Abstract
Normal human diploid cells do not spontaneously immortalize in culture, but instead enter replicative senescence after a finite number of population doublings. Ablation of key checkpoint arrest or cancer-suppressor genes, through dominantly inherited germline mutation (p53+/-, Li-Fraumeni) or viral oncogene expression (SV40 large T, HPV16/18, and E6/E7) can lead to escape from senescence, additional doublings, and entrance into crisis phase, where immortal clones emerge at low frequency. In the vast majority of cases, telomerase is reactivated and telomeres are stabilized. Here we describe the spontaneous immortalization of clinically normal fibroblasts derived from colonic stroma of a familial adenomatous polyposis (FAP) patient. The preimmortal (C26C) and the spontaneously immortalized derivative (C26Ci) cells are heterozygous for a characterized germline mutation in exon 15 of the adenomatous polyposis coli gene. Immortalization was accompanied by spontaneous reactivation of endogenous telomerase and establishment of telomeres at presenescent lengths. Normal checkpoint behavior is retained and a diploid karyotype is maintained. These cells provide a valuable new addition to the limited number of spontaneously immortalized human cell types, particularly fibroblast cells, and will be useful in experimentally determining the functional pathways in neoplastic development and in the identification of potential molecular targets for cancer chemoprevention.
Keywords: Adenomatous polyposis coli, familial adenomatous polyposis, spontaneous immortalization, telomerase, telomere
Introduction
Familial adenomatous polyposis (FAP) is a colon cancer predisposition syndrome with a population incidence of approximately 1 in 8000 [1]. The syndrome is inherited as an autosomal dominant trait with an early age of onset, and is characterized by the development of hundreds to thousands of polyps along the entire colon [2]. By their mid-30s, 95% of FAP-affected individuals have developed polyps and the mean age of cancer onset in untreated patients is 40 years. The gene responsible for FAP is adenomatous polyposis coli (APC) located on chromosome 5q21-22 [3,4]. Following the classic Knudson model, the loss of the remaining wild-type APC allele is the rate-limiting step in the adenoma-carcinoma pathway of neoplastic development [2,5]. Importantly, loss of functional APC, although central, in itself is not sufficient to progress beyond an early adenoma. Progression to carcinoma requires loss of regulation in a number of pathways including adhesion, signaling, growth factors, apoptosis, angiogenesis, and cell proliferation.
The APC gene spans over 200,000 nucleotides, including 16 exons that encode a product 2843 amino acids in length. The last exon represents 75% of the entire coded sequence and is also where mutations are frequently located [6]. Most inherited mutations lie between codons 1055 and 1309, whereas somatic mutations predominate in a mutation cluster region (MCR) localized to codons 1286 to 1513. These mutations generally take the form of nonsense or frameshift changes, which result in a truncated protein. Germline mutations between codons 1194 and 1392 tend to result in loss of heterozygozity (LOH) of the remaining wild-type allele, whereas mutations outside of this area are usually associated with secondary truncating mutations in the MCR [7]. The resulting secondary event causes a complete loss of functional APC protein, regardless of the precise position of the germline mutation.
The primary outcome of truncating mutations on the APC gene is the loss of the APC-directed degradation of β-catenin [8]. Loss of the ability to target β-catenin for degradation leads: to 1) aberrant activation of the β-catenin/T-cell factor-4 (TCF-4) signal transcription machinery, and 2) loss of the APC/β-catenin migratory function, which results in accumulation of proliferative epithelial cells in the crypt and polyp formation [9]. A major event in the development of almost all cancers is bypassing telomere-directed replicative senescence [10]. It is not clear when telomerase is reactivated in colon cancer, although it has been suggested to occur at the adenoma/carcinoma transition [11].
Normal human cells in culture do not spontaneously immortalize unless the M1 and M2 checkpoints are abrogated. The first checkpoint gate, M1, is primarily reliant on the p53 DNA damage pathway to short/unmasked telomeres. Viral oncogenes such as the SV40 large T antigen and the HPV E6/E7 proteins can bypass M1 by blocking the response pathways. To become immortal, the second checkpoint gate, M2, must also be circumvented. M2 is also called “crisis” due to the cell death that occurs after continually shortening telomeres become unstable, undergo bridge-breakage-fusion cycles, and cause apoptosis. Immortalized cells can emerge from crisis after stabilizing their telomeres, usually through the reactivation of the telomerase enzyme.
