Abstract
Malignant gliomas spawn disseminated microsatellites, which are largely refractory to currently employed therapies, resulting in eventual tumor recurrence and death. The use of tumor-tropic neural stem cells (NSCs) as delivery vehicles for therapeutic gene products represents an attractive strategy specifically focused at treating these residual neoplastic foci. We wished to elucidate the biological cues governing NSC tropism for glioma. In this context, we describe that tumortropic NSCs comprise largely of astrocytic progenitors expressing chemokine receptor 4 (CXCR4). Blocking of CXCR4 significantly inhibits NSC migration toward the tumor. These findings define specific characteristics associated with the cell populations within transplanted NSCs that demonstrate glioma-tracking behavior.
Keywords: Glioma/brain tumor, neural stem cells, migration, CXCR4, SDF-1
Abbreviations: CXCR4, chemokine receptor 4; SDF-1, stromal cell-derived factor-1; NSC, neural stem cells; NSC-LacZ, neural stem cells expressing β-galactosidase
Introduction
Despite advances in surgical techniques and adjuvant therapies, the prognosis for patients with malignant glial tumors remains dismal. The median survival following diagnosis of glioblastoma multiforme, the most common and aggressive subtype of malignant glioma, is under 1 year, with a 2-year survival rate approaching zero [1]. The failure of currently employed therapeutic approaches, which center on surgical resection followed by radiotherapy and/or chemotherapy, is rooted in the highly disseminated nature of these tumors. High-grade gliomas are highly infiltrative neoplasms, with solitary tumor cells or clusters of neoplastic cells migrating throughout the brain, often to significant distance from the main tumor. Despite aggressive therapy, it is almost impossible to successfully eliminate all of these tumor foci, which eventually serve as reservoirs for near-universal tumor recurrence, thereby contributing to the inevitable lethality of this disease. Standard adjuvant treatments including radiation and chemotherapy have, despite having modest effects on long-term survival, been unable to effect any meaningful impact on patient prognosis. The development of a successful therapeutic modality for malignant glioma will, therefore, center on the ability to devise a means of eliminating all viable intracranial neoplastic reservoirs left behind after surgical resection of the primary tumor mass. At present, this remains a daunting task given the highly disseminated nature of the disease process, and our current inability to adequately visualize and therapeutically target every remaining tumor cell.
One promising means of specifically directing treatment to migrating tumor satellites that has recently come to light involves the use of neural stem cells (NSCs). NSCs are multipotent progenitor cells that can be derived from either embryonic, fetal, neonatal, or adult tissues and are capable of long-term, sustained, in vitro propagation and terminal differentiation into neurons and glia. We and others have demonstrated that NSCs exhibit potent tropism for disseminating glioma cells in vivo. When inoculated into established intracranial gliomas in rodents, NSCs migrate away from the primary site of injection and intersperse themselves with, or track into proximity of, tumor satellites that have spread away from the primary tumor mass [2,3]. NSCs engineered to secrete tumor toxic chemokines can, in this manner, deliver these therapeutic proteins directly to these disseminated neoplastic foci with significant bioactivity. In particular, NSC populations secreting the immunostimulatory cytokines IL-12 and IL-4 as well as the proapoptotic protein TRAIL have been used to target migrating tumor pockets with resulting decreases in tumor burden and prolongation in survival [3–5]. These findings indicate that the use of NSCs represents a potentially viable tool for specific targeting of microscopic tumor nests that are otherwise refractory to currently employed therapies. However, despite promising results in preclinical murine models, the exact mechanisms governing the glioma tropic behavior of NSCs are poorly understood. Additionally, our earlier observations demonstrated that although many intratumorally inoculated NSCs demonstrated robust migratory activity and tumor-tracking capabilities, a significant proportion of transplanted NSCs did not exhibit this behavior and remained localized to the site of initial intracranial injection [3]. This could be secondary to differing phenotypic profiles within our in vivo-inoculated NSC populations, which comprise of progenitor cells at various levels of differentiation, ranging from uncommitted multipotent precursors to cells that have initiated pathways leading to assumption of either a neuronal or glial fate [6]. In this context, we hypothesized that the tumor-tropic capacity we observed within our NSC inoculae was likely exhibited by a specific subpopulation of progenitor cells at a particular stage of differentiation. We also wished to investigate whether in vivo glioma-tracking NSCs expressed any phenotypic markers such as chemokine receptors that would indicate responsiveness to known chemotactic cues related to NSC migration within the developing brain.
