Abstract
Neuroblastoma (NBL) is the most common malignant disease of infancy, and children with bone metastasis have a mortality rate greater than 90%. Two major classes of proteins, integrins and growth factors, regulate the metastatic process. We have previously shown that tumorigenic NBL cells express higher levels of the type I insulin-like growth factor receptor (IGF-IR) and that β1 integrin expression is inversely proportional to tumorigenic potential in NBL. In the current study, we analyze the effect of β1 integrin and IGF-IR on NBL cell attachment and migration. Nontumorigenic S-cells express high levels of β1 integrin, whereas tumorigenic N-cells express little β1 integrin. Alterations in β1 integrin are due to regulation at the protein level, as translation is decreased in N-type cells. Moreover, inhibition of protein synthesis shows that β1 integrin is degraded more slowly in S-type cells (SHEP) than in N-type cells (SH-SY5Y and IMR32). Inhibition of α5β1 integrin prevents SHEP (but not SH-SY5Y or IMR32) cell attachment to fibronectin and increases SHEP cell migration. Increases in IGF-IR decrease β1 integrin expression, and enhance SHEP cell migration, potentially through increased expression of αvβ3. These data suggest that specific classes of integrins in concert with IGF-IR regulate NBL attachment and migration.
Keywords: Neuroblastoma, integrins, attachment, migration, fibronectin
Introduction
Neuroblastoma (NBL) accounts for approximately 8% to 10% of all childhood cancers and is the most common malignant disease of infancy. NBL remains an understudied area, despite the fact there is a 90% mortality rate for children with NBL with bony metastatic disease [1–3]. Our laboratory has studied the role of insulin-like growth factors (IGFs) I and II, and the type I insulin-like growth factor receptor (IGF-IR) in NBL tumor biology. We have reported that IGF-I or IGF-II coupled to IGF-IR promotes growth of human NBL cells [4–9], prevents NBL apoptosis [10–15], enhances NBL cell motility [16,17], and increases NBL invasion [18]. Highly tumorigenic NBL cells express increased levels of IGF-IR [19] and inhibition of IGF-IR expression using antisense strategies inhibits tumor growth and induces regression of NBL tumors in mice [20]. These data suggest that there are distinct “biologic signatures” for lowgrade and high-grade NBL defined, in part, by IGF-IR expression and signaling.
Our recent data suggest that another family of proteins, the integrins, is also essential in defining the “biologic signatures” of high-grade and low-grade NBL [21]. The integrins are heterodimeric glycoprotein cell surface receptors comprised of α and β subunits that mediate cell-cell and cell-extracellular matrix (ECM) interactions. These interactions signal multiple cellular responses including adhesion and migration [22], and are known to participate in many aspects of tumorigenesis [23]. Primary NBL tumors with a good prognosis express several β1 integrin heterodimers, whereas NBL tumors with a poor prognosis lack integrins with β1 subunits [24]. In vitro, the absence of β1 integrin in NBL cell lines causes tumor cell detachment, whereas the expression of β1 integrin protein correlates with attached cells [25]. In parallel, low-grade differentiated NBL cell lines increase expression of α1β1, α2β1, and α3β1 integrin heterodimers [26,27]-integrins that are absent in high-grade NBL. These studies suggest that increased β1 integrin subunit expression correlates with increased NBL cell adhesion and differentiation-properties of low-grade NBL. Conversely, high-grade NBL cells have decreased integrin expression, which potentially results in abnormal or loss of integrin-mediated signaling and increased tumorigenesis.