The human cancer susceptibility syndrome, Li-Fraumeni syndrome (LFS), results from a heterozygous germline mutation in p53. Breast epithelial cells from LFS patients can spontaneously immortalize at a frequency of 10-7, whereas breast-derived fibroblasts from LFS patients immortalize much less readily [12,13]. Despite repeated attempts in the past, these are the only clear documentations of spontaneously immortalizing human cells from normal individuals or those genetically predisposed to cancer. Here we describe the spontaneous immortalization of a human fibroblast strain derived from a clinically normal colonic stroma in an FAP patient. These cells carry a four-base deletion at codon 1157 to 1158 of the APC gene, which results in frameshift and premature truncation of the protein product from one allele. These cells undergo approximately 30 population doublings (PDs) before slowing down. From three experiments with total cell number at senescence of around 1.5 x 107/cells, a solitary clone emerged, thus producing an immortalization frequency of 0.7 x 10-7. This clone displays functional endogenous telomerase activity, and maintains normal checkpoint control activity. These cells also retain APC expression because they have not developed a secondary mutation within the MCR region. This suggests that either a heterozygous APC allele may be sufficient to select for an activating mutation(s), or that loss of the second allele is not necessary for cellular immortalization. The reported early activation of telomerase in polyp development could be explained by this mechanism. Therefore, immortalization in vitro could be equated with autosomal dominant events that occur during early initiation of polyp formation in vivo, whereas complete inactivation of the APC gene is an autosomal recessive trait at the cellular level that is associated with cancer progression [14].
Materials and Methods
Cell Culture
C26C cells were derived from noncancerous colonic stromal fibroblasts of an FAP patient. The cells were maintained in four parts of Dulbecco's modified Eagle's medium to 1 part of medium 199, supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) and 50 mg/ml gentamicin sulfate. Cells were passaged 1:8 approximately once a week and split ratios were used for calculating approximate PDs where a 1:8 split was taken to represent three PDs at confluence. Cells were considered growth-arrested after 100 days in culture with no obvious proliferation. Normoxic cells were maintained in 37°C humidified incubators containing 5% CO2 and ambient oxygen (21%). To create a low-oxygen environment, Plexiglass containers with sealed lids were flushed with a mixture of 2% O2, 7% CO2, and 91% N2. Measurements with a Fyrite O2 meter indicated that oxygen concentrations gradually rose from 2% to 5% over a 5-day period. Containers were thus gassed twice per week.
Vectors and Retroviruses
Retroviral infections were performed using supernatants from PA317 cells infected with pBabePuro containing hTERT [18]. Mock infections, no retroviral supernatant, were performed as a selection control; once they had died, puromycin was removed from the media. Independent infections were performed in both low oxygen and normoxia.
UV Irradiation
Cells were irradiated according to the protocol described previously [15]. Briefly, cells were washed twice with phosphate-buffered saline (PBS), media-aspirated, and exposed to 100 J/msec UV-B without being allowed to dry. Medium was then replaced and cells were incubated for the desired time points. Following incubation, the cells were harvested and protein lysates were generated.
Western Analysis and Immunoflourescence
Chemiluminescence was used to detect signals from secondary antibody (ECL; Amersham Pharmacia, Buckinghamshire, UK). Primary antibodies recognizing p21WAF1 (Ab-1) and p53 (DO-1) were obtained from Oncogene Research Products (Boston, MA); exogenous hTERT (Geron1A4) was from Geron Corporation (Menlo Park, CA); β-catenin was from BD Transduction Laboratories (San Diego, CA); and TFIIB (C-18) was from Santa Cruz Biotechnology (Santa Cruz, CA). For immunofluorescence, cells were plated in chamber slides 48 hours prior to staining. Briefly, slides were fixed at -10°C in methanol, washed in PBS, and blocked with 10% BSA at 37°C for 1 hour. Primary antibody (e.g., β-catenin) incubation (1 hour at 37°C) was followed by PBS washes and secondary antibody incubation (1 hour at room temperature). Following washes, slides were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA) and viewed with fluorescence microscopy.