With the aim of further characterizing the tumor-tropic component of the primary NSC populations utilized in earlier therapeutic models of intracranial glioma, we now describe that tumor-tracking NSCs comprise largely of astrocytic precursors expressing significant levels of chemokine receptor 4 (CXCR4), a chemokine receptor that governs cellular migration and homing in a variety of cell types including neuronal and glial precursors in the developing brain [7,8]. It has recently been reported that the production by glioma cells of stromal cell-derived factor-1 (SDF-1), the only known ligand for CXCR4, correlated with histological grade, tumor cell survival, and invasiveness [9,10]. Based on the established roles of SDF-1 and CXCR4 in governing neuronal and glial precursor migration in the developing central nervous system (CNS) and the ability of invasive glioma cells to secrete SDF-1, we hypothesized that elaboration by disseminating tumor cells of this chemokine may play an important role in chemoattracting migratory NSC populations. We now demonstrate that the tropism of NSCs toward glioma-conditioned media in vitro can be inhibited by blocking cell surface CXCR4 receptors on NSCs, further confirming the relevance of this pathway in NSC migration. These findings delineate important characteristics of the specific cells within generalized NSC populations that exhibit the therapeutically relevant behavior of “seek-and-destroy” tumor-tropic migration. The use of these markers and further work on the characterization of these migratory subpopulations will allow for refining of NSC subsets that are increasingly responsive to cues that govern tropism for disseminated tumor satellites in vivo, and therefore allow for optimization of the therapeutic potential of NSCs in this setting.
Materials and Methods
Cells and Culture Process
The human U87MG glioma, murine GL26 glioma, NIH 3T3, and 293 human embryonic kidney cell lines were cultured in DM/F12 and Dulbecco's Modified Eagle media (DMEM) (Invitrogen, Carlsbad, CA), respectively supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Calabassas, CA), l-glutamine, and 1% penicillin/streptomycin (Invitrogen). Conditioned media from U87MG, GL26, NIH 3T3, or 293 cultures were obtained from confluent 75-cm2 culture flasks seeded 96 hours earlier with an approximately similar numbers of cells. Cryopreserved human fetal NSCs were obtained from Cambrex (Walkersville, MD) and murine NSCs were harvested from the frontoparietal regions of day 15 mouse fetuses as described earlier [3]. NSCs were cultured in DM/F12 media (Invitrogen) supplemented with B-27 growth factor (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 20 to 30 ng/ml human or murine epidermal growth factor, 20 to 30 ng/ml human basic fibroblast growth factor (Peprotech, Rocky Hill, NJ), and 2 µg/ml heparin (Sigma, St. Louis, MO). Murine NSCs were engineered to express β-galactosidase by means of in vitro infection, with the LacZ gene bearing replication-defective adenovirus as described previously [3].
Establishment of In Vivo Glioma Model and NSC Inoculation
Six- to 8-week-old C57Bl/6 mice obtained from Charles River Laboratories (Wilmington, MA) were anesthesized with intraperitoneal ketamine and xylazine and stereotactically inoculated with 5 x 104 GL26 cells in 3 µl of 1.2% methylcellulose/MEM in the right corpus striatum as reported previously [11]. At day 7 postimplantation, animals received intratumoral inoculations of 2 x 105 neural stem cells expressing β-galactosidase (NSC-LacZ) in 5 µl of serum and virus-free media, injected directly into established tumor using the same burr hole and stereotactic coordinates. Animal use was performed in strict accordance with Institutional Animal Care and Use Committee guidelines in force at Cedars-Sinai Medical Center.