The current study expands our investigations on the role of integrin and IGF-IR expression and activation in human NBL cells by examining NBL cell attachment to, and migration across, ECM. In a panel of eight human NBL cell lines, less tumorigenic adherent NBL cells express high levels of β1 integrin, whereas more tumorigenic, less adherent NBL cells lack β1 integrin expression. Higher β1 integrin levels in less tumorigenic cells are due to changes in protein turnover, not changes in transcription. β1 Integrin levels also correlate with the degree of cell attachment to two ECM components: fibronectin and collagen IV. Introduction of recombinant β1 integrin into less adherent NBL cell lines significantly increases their attachment to fibronectin and collagen IV. Inhibition of α5β1 integrin, which prevents attachment to fibronectin and collagen IV, allows migration of nontumorigenic NBL cells toward 10% calf serum (CS). In NBL cells with high IGF-IR expression, migration is increased, which likely results from the upregulation of the αvβ3 integrin. Our results suggest that integrin and IGF-IR expression profiles regulate NBL cell attachment to the ECM, which in turn modifies NBL cell migration and may determine NBL metastatic potential.
Materials and Methods
Materials
Falcon brand tissue culture supplies were purchased from BD Biosciences (Bedford, MA). Chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Cell Culture and Transfection
SHEP, SH-SY5Y, IMR32, and SK-N-AS cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% CS (Hyclone, Logan, UT). SH-IN, SK-N-BE-2-C, SK-NBE-2-M17, SMS-KCN-S, and SMS-KCN-69n cells obtained from Dr. Valerie Castle's laboratory at the University of Michigan (Ann Arbor, MI) were grown in minimal essential media (MEM) (Invitrogen Corporation) and F12 (1:1) supplemented with 10% fetal calf serum (FCS) (Hyclone). All NBL cell lines were incubated at 37°C in a humidified atmosphere with 10% CO2. Cells were routinely dissociated with trypsin-EDTA (Invitrogen Corporation) for subculture.
SH-SY5Y and IMR32 cells were stably transfected with the human β1 integrin GeneStorm clone RG000381 in a pcDNA3.1-zeo vector, purchased from Invitrogen Corporation. The GeneStorm clones are sequenced for identity and confirmed to produce protein before shipment. Each clone is also tagged to allow for detection of the exogenous protein. Transfections were performed with the pcDNA3.1/β1 integrin vector or a control pcDNA3.1 vector using Lipofectamine 2000 following the manufacturer's instructions (Invitrogen Corporation). Cells were selected in DMEM containing 10% CS and 250 µg/ml zeocin (Invitrogen Corporation) and maintained in DMEM containing 10% CS and 100 µg/ml zeocin. SHEP/IGF-IR cell clones [11] were maintained in DMEM containing 10% CS and 250 µg/ml G418 (Invitrogen Corporation).
Western Immunoblotting
Western blot analyses were performed as previously described [8]. Cell lysates were collected using modified RIPA buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate) containing 10 µg/ml leupeptin, 10 µg/ml aprotonin, and 100 µg/ml PMSF. Five micrograms of protein was loaded and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to nitrocellulose membranes (Hybond; Amersham Biosciences Corp., Piscataway, NJ), the membranes were incubated in a blocking solution composed of 5% Carnation nonfat dry milk (Nestle USA, Inc., Solon, OH) dissolved in Trisbuffered saline (TBS) containing 0.1% Tween-20 (TBST) for 2 hours at room temperature or 4°C overnight. The primary antibodies used were anti-β1 integrin MAB1973Z, anti-α2 integrin MAB1950Z, anti-α3 integrin MAB1952Z, anti-α4 integrin MAB16983Z, anti-α5 integrin MAB1956Z, anti-αv integrin MAB1953Z, anti-β1 integrin MAB2000, anti-β3 AB1932 (1:1000; all Chemicon International, Inc., Temecula, CA), anti-V5 (1:1000; Invitrogen Corporation), and anti-glyceraldehyde-3-phosphate dehydrogenase (1:10,000; Chemicon International, Inc.). Blots were incubated with primary antibodies diluted in blocking solution for 2 hours at room temperature or overnight at 4°C. After extensive washing in TBST, blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG secondary antibody (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 hour at room temperature. Blots were developed with the Phototope-HRP Western Blot Detection Kit (Cell Signaling Technology, Inc., Beverly, MA) according to the manufacturer's instructions, and exposed to Hyperfilm-ECL film (Amersham Biosciences Corp.). Blots shown are one of at least three independent experiments performed.