Telomerase Activity and Telomere Length Analysis
Telomerase activity was determined using the TRAP assay as described [16]. Telomere length was measured after DNA extraction at multiple PD levels, digestion with the restriction enzyme cocktail (AluI, CfoI, HaeIII, HinfI, MspI, and RsaI), and resolution by agarose gel electrophoresis as detailed elsewhere [17]. High-molecular-weight telomeric DNA was resolved using programmed pulse field gel electrophoresis (FIGE Mapper System; Bio-Rad, Hercules, CA). Telomeric DNA was visualized through hybridization of radiolabelled (T2AG3)3 and autoradiograph exposure. Signals were analyzed with the program Telorun (http://www.swmed.edu/home_pages/cellbio/shay-wright/research/sw_lab_methods) using weighted mean calculations designed to normalize signal intensity relative to the digestion product size.
Detection of APC Mutation and Expression
DNA sequence analysis of the entire APC gene was performed on the spontaneously immortalized C26Ci cell line. Polymerase chain reaction (PCR) amplification reactions were performed using DNA extracted from cultured C26Ci cells. Fluorescent dye-labeled sequencing was used to sequence (both forward and reverse direction) the amplified products. This covered approximately 8532 bp comprising 15 exons and approximately 420 adjacent noncoding intronic base pairs. Noncoding intronic regions analyzed did not extend by more than 20 bp proximal to the 5′ end, or 10 bp distal to the 3′ end of each exon. Chromatographic tracings of amplicons were evaluated by computer analysis and compared to the normal APC sequence.
To confirm the sequencing results, the reported mutation was reanalyzed by PCR analysis. DNA was isolated from C26C and C26Ci cells using standard techniques. Two primers were designed for allele-specific (normal or mutant) sequences, which, for amplification, were used with a single reverse primer. Primer sequences are: TGAAGAAGAACAGCATGAAGAAGAAGAGAG (normal), TGAAGAAGAACAGCATGAAGAAGAAGAC (mutant), and TGCTATTTGCAGGGTATTAGCAGAATCTGC (reverse). Stringent reaction conditions were used with an annealing temperature of 66°C for 60 seconds. The human β-globin gene was used as a positive control. Control primer sequences are: CAATGTATCATGCCTCTTTGCACC and GAGTCAAGGCTGAGAGATTGCAGG. The reaction products (472 bp for APC and 860 bp for β-globin products) were analyzed using 2% agarose gel electrophoresis.
For expression analysis, cells were harvested at log phase of growth and RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) using the provided methodology. Purity of RNA was determined by the ratio of A260/A280, where values greater than 2.0 were taken as evidence of sufficient purity. One-step reverse transcription-polymerase chain reaction (RT-PCR) was performed using Superscript One-Step RT-PCR with Platinum Taq (Invitrogen) using primers designed to be exon spanning-specific (Table 1). Products were resolved using 1.2% to 2% agarose gel electrophoresis. Anticipated product sizes from RNA and genomic PCR product are listed in Table 2.
Table 1.
Primers Used in RT-PCR.
| Exon | Forward | Reverse | |
| 10 | 5′-GTCCTGCTGTGTGTGTTC-3′ | ||
| 11 | 5′-ACTGTGAAATGTATGGGC-3′ | 5′-CCATTCCAGCATATCGTC-3′ | |
| 12 | 5′-TGCAGCCTTTCATAGAGC-3′ | ||
| 13 | 5′-TTTGTCTTGGCGAGCAGA-3′ | ||
| 14 | 5′-ATCAACCCTCAAAAGCGT-3′ | 5′-CAAGCTGGACACATTCCG-3′ | |
| 15 | 5′-GCATCCTTGTACTTCGCA-3′ | ||
| 15del | 5′-TTCGGTTTTACTGCTTTG-3′ |
The 15del primer is 3′ to the familial truncation mutation present at codons 1157 to 1158.
Table 2.