Histological Visualization of Tumor-Bearing Brain Sections and Immunohistochemical Analysis of Glioma Tropic NSC Phenotypes
Brains harvested from NSC-LacZ inoculated tumor-bearing animals were frozen on dry ice, sectioned using a cryostat, mounted on slides, and air-dried. For histological visualization of LacZ-expressing NSCs, sections were stained with X-gal as per routine protocol and then counterstained with neutral red. Adjacent tissue sections were fixed in acetone. Staining was performed as per standard immunohistochemistry protocols using primary antibodies against β-galactosidase, Sox-2, SSEA-1, A2B5, E-NCAM, β-III tubulin, glial fibrillary acidic protein (GFAP), CNPase, PDGFRα (Chemicon, Temecula, CA), CXCR4 (Torrey Pines Biolaboratories, Houston, TX), EAAT1, and EAAT2 (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary staining was performed using antibodies conjugated with the fluorophores FITC or Cy3 (Chemicon). Following staining, slides were mounted in aqueous mounting media (ICN Biochemicals, St. Louis, MO) and visualized under a fluorescence microscope.
In Vitro Chemotaxis Experiments
All chemotaxis experiments were performed using a chemotaxis chamber system (Neuro Probe, Gaithersburg, MD) consisting of pairs of culture wells separated by a 5-µm porous polycarbonate membrane. Lower wells were filled with either GL26- or U87MG-conditioned media harvested as described above. Fresh DMEM supplemented with 10% FBS and 1% penicillin/streptomycin was used as a control (unconditioned media). Following placement of the intervening porous membrane, approximately 1.5 x 105 disaggregated human or murine NSCs were added to the top chambers. The chamber system was incubated at 37°C for 4 hours, after which media from lower wells were collected and quantitatively analyzed for cell content using flow cytometry against a defined number of fluorescent beads (BD Pharmingen, San Diego, CA). This allowed for quantification of the percentage of cells added to each top chamber that had migrated to the bottom chamber. For neutralization assays, anti-SDF-1 (250 µg/l) (neutralizing both known α and β isoforms of the chemokine) and anti-CXCR4 (40 µg/ml) monoclonal antibodies (R&D Systems, Minneapolis, MN) were incubated with tumor-conditioned media or NSCs, respectively, for 30 minutes at room temperature prior to the assay. Control samples were incubated with identical concentrations of an isotype-matched nonspecific antibody (BD Pharmingen). All experiments were performed in triplicate.
Results
NSCs That Migrate to Sites of Disseminating Tumor Comprise of Astrocytic Precursors
We wished to determine whether the tumor tropism exhibited by inoculated primary NSCs for in vivo-disseminating glioma was localized to a particular subpopulation at a specific stage of differentiation. We histochemically analyzed brain tissues from glioma-bearing animals that had received intratumoral inoculations of NSC-LacZ. Routine X-gal staining revealed a significant proportion of β-galactosidase+ cells that had migrated away from the site of inoculation into proximity of islets of tumor cells (readily identifiable following a neutral red counterstain) that were disseminating into and through normal brain parenchyma (Figure 1), similar to findings reported by us previously [3]. At the same time, a residual population of NSC-LacZ remained localized to the site of initial inoculation and did not exhibit this migratory, tumor-tropic activity. We then subjected mirror sections of the abovementioned samples (i.e., analogous histological samples that were not more than 20 to 30 µm removed from the original samples visualized with X-gal staining) to immunofluorescent histochemistry with a panel of antibodies specific for markers reflective of proteins expressed at varying stages of NSC differentiation. These included the transcription factor Sox-2 and the cell surface antigen