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RNA was isolated from six-well plates using the TRIzol Reagent (Invitrogen Corporation) according to the manufacturer's instructions. One microgram of RNA was used in the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen Corporation) with random hexamers according to the manufacturer's instructions. cDNA was amplified using the β1 integrin forward primer 5′-GATCAGTTCAGTTTGCTGTGTG-3′ and the β1 integrin reverse primer 5′-TGTGCTAATGTAAGGCATCACAG-3′ in a standard PCR reaction (94°C for 2 minutes; 30 cycles of 94°C for 1 minute, 60°C for 1 minute, and 70°C for 30 seconds; 70°C for 10 minutes). Control primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were included in the reactions. The GAPDH forward primer was 5′-GTGAAGGTCGGAGTCAACG-3′ and the GAPDH reverse primer was 5′GGTGAAGACGCCAGTGGACTC-3′. The cDNA fragments were separated on 1.5% agarose gels and imaged with a Polaroid camera (Polaroid Corporation, Waltham, MA).
Quantitative polymerase chain reaction (QPCR) was performed using the Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA). Briefly, fresh total RNA was harvested as described for semiquantitative RT-PCR and cDNA produced using the AMV reverse transcriptase kit (Promega, Madison, WI) with oligo-dT primer, as per manufacturer's recommendations. The integrity of cDNA was determined by conventional PCR for GAPDH. For QPCR, aliquots of 2 ng of cDNA were combined with β1 integrin primers (150 mM each) and SYBR green master mix in a final volume of 25 µl in a Smart Cycler Tube, 25 µl (Cepheid, Sunnyvale, CA). Two different primer sets were used, with each set designed to produce a 180-bp product. Primer sequences are: set 1 forward, 5′-CCCTTGCACAAGTGAACAGA-3′, set 1 reverse, 5′-ACATTCCTCCAGCCAATCAG-3′; set 2 forward, 5′-CCCTTGCACAAGTGAACAGA-3′, set 2 reverse, 5′-CATTCCTCCAGCCAATCAGT-3′. QPCR was carried out in a Smart Cycler (Cepheid) for 35 cycles. The presence of final PCR products was confirmed by separation on a 1.2% TAE agarose gel. Experiments were performed in duplicate for each set of primers.
β1 Integrin Turnover
β1 Integrin turnover was studied according to the method of Hotchin et al. [28]. NBL cell lines were serum-starved for 4 hours and then treated for 0, 2, 6, or 24 hours with 5 µg/ml cycloheximide (Sigma-Aldrich Corp.) [12]. Cell lysates were collected for SDS-PAGE and Western immunoblotting as described. β1 Integrin levels were measured with computer-assisted densitometry using NIH Image as previously described [29]. β1 Integrin turnover rates were calculated by plotting the percentage control of optical density (at time 0) of β1 integrin against time.
Pulse-Chase Experiments
Pulse-chase experiments were performed using the method of Hotchin et al. [28]. NBL cell lines were serumstarved for 4 hours and then cultured in DMEM lacking cysteine and methionine (Invitrogen Corporation) for 1 hour. Cells were pulsed for 1 hour with 50 µCi/ml [35S]cysteine and [35S]methionine (Pro-mix L[35S] in vitro cell labeling mix; Amersham Biosciences Corp). Medium containing DMEM with 10% CS was replaced and the cells were incubated for 0, 2, 6, or 24 hours. Cell lysates were collected in modified RIPA buffer. Four hundred micrograms of protein was precleared with 30 µl of protein A/G agarose (Santa Cruz Biotechnology, Inc.) and immunoprecipitated with 2 µg of anti-β1 integrin MAB 2000 antibody (Chemicon International, Inc.) overnight at 4°C with end-over-end rotation. Thirty microliters of protein A/G agarose was added to the lysates for 4 to 5 hours at 4°C with end-over-end rotation. After extensive washing in modified RIPA buffer, 30 µl of 2 x SDS sample buffer (20 mM Tris, pH 8.0, 2 mM EDTA, 2% SDS, 20 mM dithiothreitol, 0.02% bromophenol blue, and 4% glycerol) was added to the beads, which were boiled and subjected to SDS-PAGE on 4% to 20% gradient gels (Bio-Rad Laboratories, Hercules, CA). The gels were fixed in 50% methanol with 10% acetic acid for 30 minutes at 4°C and then placed in gel-drying solution (7% methanol, 7% acetic acid, and 1% glycerol) for 5 minutes. The gels were dried for 90 minutes at 80°C and then exposed to X-OMAT AR film (Eastman Kodak Company, Rochester, NY).