Expression and Genomic PCR Product Sizes.
| Primer Pair | RNA Product Size (nt) | DNA Product Size (nt) |
| Ex10F–Ex11R | 172 | 5287 |
| Ex11F–Ex12R | 218 | 813 |
| Ex13F–Ex14R | 286 | 6264 |
| Ex14F–Ex15R | 473 | 2862 |
| Ex14F–Ex15Rdel | 1881 | 4270 |
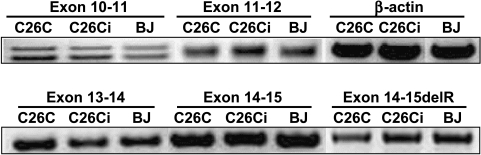
The exon-spanning primer strategy allows immediate distinction between expressed and processed mRNA and genomic DNA based on product size following electrophoresis. PCR products are shown in Figure 5.
Results
Growth Curves of FAP Fibroblasts
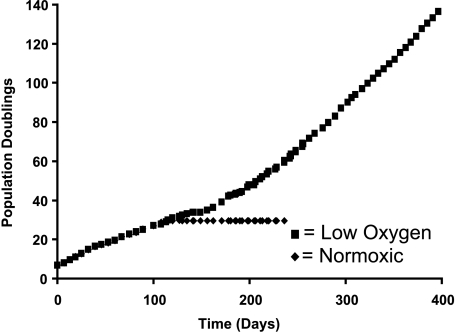
Primary cultures of normal human fibroblasts proliferate for between 30 and 80 PDs, depending on cell strain and donor age, after which time they undergo growth arrest called replicative senescence. Low oxygen (2–5% oxygen), which resembles that seen in the physiologic setting, can delay the onset of senescence and prolong proliferative cellular lifespan by up to 40% [18]. Cultures of clinically normal colonic stromal fibroblasts derived from an FAP patient, termed C26C, underwent approximately 30 PDs before undergoing growth arrest (Figure 1). These cells were grown in standard tissue culture conditions with ambient room oxygen concentrations (21%). A minimal extension of lifespan (10%) was seen when C26C was cultured in low oxygen, but under these conditions, a clonal population of cells emerged from the growth-arrested population at a frequency estimated to be around 0.7 x 10-7 (one clone from 1.5 x 10-7 cells), over an approximately 45-day period. These cells, when pooled (termed C26Ci), continued to proliferate and, when transferred back into the room oxygen, did not engage a growth arrest stress response pathway. After 150+ PDs, these cells were deemed immortal as they had demonstrated a greater than 400% increase in proliferative lifespan.
Figure 1.
Growth curves of FAP colonic fibroblasts. Normoxic (◆) and low-oxygen (■) cell growth curves are shown. The x- and y-axes values are time (days) and PDs, respectivel
Telomere Length and Telomerase Activity of FAP Fibroblasts
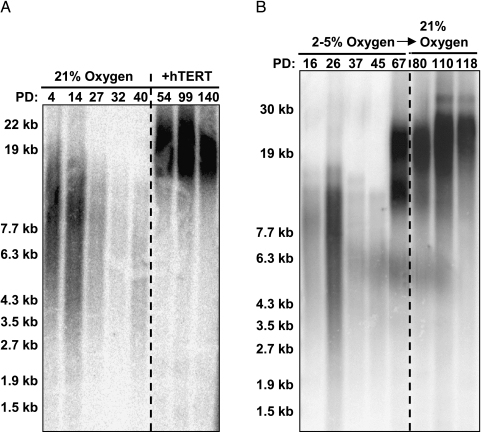
A prerequisite of cellular immortalization is the almost universal reactivation of telomerase and the bypass of telomere-directed senescence. Growth arrest in C26C cells occurred with telomeres of around 6 kb, and this was irrespective of the oxygen concentration used for the primary culture of the cells (Figure 2, A and B). Direct immortalization with ectopic hTERT was readily achievable in both low oxygen and room oxygen (data not shown). The spontaneously immortalized pooled clones displayed greatly elongated telomeres by the first assayable point (67 PDs) of around 15 to 20 kb, and they were maintained at approximately 20 kb thereafter (Figure 2B). Transfer of the immortal cells into normoxia did not reduce telomere length, which instead continued to elongate at a low rate. Assay of selected time points of the parental populations for telomerase activity showed that normoxic cells were negative throughout their lifespan (Figure 3). Although low-oxygen populations were also negative initially, cells assayed following the emergence of the immortal clone were positive for telomerase activity, functional activity as reflected by the elongated telomeres, and correctly spliced hTERT message (data not shown). This identifies the mechanism of immortalization of these cells as reactivation of the telomerase enzyme.