SSEA-1, known to be expressed in uncommitted neural precursors; A2B5 and E-NCAM, indicative of NSCs that have initiated differentiation pathways toward astrocytic and neuronal fates, respectively; GFAP, expressed in cells of astroglial lineages; EAAT1 and EAAT2, glutamate transporter-related proteins found in functional, differentiated astroglial cells; PDGFRα, expressed in oligodendroglial precursors; CNPase, found in differentiated oligodendrocytes; and β-III tubulin, expressed in precursor as well as differentiated neuronal cells [6,12–15]. We specifically focused on expression of these markers in β-galactosidase+ NSCs that had dispersed from the primary inoculation tract and were now migrating in conjunction with, or in proximity to, disseminating tumor satellites, as observed on earlier X-gal-stained mirror sections. Our findings (summarized in Table 1) indicated that although populations of NSCs expressing Sox-2 and SSEA-1 existed in the vicinity of the initial injection tract, the majority of β-galactosidase expressing NSCs that were seen migrating along with glioma outgrowths and satellites were negative for these markers (not shown). Additionally, these tumor-tropic NSC populations were strongly positive for A2B5 and GFAP (Figure 2), although negative for the oligodendroglial-associated proteins PDGFRα and CNPase (not shown) as well as the neuronal marker β-III tubulin (not shown), clearly indicating differentiation toward astrocytic lineages. At the same time, these cells were negative for the glial-specific glutamate transporter-related proteins EAAT1 and EAAT2, known to be expressed in differentiated astrocytes [13]. Conversely, populations of β-galactosidase+ cells with differentiated morphologies that expressed EAAT1 and EAAT2 along with GFAP and A2B5 could be observed in the vicinity of the initial injection tract within the main tumor mass (not shown), confirming that complete astrocytic differentiation of inoculated precursors was, in fact, taking place. However, the absence of EAAT1/EAAT2 expression in glioma-tracking β-galactosidase+ cell populations, in conjunction with expression of A2B5 and clear absence of fully differentiated morphology, indicates that tumor-tropic cell populations likely comprised of progenitor cells that had initiated, but not completed, pathways toward astrocytic differentiation.
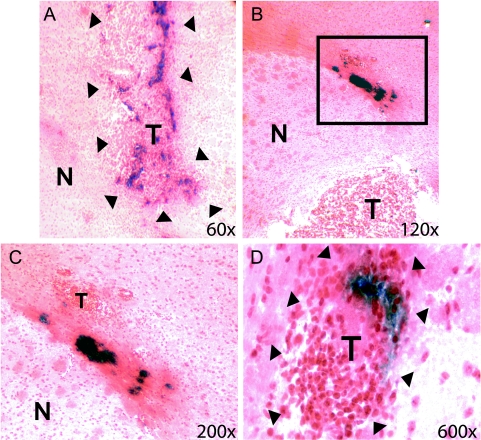
Figure 1.
NSCs are tropic for disseminating glioma in vivo. β-Galactosidase-expressing NSCs were inoculated into established intracranial GL26 tumors in C57Bl/6 mice. Histological brain sections were then processed with routine X-gal staining, resulting in the development of a blue to dark blue precipitate within NSC-LacZ. Sections were then counterstained with neutral red. Tumor tissue could be identified by intense red staining of neoplastic nuclei and visible dense aggregates of tumor cells. T designates tumor, and N represents normal tissue. (A) Low-power image illustrating the presence of nonmigratory NSC-LacZ within main tumor mass (T), demarcated by arrows. (B) Panel illustrates NSC-LacZ that have moved out of the main tumor mass and are moving into the proximity of tumor cell islets that are migrating along the grey matter/white matter boundary, likely along a white matter tract (inset box). Note that migratory NSC-LacZ are still aggregated in neurosphere-like accumulations. (C) Panel represents a high-power magnification of the inset box in (B). Dark blue NSC-LacZ aggregates are clearly visible in close proximity to a disseminating tumor satellite (T). (D) High-power image of an independent tumor satellite (demarcated by arrowheads) at significant distance from primary tumor site. Blue NSC-LacZ are visible within the tumor, clearly indicating that NSC-LacZ are capable of extensive migratory activity in vivo and can intercalate themselves into disseminated tumor islets.
Table 1.