Attachment Assays
Cells were dissociated in trypsin-EDTA for 5 minutes, and then centrifuged for 5 minutes at 3000 rpm (18771g) in a Sorvall RT6000B (Kendro Laboratory Products, Asheville, NC) centrifuge in the presence of 2% CS to inactivate the trypsin. Cells were then rinsed twice in Hank's balanced salt solution (HBSS) lacking calcium and magnesium (BioWhittaker, Walkersville, MD) and resuspended in DMEM. A total of 6 x 104 cells was plated in triplicate on fibronectin, collagen IV, vitronectin, or bovine serum albumin (BSA) in DMEM with and without 25 µg/ml anti-α5β1 integrin-blocking antibody MAB1969 (Chemicon International, Inc.) using the CytoMatrix Screen Kit (Chemicon International, Inc.) according to the manufacturer's instructions and incubated at 37°C, 10% CO2 for 1 hour. Cells were rinsed three times with phosphate-buffered saline (PBS) containing calcium and magnesium and stained for 5 minutes with 0.2% crystal violet dissolved in 10% ethanol. After two washes with PBS (no calcium or magnesium), crystal violet was eluted with 0.1 M NaH2PO4 containing 50% ethanol for 15 minutes and the optical densities were measured in a Multiskan Ascent platereader (LabSystems, Helsinki, Finland) at 540 nm.
To verify that the cell types did not absorb the crystal violet dye differently, a standard curve was generated for each cell line. Between 1 x 103 and 7.5 x 104 cells were plated in 96-well plastic tissue culture plates and centrifuged for 5 minutes. Cells were then stained with crystal violet and rinsed, and the dye was eluted. Average optical densities were calculated and plotted against cell numbers.
Migration Assays
Cells were dissociated in trypsin-EDTA for 5 minutes, and then centrifuged for 5 minutes at 3000 rpm (1877g) in a Sorvall RT6000B centrifuge in the presence of 2% CS to inactivate the trypsin. Cells were then rinsed twice in HBSS and resuspended in DMEM. Migration assays were performed by plating 6 x 104 cells in 100 µl of DMEM with or without 25 µg/ml α5β1 integrin-blocking antibody in triplicate in Corning 6.5-mm transwells with 3-µm pores (Fisher Scientific International, Inc., Hampton, NH). Ten percent CS in DMEM was added to the bottom well to stimulatemigration of NBL cells through the transwell membrane. Cells were incubated for 18 hours at 37°C and 10% CO2. Cells on the inside of the transwell were removed by gently swabbing with a cotton swab. Cells that had migrated through the transwell were stained with 0.2% crystal violet in 10% ethanol for 5 minutes, rinsed with PBS, and eluted with 0.1 M NaH2PO4 containing 50% ethanol for 15 minutes. Optical densities were measured on a Multiskan Ascent platereader (LabSystems) at 540 nm.