Figure 2.
Telomere elongation in C26C and C26Ci cells. (A) C26C cells cultured in room oxygen display telomere shortening with increasing lifespan, which is reversed by reconstituting telomerase activity with exogenous hTERT expression. (B) C26C cells cultured in low oxygen have telomeres that shorten in a normal manner but elongate following the emergence of a variant (C26Ci) population, which has reactivated endogenous telomerase activity. C26Ci telomere length is not sensitive to room oxygen following variant selection in low oxygen. Molecular weights are indicated on the left-hand axis; PDs and culture conditions are indicated on the upper x-axis.
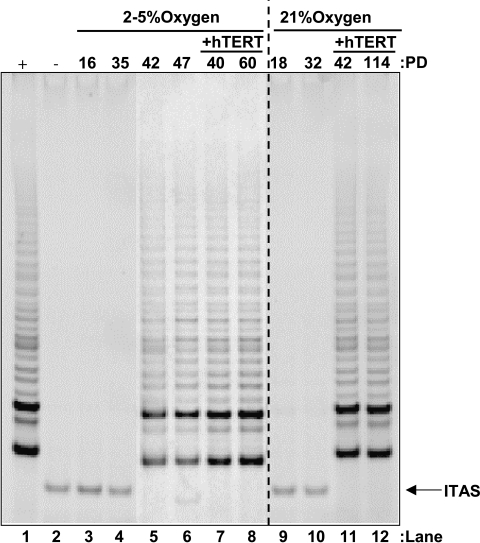
Figure 3.
Telomerase activity is coupled to C26Ci variant emergence. Lanes 1 and 2 indicate positive and negative control. Control populations of cells do not display telomerase activity (lanes 3–4 and 9–10), but following variant emergence in low oxygen, it becomes detectable (lanes 5–6). Exogenous hTERT expression reconstitutes telomerase activity in both low and room oxygen (lanes 7–8 and 11–12). Upper axis indicates PD, oxygen conditions, and the presence or absence of exogenous hTERT. Internal telomerase amplification standard (ITAS) is indicated on the lower right-hand axis.
DNA Damage and Stress Response Pathway Status
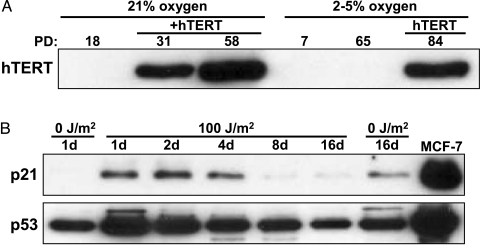
Spontaneously immortalized cells have generally deregulated three pathways: telomere maintenance, p53 DNA damage responses, and p16/pRB stress response pathways. This can occur through viral oncogene overexpression, or familial cancer susceptibility syndromes that carry autosomal dominant mutations in key genes such as p53+/- in Li-Fraumeni [19]. The spontaneously immortal FAP cells in the present study had deregulated telomerase such that its activity was now detectable by the TRAP assay. Western blot analysis confirmed that this was due to endogenous telomerase; an antibody that is not sufficiently sensitive to detect endogenous hTERT did not give any signal in our spontaneously immortal cells (Figure 4A), whereas C26C cells infected with retroviral hTERT in both low and room oxygen displayed abundant exogenous hTERT protein (Figure 4A). To determine the status of the p53 DNA damage response, the immortal cells were exposed to 100 J/m2 of damaging UV-B light (Figure 4B). Growth arrest and upregulation of p21 and p53 established that these pathways remained functional. In addition to maintaining intact damage and stress response pathways, immortal C26C cells were confirmed as retaining a normal human diploid complement of chromosomes (data not shown).
Figure 4.