Expression of Protein Markers Associated with Differentiation of NSCs on In Vivo Intratumorally Inoculated NSC-LacZ.
| Differentiation Stage-Related Marker |
Differentiation Stage | Staining on Non-migratory NSC-LacZ |
Staining on Glioma-Tropic NSC-LacZ |
| Sox-2 | Multipotent NSCs | Weak, scattered cells | Negative |
| SSEA-1 | Multipotent NSCs | Weak, scattered cells | Negative |
| A2B5 | Glial-restricted precursor, astrocyte-restricted precursor, astrocyte | Positive | Positive |
| E-NCAM | Neuronal precursor, neuron | Weak, scattered cells | Negative |
| PDGFRα | Oligodendroglial precursor, oligodendrocyte | Negative | Negative |
| GFAP | Astroglial precursor, astrocyte | Strongly positive | Strongly positive |
| β-III tubulin | Neuron | Weak, scattered cells | Negative |
| CNPase | Oligodendrocyte | Weak, scattered cells | Negative |
| EAAT1/EAAT2 | Differentiated glia (primarily astrocytes) | Positive | Negative |
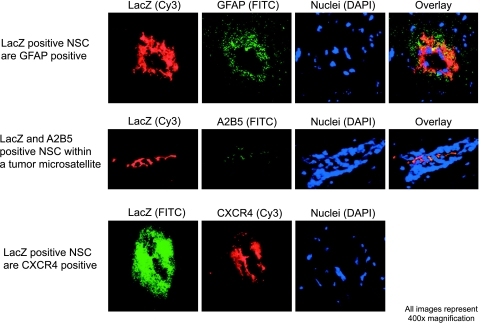
Figure 2.
NSCs tracking tumor outgrowths and satellites in vivo are likely astrocytic precursors and express CXCR4. β-Galactosidase-expressing NSCs tracking disseminated glioma on histological sections of treated tumor-bearing brains were identified and sections were stained using fluorescent histochemistry for a variety of neuronal differentiation-and glial differentiation-specific markers. NSCs within tumor outgrowths and satellites were positive for the astrocytic precursor markers A2B5 and GFAP (top and center rows, respectively) and negative for the neuronal commitment and differentiation markers E-NCAM and β-III tubulin (not shown). Additionally, tumor-tracking NSCs strongly expressed CXCR4, the cell surface receptor for SDF-1.
Tumor-Tracking NSCs Strongly Express CXCR4
Based on the demonstrated ability of SDF-1 secretion from invasive glioma cells in promoting tumor invasiveness and survival [10,16], as well as the established role of this chemokine and its receptor CXCR4 in governing neuronal and glial precursor migration within the developing brain [7,8], we wished to investigate whether tumor-tracking NSC-LacZ populations expressed CXCR4. We demonstrated that, although weak CXCR4 expression was visible both on glioma cells as well as within NSC-LacZ populations remaining within the main tumor mass (not shown), NSC-LacZ that were tracking tumor outgrowths and satellites strongly expressed this protein (Figure 2), indicating a potential role for this receptor in governing NSC responsiveness to glioma-elaborated chemotactic cues.
NSC Migration Toward Tumor-Conditioned Media In Vitro Can Be Inhibited by Blocking NSC Surface CXCR4 Receptors
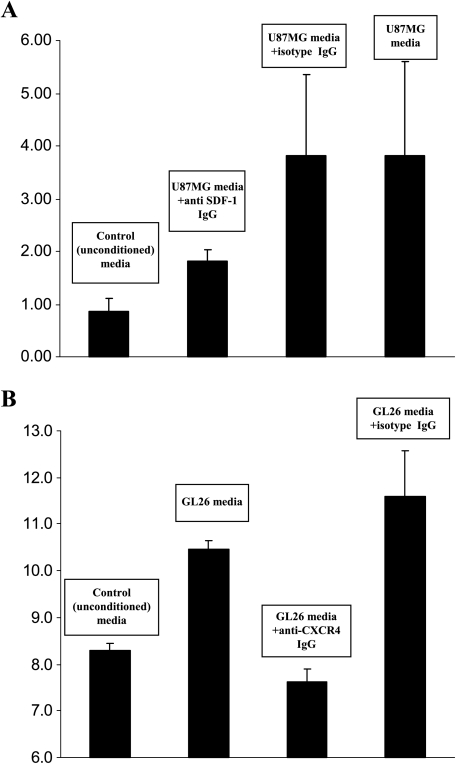
Based on our observation that tumor-tropic NSC populations in vivo strongly expressed CXCR4, we wished to determine whether this receptor played a role in NSC chemotaxis toward glioma. In a two-chamber-based experimental system wherein tumor-conditioned media was separated from human and murine NSCs by a porous membrane, we observed that NSC migration toward glioma supernatant was significantly higher than that observed toward unconditioned media (Figure 3), indicating chemotaxis toward a soluble factor present in tumor-conditioned media. In contrast, there was no significant migration of NSC migration toward conditioned media from the NIH 3T3 cell