αvβ3 Integrin Surface Expression and Binding Assay
αvβ3 Integrin expression was analyzed by Western immunoblotting (for total protein levels) and flow cytometry (for cell surface expression). For Western immunoblotting, cells were harvested and run as described above, then immunoblotted using an anti-αvβ3 antibody. For flow cytometry, cells were harvested with 1 mM EDTA and incubated with anti-αvβ3 (MAB1976F; Chemicon International, Inc.), then fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody (Invitrogen Corporation) and fluorescence were measured using a flow cytometer [30].
To analyze functional binding through the αvβ3 integrin, cellular adhesion of SHEP/vector and SHEP/IGF-IR cells mediated by αvβ3 integrin was measured using an Integrin-Mediated Cell Adhesion Kit from Chemicon International, Inc. Analysis was performed as per manufacturer's protocol. Briefly, a cell suspension containing 350,000 cells/ml in PBS detached from the plate using 5 mM EDTA was prepared. One hundred microliters of cell suspension was added to the control plate and the 96-well capture plate. The capture plate contained a monoclonal antibody against human αvβ3 immobilized to the plate with goat anti-mouse antibody. Cells were incubated on the plate for 2 hours, then rinsed with PBS containing 1 mM Ca‘2+/Mg2+. After rinsing, cells were stained for 5 minutes using the provided Cell Stain Solution, rinsed five times, and air-dried. Wells were then incubated in the provided extraction buffer for 5 minutes, and absorbance was read at a wavelength of 560 nm using a LabSystems Fluoroskan Ascent FL fluorimeter (LabSystems, Franklin, MA). Results are presented as the mean optical density ± SEM relative to control.
Results
Integrin Profile in Human NBL Cell Lines
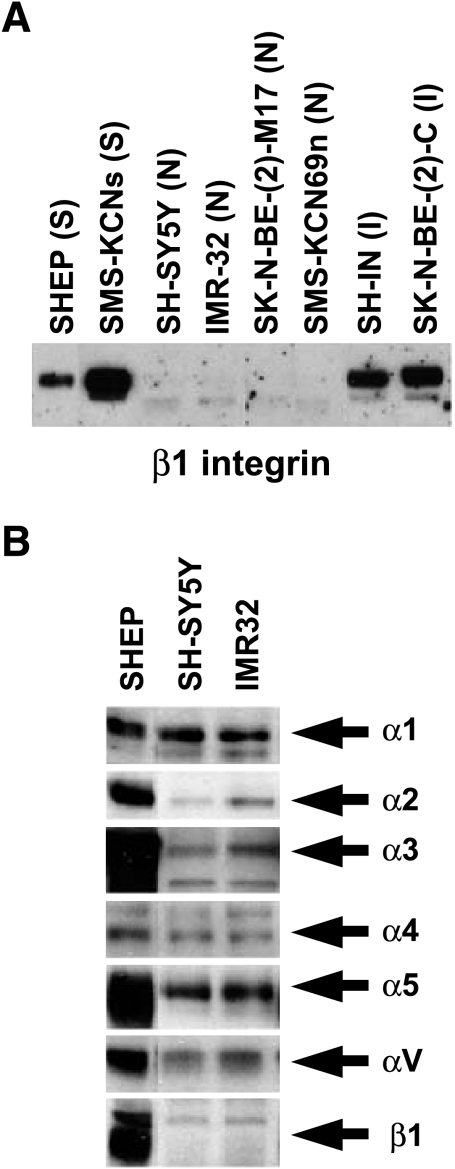
β1 Integrin expression in eight NBL cell lines was compared with known NBL cell phenotypes. Human NBL tumors contain several morphologically distinct cell types, and these have been subcloned into cell lines with unique characteristics [31]. S-type cells (SHEP cells) are highly substrateadherent, grow in monolayer cultures, do not form tumors in nude mice, and have a finite lifespan [31]. N-type cells (SH-SY5Y and IMR32 cells) exhibit small, rounded cell bodies, with many lamellipodia and neurites extending from the soma. N-type cells are tumorigenic in nude mice and have an infinite lifespan [31]. We chose to test two S-type lines, two I-type lines (intermediate morphology and tumorigenic properties) [31], and four N-type lines. S-type (SHEP and SMS-KCN-S) and I-type (SH-IN and SKN-BE-2-C) NBL cells have high β1 integrin levels, whereas N-type (SH-SY5Y, IMR32, SK-N-BE-2-M17, and SMS-KCN-69n) cell lines have low levels of β1 integrin (Figure 1A).