C26Ci lacks exogenous hTERT expression and upregulate DNA damage and proteins in response to UV-B. (A) Lysates from room-oxygen and lowoxygen cells show that only hTERT-immortalized cells, and not C26Ci variants, display overexpressed exogenous protein. Upper axis indicates the lysate PD, the presence of exogenous hTERT, and oxygen concentration. The hTERT band is indicated. (B) In addition to lacking exogenous hTERT expression, the C26Ci variant cells upregulate p53 and p21 acutely in response to damaging UV-B irradiation. MCF7 is included as positive control for p53 and p21. The upper axis indicates the time passed in days, post-UV-B exposure, of cell harvesting. Days 1 and 16 untreated samples are included as controls. Slight upregulation of p53, p21, and that untreated at day 16 is likely to be due to dish confluency.
Retention of Wild-Type APC Sequence and Expression in Immortal Cells
DNA sequencing showed a 4-bp deletion at codon 1157 to 1158, which causes a premature truncation of the APC protein at codon 1163. This mutation corresponds to the patient's original germline mutation. No other deleterious mutations were identified by DNA sequencing. PCR analysis showed the presence of both mutant and wild-type APC alleles at codon 1157 to 1158 in DNA from both C26C and C26Ci cells. This confirmed the presence of the mutation identified by DNA sequencing and indicated that C26Ci cells do not have LOH at the APC locus. PCR analysis also showed amplification of the β-globin product using DNA from both C26C and C26Ci cells.
To further characterize the C26Ci cells, we sought to establish whether APC expression was retained following spontaneous immortalization. The inherited germline mutation in C26C cells lies immediately prior to codons 1194 to 1392 at 1157 to 1158. Familial mutations within 1194 to 1392 are predicted to undergo loss of the second allele as the secondary somatic mutation; those outside this region are most likely to display a truncating mutation within the MCR as a second event [7]. We confirmed the retention of wild-type APC expression. One-step RT-PCR using exon-specific primers confirmed expression from exons 11 to 15 (Table 1 and Figure 5). Primers designed to amplify exon 15 regions in both 5′ and 3′ GAGA deletion also determined that loss of the second allele had not taken place. This shows that silencing of the remaining wild-type allele by promoter methylation had not occurred.
Figure 5.
C26Ci retains wild-type APC expression. RT-PCR products derived from exon-straddling primers show that wild-type APC expression is retained in the C26Ci variant line from exon 10 until beyond the heterozygous truncating mutation. Expected expression product sizes and potential DNA contaminant product sizes are listed in Table 2.
Lack of β-Catenin Redistribution
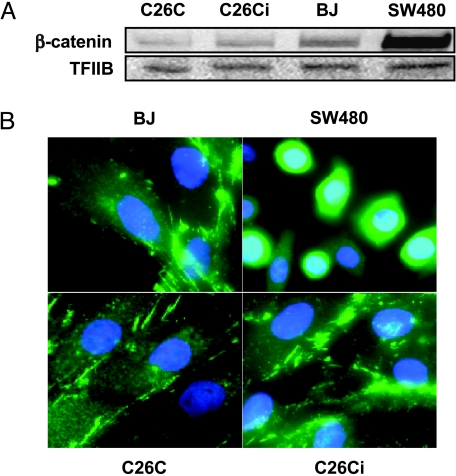
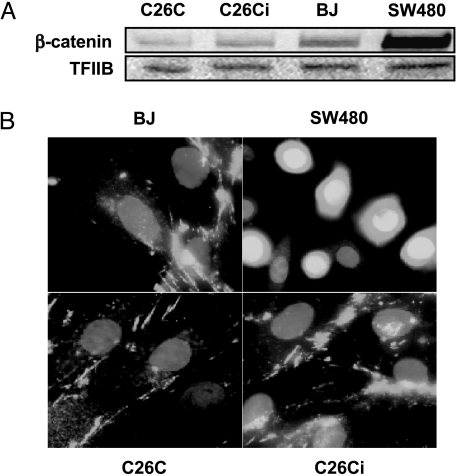
The targeted degradation of β-catenin is an important function of APC. Loss of APC leads to β-catenin-derived activation of transcriptional pathways through TCF-4 function and a loss of migratory activity resulting in polyp formation [9]. Western blot analysis determined that C26Ci cells did not have an abnormal accumulation of β-catenin protein (Figure 6A). Nuclear localization of β-catenin results in TCF-4 activation. To rule this out, we performed immunofluorescence on both mortal and immortal C26C. We observed a stress fiber cytoplasmic-associated staining pattern, which was not altered with immortalization and was similar to the normal mortal human fibroblast strain, BJ. Nuclear localization was observed in SW480 human colon carcinoma cells, which served as a positive control [20]. Alteration of the β-catenin degradation pathway allowing nuclear translocation and aberrant activation of transcription factors does not therefore appear to be the mechanism of immortalization.