line or from the 293 human embryonic kidney cell line, further confirming the tumor-specific nature of NSC migration. With the aim of determining whether neutralization of SDF-1 in tumor supernatant would inhibit NSC migration toward glioma-conditioned media, we incubated an anti-SDF-1 antibody with human U87MG glioma tumor supernatant and then utilized this in a chemotaxis assay with human fetal NSCs. We found that in comparison to the significant NSC chemotaxis seen toward the U87MG supernatant incubated with an isotype-matched nonspecific antibody, addition of the anti-SDF-1 neutralization antibody markedly decreased NSC migration (Figure 3A), although this difference did not meet statistical significance (P = 0.09; t-test). The addition of anti-SDF-1 neutralization antibody did not significantly decrease murine fetal NSC migration toward GL26 glioma-conditioned media (data not shown). However, following incubation with an anti-CXCR4-blocking antibody, a significant decrease in NSC migration toward glioma-conditioned media was seen both in the case of murine (Figure 2) as well as human (not shown) fetal NSCs (P = 0.022 and P = 0.003, respectively; t-test). In contrast, NSCs incubated with an isotype-matched nonspecific antibody did not exhibit decreased migration toward tumor-conditioned media when compared to untreated NSCs (Figure 3B). These data indicate that blocking of CXCR4 significantly inhibits NSC taxis toward glioma supernatant, suggesting an important role for this receptor in the tumor-tropic behavior exhibited by these cells. Our inability, however, to observe a statistically verifiable difference following neutralization of SDF-1 in tumor supernatants may indicate either suboptimal neutralization of soluble chemokine or presence within the tumor-conditioned media of secondary ligands capable of inducing chemotaxis through the CXCR4 pathway.
Figure 3.
NSCs demonstrate migratory tropism toward glioma-conditioned media in vitro. Human and murine fetal NSCs were placed in the upper well of a two-well chemotaxis chamber system, separated from a lower well containing various media/culture supernatants by a polycarbonate membrane with multiple 5-µm pores. Following incubation at 37° for 4 hours, media from the lower chambers were harvested and cells were quantified. Y-axis depicts percentage of NSCs that migrated into the lower chambers. (A) Results indicated that human fetal NSCs demonstrated minimal migratory activity toward normal unconditioned medium, whereas movement toward U87MG glioma supernatant was significantly higher (P = .005; t-test). Dilution of glioma media resulted in a significant decrease in NSC chemotaxis (not shown), indicating that NSC translocation was likely due to a tumorelaborated soluble factor. Addition of a neutralizing antibody against one such potential factor, SDF-1, reduced chemotaxis noticeably compared to NSCs treated with nonspecific isotype IgG, albeit not to a statistically significant extent (P = .09; t-test). (B) Murine fetal NSCs demonstrated enhanced migratory activity toward GL26-conditioned medium compared to control media (P = .0001; t-test). Addition of an anti-CXCR4 neutralization antibody significantly decreased NSC translocation toward glioma-conditioned media compared to NSCs treated with nonspecific isotype IgG (P = .003; t-test).