Figure 1.
Integrin expression in NBL cell lines. (A) β1 Integrin Western blot of SHEP (S-type), SMS-KCN-S (S-type), SH-SY5Y (N-type), IMR32 (N-type), SKN-BE-2-M17 (N-type), SMS-KCN-69n (N-type), SH-IN (I-type), and SKN-BE-2-C (I-type) NBL cell lines. I-type cells are intermediate in morphology and tumorigenic characteristics between S-type and N-type. (B) Western blots of SHEP, SH-SY5Y, and IMR32 cells. α1 = 150 kDa; α2 = 160 kDa; α3 = 150 kDa; α4 = 150 kDa; α5 = 150 kDa; αv = 125 kDa; β1 = 130 kDa. Blots are one representative example of three or more separate experiments.
We additionally characterized the expression of α1, α2, α3, α4, α5, and βv integrin in SHEP, SH-SY5Y, and IMR32 human NBL cell lines (Figure 1B). SHEP cells are the least tumorigenic S-type cells [31] and express the highest levels of the α3, α5, and β1 integrin subunits. The SH-SY5Y and IMR32 cells are the most tumorigenic NBL cell lines [31,32] used in this study, and they express very little α2 and β1 integrin and moderate amounts of α3 and α5 integrin (Figure 1B). No correlation between the expression of the remaining integrin subunits examined (α1, α4, and αv) and known relative tumorigenicities [31,32] was observed (Figure 1B).
Upregulated β1 Integrin in SHEP Cells Is Due to Changes in Turnover of Protein
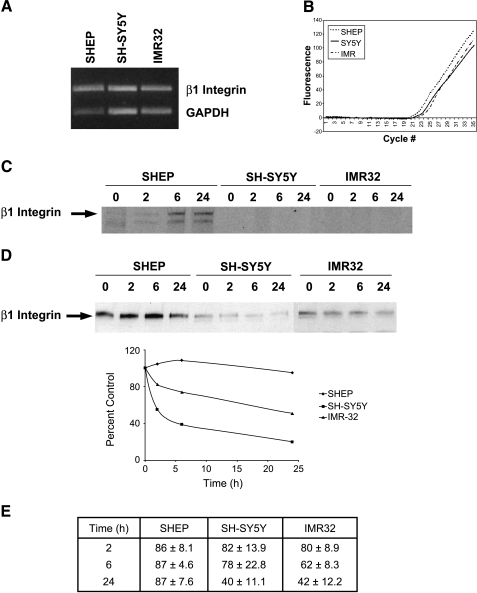
The observed differences in β1 integrin protein levels among the NBL cell lines may be due to either increased transcription or changes in β1 integrin protein turnover. SHEP (S-type) and SH-SY5Y (N-type) cells are derived from the same parental NBL tumor [31]. We examined the differences in β1 integrin expression in these paired cell lines, as well as in another unrelated tumorigenic N-type cell line, IMR32 cells. mRNA levels of β1 integrin, measured using semiquantitative RT-PCR, were unchanged among SHEP, SH-SY5Y, and IMR32 cells, suggesting that transcription of the β1 integrin gene is not different among these cell lines (Figure 2A). To confirm the semiquantitative PCR results, we performed quantitative, real-time PCR using two sets of primers to two different portions of the β1 integrin molecule. Fluorescence, indicating the amount of β1 integrin transcript formed, was similar for all three cell lines, amplified above background at 25 cycles and increasing to saturated fluorescence over a similar number of cycles (Figure 2B). Therefore, the differences in β1 integrin protein levels detected are not due to differences in transcription in the three NBL cell lines.