Figure 6.
β-Catenin does not undergo upregulation or nuclear localization. (A) Lysates from preimmortal (PD11), immortal (PD52), and BJ all express similar levels of β-catenin. Levels of β-catenin do not become upregulated following spontaneous immortalization. SW480 is included as a positive control for β-catenin accumulation. TFIIB is included as a loading control. (B) Immunofluorescence of C26C, C26Ci, and BJ control cells display a cytoplasmic stress-fiber-associated staining pattern and did not show obvious nuclear localization. SW480 (positive control) showed nuclear localization. Nuclei are stained with 4′,6 diamidino-2-phenylindole (DAPI). Note that there is more intense nuclear staining in the β-catenin-positive SW480 cells compared to the cytoplasmic-only staining of the other cell types.
Discussion
This is the first report demonstrating the spontaneous immortalization of human FAP colonic fibroblasts in cell culture. This immortalization is dependent on a concurrent reactivation of telomerase to prevent continued telomere shortening. We found that these cells retained the heterozygous state for APC and that cell cycle checkpoint control was intact, with no apparent deregulation of the p53/p21 pathways and the retention of diploid karyotype (data not shown). The exact mechanism by which the colonic FAP fibroblasts reactivate telomerase activity remains undetermined, but does not appear to be through a β-catenin-dependent transcriptional mechanism.
Telomerase expression is sufficient to immortalize cells without leading to transformation or increased tumorigenic potential [21]. Spontaneous immortalization of human cells is, by any description, a rare event. As far as we could determine, there are no published papers of spontaneous immortalization occurring without an accompanying genetic lesion or retroviral oncogene intervention. The first category is defined by those cell lines emerging from LFS studies, where p53 heterozygozity enables cells to escape from M1 (or telomere-directed senescence) into M2 (or telomere-directed crisis) and to spontaneously immortalize at a frequency of around 5 x 10-7 [13]. The second category is defined by those cells expressing retroviral oncogenes (SV40 T antigen and HPV16/18 E6/E7) whose purpose is to deregulate cell cycle checkpoint control pathways allowing unchecked proliferation. These same deregulated checkpoint controls prevent M1 recognition, allowing progression into M2 from which spontaneously immortal clones can emerge at a frequency of 1 x 10-7 [22]. In these instances, telomeres continue to shorten and may participate in the genetic instability that occurs at M2 when end fusions are converted to chromosomal abnormalities including translocations and deletions, leading to what has been called mitotic catastrophe.
The spontaneously immortal FAP fibroblasts are best characterized as meeting the previously described first category. The emergence of clones did not, however, appear to be accompanied by any of the hallmarks associated with crisis. This suggests that either the cells had an accompanying undefined mutation that preexisted at M1, or that APC heterozygozity is in itself sufficient to be permissive for secondary mutation(s). Clearly, APC heterozygozity increases the likelihood of secondary event(s) at a relatively low probability in vivo and facilitates spontaneous immortalization in vitro.
Early deregulation and reexpression of telomerase activity are associated with aggressive FAP (carcinoma present), but not with benign cases (no carcinoma) [23]. Loss of functional APC and targeted β-catenin degradation result in relieved autorepression of the hTCF-4 transcription factor and activation of its gene targets [9]. These include c-myc, known to be an activator of hTERT transcription. However, a number of other recently identified repressors of hTERT transcription fall into well-characterized tumor-suppressor gene or oncogene pathways whose deregulation could also be responsible for telomerase reactivation [24]. These included a transcriptional target of the transforming growth factor (TGF)-β pathway: the transcription factor SIP1; Mad1, which regulates the activation of c-myc by hTERT; and Menin, which is, itself, a well-characterized tumor-suppressor gene. It remains unclear if deregulation of any one of these individual pathways is sufficient for telomerase reactivation in vivo as is the case in the tissue culture environment. A secondary inactivating mutation in any one of these pathways might therefore result in telomerase reactivation and immortalization of the targeted cells. Irrespective of the exact mechanism by which these cells have deregulated telomerase, an immortalization event has occurred. These cells provide a valuable new addition to the limited number of spontaneously immortalized human cell types, particularly fibroblast cells, and will be useful in experimentally determining the functional pathways in neoplastic development and in identifying potential molecular targets for cancer chemoprevention.