Discussion
In the setting of malignant glioma, the use of tumor-tropic NSCs represents a promising approach capable of delivering tumoricidal therapeutic agents directly to disconnected neoplastic foci. The ability of NSCs to track to isolated sites of disseminated tumor is fundamental to the success of this approach, and defining the mechanisms governing this behavior will prove critical to refining this therapeutic option for potential clinical translation. Recently, it has been reported that high-grade gliomas secrete significant levels of SDF-1, and that the expression of this protein and the CXCR4 receptor correlated with the histological grade and invasive capacity of these tumors, as well as tumor cell survival [9,10,16]. Additionally, the interaction of CXCR4 and SDF-1 is a known factor involved in the migration of NSCs [7], including astrocytic precursors, in the external granular layer of the developing cerebellum [8]. We now demonstrate that glioma-tracking populations of NSCs within intratumorally injected primary NSC inoculae comprise of cells with phenotypic expression profiles characteristic of astrocytic precursors, as well as strong expression of CXCR4, and that the tumor-tropic capacity of these cells can be inhibited by neutralization of surface CXCR4. These findings clearly point to astrocytic progenitors as the candidate cells exhibiting tumor-tracking capacity within transplanted NSCs, which is in keeping with earlier findings that immature astrocytes have the potential to migrate toward pathology in the brain [17]. In this context, it is important to note, however, that Rao [12] has described the absence of GFAP along with expression of A2B5 as characteristic of astrocyterestricted precursors within populations of NSCs. Our results, in contrast, demonstrate expression of both A2B5 and GFAP within tumor-tropic NSCs along with an absence of differentiated morphology and negligible expression of the glutamate transporters EAAT1/EAAT2, which are associated with functionally differentiated glial progeny. This may indicate that tumor-tropic NSCs exhibit an astrocytic precursor phenotype at a more advanced stage of differentiation than that reported by Rao. Similarly, the expression of GFAP by these tumor-tracking populations also indicates that these cells are at a level of fate commitment beyond that of the previously described bipotent O-2A astroglial- and oligodendroglial-restricted precursor [18]. Morphological immaturity and the absence of EAAT1/EAAT2 indicate that these cells have, however, not reached astrocytic maturity. In this context, it is likely that complete differentiation of these tumor-tropic populations occurs soon after the 1-week time point analyzed in our experiment, although we have previously described an abundance of morphologically undifferentiated, transplanted tumor-tropic cells up to 18 days following intratumoral inoculation of NSCs [3]. Of additional interest was our observation that expression of the neuronal markers E-NCAM and β-III tubulin was found in a very small proportion of transplanted NSC-LacZ. Although the corpus striatum has been shown to have a predominantly gliogenic influence on transplanted neural precursors [19], the paucity of neuronal differentiation we noted may potentially indicate the presence of factors inhibiting neurogenesis within the tumor microenvironment.
Based on the known chemotactic involvement of CXCR4 in neuronal and glial precursor migration in the developing CNS [7,8], we wished to investigate whether this receptor played a functional role in NSC tropism toward tumor. Our observation that blocking of CXCR4 inhibits NSC tropism for glioma in vitro, which was consistently reproducible following repeated assays, fundamentally supports the involvement of this receptor in mediating this behavior. It is important to note, however, that in spite of demonstrating marked reductions in mean NSC translocation following neutralization of SDF-1, we were unable to validate these differences statistically, despite repeated experimentation confirming this inhibitory effect. This may represent a technical issue involving suboptimal neutralization of soluble chemokine versus more efficient blocking of cell surface CXCR4, or these findings may point to a role for additional, as of yet unidentified soluble ligand(s) for CXCR4, possibly further isoform variants of SDF-1 apart from the α and β subtypes we neutralized. Nevertheless, as SDF-1/CXCR4-mediated astrocytic precursor dissemination may play a key role in the establishment of a glial scaffold in the developing brain and the elaboration of SDF-1 by disseminating glioma cells is implicated as a key factor in their invasive properties, it is likely that similar mechanisms govern the tropism of NSCs for migrating glioma cells.