Figure 2.
Differences in β1 integrin expression are due to decreased turnover in SHEP cells. (A) Semiquantitative RT-PCR of β1 integrin (top band) and GAPDH (bottom band) in SHEP, SH-SY5Y, and IMR32 cells. mRNA levels remain unchanged among the different cell lines. The agarose gel shown is one representative example of four separate experiments. (B) Quantitative, real-time PCR of β1 integrin in SHEP, SH-SY5Y, and IMR32 cells. (C) Pulse-chase labeling of nascent β1 integrin protein in SHEP, SH-SY5Y, and IMR32 cells at 0, 2, 6, and 24 hours. (D) Western blot of β1 integrin after treatment with 5 µg/ml cycloheximide for 0, 2, 6, and 24 hours to inhibit protein synthesis in SHEP, SH-SY5Y, and IMR32 cells. Percent control of optical density for each cell line is plotted against time in the graph. Blot and graph shown are one representative example from four separate experiments. (E) Table representing the results from all four experiments performed in (D). Results are expressed as optical density ± SEM.
We used two methods to ascertain the turnover of β1 integrin in SHEP, SH-SY5Y, and IMR32 cells. Pulse-chase experiments were performed to measure the half-life of the β1 integrin protein. β1 Integrin protein appears within 2 hours in SHEP cells and lasts at least 24 hours (Figure 2C). However, β1 integrin was not detectable in SH-SY5Y or IMR32 cells within 24 hours. Next, cells were treated with 5 µg/ml cycloheximide to abolish protein synthesis and examine β1 integrin protein degradation. Our laboratory and others have previously demonstrated that this concentration of cycloheximide is sufficient to block 95% of protein synthesis within 1 to 3 hours in NBL cells [12,28]. Western blots of β1 integrin show that expression does not change within 24 hours (Figure 2D) in SHEP cells, but that its expression decreases between 2 and 24 hours in SH-SY5Y and IMR32 cells following cycloheximide treatment. In fact, β1 integrin levels fall to 40% of control levels in SH-SY5Y and IMR32 cells, while only decreasing to 83% by 24 hours in SHEP cells (Figure 2E). These data indicate increased degradation of β1 integrin protein in N-type cells, leading to decreased protein half-life.
β1 Integrin Expression Increases Attachment to Fibronectin and Collagen IV
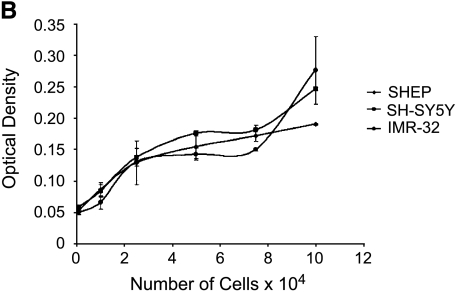
Because integrins are involved in cell-ECM interactions, we next examined the effect of β1 integrin levels on adhesion in S-type and N-type NBL cell lines. The α5β1 integrin heterodimer is one β1-containing integrin receptor that binds to fibronectin, whereas the α1β1, α2β1, a3β1, and αvβ1 integrin heterodimers all bind to collagen IV [33]. Vitronectin predominantly binds to αvβ3 integrin, not β1 heterodimers [33], and was therefore used as a control. SHEP cells adhere more strongly to fibronectin and collagen IV than to vitronectin (Figure 3A; P = .007 and P < .0001, respectively). SH-SY5Y cells and IMR32 cells also adhere to fibronectin and collagen IV, but with less affinity than SHEP cells (Figure 3A; SH-SY5Y and IMR32: P = .007 and P = .003, respectively, for fibronectin compared with SHEP cells on fibronectin). Given the strong attachment of NBL cells to fibronectin, we next assayed attachment in the presence and absence of an α5β1 integrin-blocking antibody, as this integrin is the predominant binding partner for fibronectin (Figure 3A). The specific antibody used block binding of α5β1 integrin to fibronectin by inducing a conformational change in the α5β1 integrin heterodimer [34]. The α5β1 integrin-blocking antibody decreased attachment of SHEP cells to fibronectin by about 75% (P = .002), suggesting that SHEP NBL cell attachment to fibronectin was indeed primarily through the α5β1 integrin receptor. However, SH-SY5Y and IMR32 NBL cell attachment to fibronectin was not through the α5β1 integrin receptor, as the α5β1 integrin-blocking antibody did not affect attachment of these cells to fibronectin. Additionally, SH-SY5Y cells and IMR32 cells adhered to vitronectin significantly more than SHEP cells (P = .0001 and P = .003, respectively). The differences in attachment among the three cell lines were not due to variations in the cells' ability to bind the crystal violet stain because standard curves comparing cell number with the optical density of eluted crystal violet overlapped (Figure 3B). These results suggest that β1 integrin (especially the α5β1 integrin heterodimer), is important in attachment of less tumorigenic S-type cells to fibronectin and that N-type NBL cell lines expressing decreased amounts of β1 integrin are less adhered to fibronectin.