Acknowledgements
We thank Kenia Gandia and Anita Sanchez for excellent technical assistance. We also thank Zhenqiang Gao for his effort toward establishment of the parental cell line and Moreh Salunek for technical assistance in the DNA sequencing.
Footnotes
This work was supported by postdoctoral grants from the Department of Defense Breast Cancer Research Program (DAMD17-01-1-0420 to N.R.F.) and the National Cancer Institute (DCP, CN-85143, and CN-85139).
References
- 1.Bisgaard ML, Fenger K, Bulow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121–125. doi: 10.1002/humu.1380030206. [DOI] [PubMed] [Google Scholar]
- 2.Fearnhead NS, Wilding JL, Bodmer WF. Genetics of colorectal cancer: hereditary aspects and overview of colorectal tumorigenesis. Br Med Bull. 2002;64:27–43. doi: 10.1093/bmb/64.1.27. [DOI] [PubMed] [Google Scholar]
- 3.Bodmer WF, Bailey CJ, Bodmer J, Bussey HJR, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P, Sheer D, Solomon E, Spurr NK. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 4.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Lepaslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 6.Beroud C, Soussi T. APC gene: database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996;24:121–124. doi: 10.1093/nar/24.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, Frayling I, Efstathiou J, Pack K, Payne S, Roylance R, Gorman P, Sheer D, Neale K, Phillips R, Talbot I, Bodmer W, Tomlinson I. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's “two-hit” hypothesis. Nat Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 8.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 9.Bright-Thomas RM, Hargest R. APC, beta-catenin and hTCF-4; an unholy trinity in the genesis of colorectal cancer. Eur J Surg Oncol. 2003;29:107–117. doi: 10.1053/ejso.2002.1331. [DOI] [PubMed] [Google Scholar]
- 10.Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang R, Cheng AJ, Wang JY, Wang TC. Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res. 1998;58:4052–4054. [PubMed] [Google Scholar]
- 12.Gollahon LS, Kraus E, Wu TA, Yim SO, Strong LC, Shay JW, Tainsky MA. Telomerase activity during spontaneous immortalization of Li-Fraumeni syndrome skin fibroblasts. Oncogene. 1998;17:709–717. doi: 10.1038/sj.onc.1201987. [DOI] [PubMed] [Google Scholar]
- 13.Herbert BS, Wright AC, Passons CM, Wright WE, Ali IU, Kopelovich L, Shay JW. Effects of chemopreventive and antitelomerase agents on the spontaneous immortalization of breast epithelial cells. J Natl Cancer Inst. 2001;93:39–45. doi: 10.1093/jnci/93.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Kopelovich L. Heritable colorectal cancer and cancer genes: systemic expressions. Mol Carcinog. 1993;8:3–6. doi: 10.1002/mc.2940080104. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- 16.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 17.Ouellette MM, Liao M, Herbert BS, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- 18.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell. 2003;2:235–243. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 19.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, Friend SH. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 20.Rice PL, Kelloff J, Sullivan H, Driggers LJ, Beard KS, Kuwada S, Piazza G, Ahnen DJ. Sulindac metabolites induce caspase- and proteasome-dependent degradation of beta-catenin protein in human colon cancer cells. Mol Cancer Ther. 2003;2:885–892. [PubMed] [Google Scholar]
- 21.Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 22.Shay JW, Van Der Haegen BA, Ying Y, Wright WE. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- 23.Ikeguchi M, Makino M, Kaibara N. Telomerase activity and p53 gene mutation in familial polyposis coli. Anticancer Res. 2000;20:3833–3837. [PubMed] [Google Scholar]
- 24.Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003;113:881–889. doi: 10.1016/s0092-8674(03)00430-6. [DOI] [PubMed] [Google Scholar]