The level of NSC migration we observed toward glioma-conditioned media in vitro was significantly lower than that qualitatively predictable based on our previously described in vivo migration patterns [3]. This is, however, in conjunction with our finding that tumor-tropic behavior is exhibited principally by cells that are progressing toward astrocytic differentiation. As the cells utilized in our in vitro experiments comprised chiefly of NSCs cultured in conditions designed to favor maintenance of an undifferentiated state, although early evidence of eventual neuronal or glial directionality may still be discernable [12], a lower percentage of committed and actively differentiating astrocytic precursors would be expected in these populations. Following in vivo transplantation, however, NSCs respond to predominantly gliogenic cues inherently present in the corpus striatum, increasing the numbers of astrocytic progenitors potentially responsive to chemotactic signals emanating from disseminating tumor cells. This is further supported by our observation that NSC populations maintained in culture contain numerous cells positive for the uncommitted precursor marker Sox-2 (M. Ehtesham, unpublished results), whereas expression of this factor was very weak in transplanted NSC-LacZ 1 week following in vivo inoculation (Table 1). These findings may support a rationale for intratumoral transplantation of partially differentiated CXCR4+ astrocytic progenitors rather than generalized pools of NSCs. In particular, engineering A2B5/GFAP+ astrocytic precursors to overexpress CXCR4 may prove an attractive strategy to promote glioma tropism and improve the therapeutic potential of these cells. However, given that cerebral white matter—the most relevant site for inoculation of tumor therapeutic NSCs—is known to already provide an environment favoring differentiation of NSCs into astrocytes, it is unclear whether introduction of partially differentiated cells would provide any tangible benefit as opposed to the use of uncommitted NSCs.
Also of interest was our finding that primary murine fetal NSCs exhibited significantly more migration, even toward unconditioned media, as opposed to human fetal NSCs. This may be explained by the differing origins of these cultures. Murine NSCs were derived from primary fetal tissue, whereas human fetal NSCs were cultured from a several-year-old, cryopreserved, commercially available stock. It is possible that freshly generated primary murine cells displayed a more active migratory capacity as opposed to the human NSCs, whose biological activity may have been hampered secondary to prolonged cryogenic storage. Additionally, human NSCs were subjected to repeated passaging prior to their storage, which may also have altered their phenotypic characteristics, making them less responsive to glioma-elaborated chemotactic cues.
Given the abysmal prognoses associated with high-grade gliomas, there is an urgent need to develop novel therapies with translational potential. Currently, the use of NSCs as therapeutic delivery vehicles has offered encouraging results in preclinical models. The use of this technology in patients is still, however, hampered by significant limitations, key among which is the isolation of clinically viable and legally utilizable sources of tumor-tropic neural progenitors. Progress is, however, being made on this front as exemplified by recent reports by our group and others regarding alternative tissue sources from which multipotent neural precursors can be derived [20,21]. Additionally, preliminary evidence indicates that these cells may also be tactic for migrating glioma cells (P. Kabos, unpublished results). The identification of CXCR4 as a key element governing the process of neural precursor migration toward glioma cells may allow for more efficient isolation of potentially tumor-tropic cells from these alternative tissues, thereby hastening the therapeutic testing of glioma-tracking neural precursors in a clinical setting.
Acknowledgements
We are grateful to Sebastian Wachsmann-Hogiu, Renato DeAraujo, and Daniel H. Farkas for their kind assistance in obtaining fluorescent images of histochemically stained brain sections.
Footnotes
This work was supported, in part, by National Institutes of Health grant NS02232 to J.S.Y.
References
- 1.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neuro-Oncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 2.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 4.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 5.Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, Yu JS. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 6.Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- 7.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 8.Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 9.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 10.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1 alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63:1969–1974. [PubMed] [Google Scholar]
- 11.Ehtesham M, Samoto K, Kabos P, Acosta FL, Gutierrez MA, Black KL, Yu JS. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 12.Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland ML, Delaney TA, Noebels JL. Glutamate transporter mRNA expression in proliferative zones of the developing and adult murine CNS. J Neurosci. 1996;16:2191–2207. doi: 10.1523/JNEUROSCI.16-07-02191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai J, Limke TL, Ginis I, Rao MS. Identifying and tracking neural stem cells. Blood Cells Mol Dis. 2003;31:18–27. doi: 10.1016/s1079-9796(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 15.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNSstemcells, identifying themas nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 17.Smith GM, Silver J. Transplantation of immature and mature astrocytes and their effect on scar formation in the lesioned central nervous system. Prog Brain Res. 1988;78:353–361. doi: 10.1016/s0079-6123(08)60304-0. [DOI] [PubMed] [Google Scholar]
- 18.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 19.Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 21.Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002;178:288–293. doi: 10.1006/exnr.2002.8039. [DOI] [PubMed] [Google Scholar]