1016/S0002-9440(10)65743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong JW, Leahy A, Stuhlmann H. Retroviral promoter-trap insertion into a novel mammalian septin gene expressed during mouse neuronal development. Mech Dev. 1999;86:183–191. doi: 10.1016/s0925-4773(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 17.Strehl S, Borkhardt A, Slany R, Fuchs UE, Konig M, Haas OA. The human LASP1 gene is fused to MLL in an acute myeloid leukemia with t(11;17)(q23;q21) Oncogene. 2003;22:157–160. doi: 10.1038/sj.onc.1206042. [DOI] [PubMed] [Google Scholar]
- 18.Jackisch BO, Hausser H, Schaefer L, Kappler J, Muller HW, Kresse H. Alternative exon usage of rat septins. Biochem Biophys Res Commun. 2000;275:180–188. doi: 10.1006/bbrc.2000.3287. [DOI] [PubMed] [Google Scholar]
- 19.Xue J, Wang X, Malladi CS, Kinoshita M, Milburn PJ, Lengyel I, Rostas JA, Robinson PJ. Phosphorylation of a new brain-specific septin, G-septin, by cGMP-dependent protein kinase. J Biol Chem. 2000;275:10047–10056. doi: 10.1074/jbc.275.14.10047. [DOI] [PubMed] [Google Scholar]
- 20.Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, Book-Melamed N, Timberg R, Duckett CS, Lechleider RJ, Steller H, Orly J, Kim SJ, Roberts AB. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol. 2000;2:915–921. doi: 10.1038/35046566. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 22.Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109(Pt 12):2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 24.Ponten J, Westermark B. Properties of human malignant glioma cells in vitro. Med Biol. 1978;56:184–193. [PubMed] [Google Scholar]
- 25.Westermark B, Ponten J, Hugosson R. Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand A. 1973;81:791–805. doi: 10.1111/j.1699-0463.1973.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 26.Rutka JT, Giblin JR, Hoifodt HK, Dougherty DV, Bell CW, McCulloch JR, Davis RL, Wilson CB, Rosenblum ML. Establishment and characterization of a cell line from a human gliosarcoma. Cancer Res. 1986;46:5893–5902. [PubMed] [Google Scholar]
- 27.Rutka JT, Giblin JR, Dougherty DY, Liu HC, McCulloch JR, Bell CW, Stern RS, Wilson CB, Rosenblum ML. Establishment and characterization of five cell lines derived from human malignant gliomas. Acta Neuropathol (Berl) 1987;75:92–103. doi: 10.1007/BF00686798. [DOI] [PubMed] [Google Scholar]
- 28.Dirks PB, Patel K, Hubbard SL, Ackerley C, Hamel PA, Rutka JT. Retinoic acid and the cyclin dependent kinase inhibitors synergistically alter proliferation and morphology of U343 astrocytoma cells. Oncogene. 1997;15:2037–2048. doi: 10.1038/sj.onc.1201392. [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Surka M, Howard J, Trimble WS. Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other septin proteins. Cell Motil Cytoskelet. 1999;43:52–62. doi: 10.1002/(SICI)1097-0169(1999)43:1<52::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Trimble WS. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J Membr Biol. 1999;169:75–81. doi: 10.1007/s002329900519. [DOI] [PubMed] [Google Scholar]
- 31.Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- 32.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng XR, Jia Z, Zhang Y, Ware J, Trimble WS. The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol. 2002;22:378–387. doi: 10.1128/MCB.22.1.378-387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begemann M, Kashimawo SA, Choi YA, Kim S, Christiansen KM, Duigou G, Mueller M, Schieren I, Ghosh S, Fabbro D, Lampen NM, Heitjan DF, Schiff PB, Bruce JN, Weinstein IB. Inhibition of the growth of glioblastomas by CGP 41251, an inhibitor of protein kinase C, and by a phorbol ester tumor promoter. Clin Cancer Res. 1996;2:1017–1030. [PubMed] [Google Scholar]
- 35.Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993;21:4411–4412. doi: 10.1093/nar/21.18.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cid VJ, Adamikova L, Sanchez M, Molina M, Nombela C. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology. 2001;147:1437–1450. doi: 10.1099/00221287-147-6-1437. [DOI] [PubMed] [Google Scholar]
- 39.Polakis P, McCormick F. Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target. J Biol Chem. 1993;268:9157–9160. [PubMed] [Google Scholar]
- 40.Field CM, Kellogg D. Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- 41.Fishkind DJ, Wang YL. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993;123:837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto JJ, Schroeder TE. Association of actin and myosin in the contractile ring. Ann NY Acad Sci. 1990;582:179–184. doi: 10.1111/j.1749-6632.1990.tb21678.x. [DOI] [PubMed] [Google Scholar]
- 43.Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippincott J, Li R. Dual function of Cyk2, a cdc15/PSTPIP family protein, in regulating actomyosin ring dynamics and septin distribution. J Cell Biol. 1998;143:1947–1960. doi: 10.1083/jcb.143.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakui M, Yamaguchi A, Sakurai D, Ogasawara K, Yokochi T, Tsuchiya N, Ikeda Y, Tokunaga K. Genes highly expressed in the early phase of murine graft-versus-host reaction. Biochem Biophys Res Commun. 2001;282:200–206. doi: 10.1006/bbrc.2001.4550. [DOI] [PubMed] [Google Scholar]
- 47.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, Jones M, Tyson K, Bassi C, Scarpa A, Lemoine NR. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Tanaka T, Matsuzaki S, Seto Y, Matsuda T, Komori K, Itoh J, Kijima H, Tamai K, Shibayama M, Hashimoto Y, Nakazawa H, Toma H. Rapid and quantitative detection of human septin family Bradeion as a practical diagnostic method of colorectal and urologic cancers. Med Sci Monit. 2003;9:MT61–MT68. [PubMed] [Google Scholar]
- 49.Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- 50.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 51.Gladfelter AS, Bose I, Zyla TR, Bardes ES, Lew DJ. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol. 2002;156:315–326. doi: 10.1083/jcb.200109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachikawa H, Bloecher A, Tatchell K, Neiman AM. A Gip1p-Glc7p phosphatase complex regulates septin organization and spore wall formation. J Cell Biol. 2001;155:797–808. doi: 10.1083/jcb.200107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr Biol. 1999;9:738–745. doi: 10.1016/s0960-9822(99)80333-9. [DOI] [PubMed] [Google Scholar]
- 54.Assaad FF. Of weeds and men: what genomes teach us about plant cell biology. Curr Opin Plant Biol. 2001;4:478–487. doi: 10.1016/s1369-5266(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 55.Kalikin LM, Sims HL, Petty EM. Genomic and expression analyses of alternatively spliced transcripts of the MLL septin-like fusion gene (MSF) that map to a 17q25 region of loss in breast and ovarian tumors. Genomics. 2000;63:165–172. doi: 10.1006/geno.1999.6077. [DOI] [PubMed] [Google Scholar]
- 56.Russell SE, McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Petty EM, Kalikin LM, Church SW, McIlroy S, Harkin DP, Keilty GW, Cranston AN, Weissenbach J, Hickey I, Johnston PG. Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res. 2000;60:4729–4734. [PubMed] [Google Scholar]
- 57.Osaka M, Rowley JD, Zeleznik-Le NJ. MSF (MLL septin-like fusion), a fusion partner gene of MLL, in a therapy-related acute myeloid leukemia with a t(11;17)(q23;q25) Proc Natl Acad Sci USA. 1999;96:6428–6433. doi: 10.1073/pnas.96.11.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]