Figure 3.
Attachment of NBL cell lines to ECM proteins. (A) SHEP, SH-SY5Y, and IMR32 cells were allowed to adhere to fibronectin, collagen IV, or vitronectin for 1 hour in the presence or absence of the α5β1-blocking antibody, and then stained with crystal violet. Optical density at 540 nm is plotted against cell line and condition. Error bars show SEM. The graphs are one representative example of two separate experiments, plated in triplicate. (B) SHEP, SH-SY5Y, and IMR32 cells absorb the crystal violet stain equally. Optical density is plated against the number of cells. Error bars show SEM. The graphs are one representative example of two separate experiments, plated in triplicate.
Overexpression of β1 Integrin in N-Type NBL Cell Lines Increases Attachment
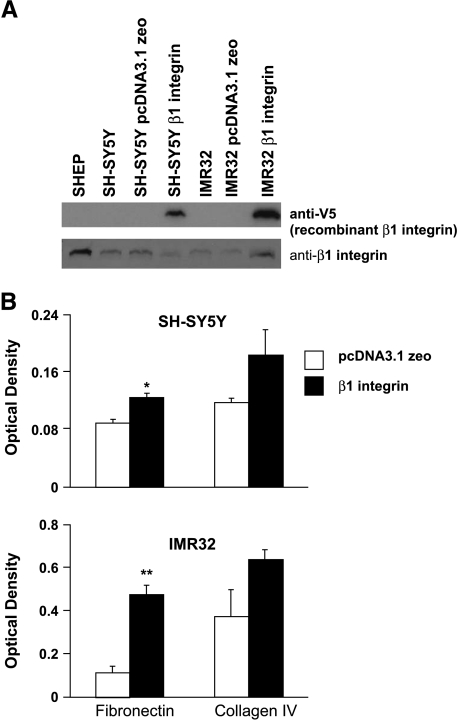
Our hypothesis is that β1 integrin expression in NBL cells decreases cell migration through increased cell attachment, leading to decreased tumorigenic potential. We therefore stably transfected SH-SY5Y and IMR32 cells with recombinant human β1 integrin containing an N-terminal V5 epitope tag or with empty pcDNA3.1 zeo vector and investigated attachment. Both N-type NBL cell lines express recombinant β1 integrin, although the IMR32 cells express more than the SH-SY5Y cells (Figure 4A, upper blot). Moreover, the IMR32 cells overexpress the total amount of β1 integrin (recombinant and endogenous), whereas the SH-SY5Y cells do not (Figure 4A, bottom blot). β1 Integrin-transfected N-type cell lines grow more slowly than the vector-transfected controls (our observations). In fact, stable clones expressing high levels of recombinant β1 integrin cease cell division and die. These results were similar to those obtained when SHEP cells were stably transfected with β1 integrin (our unpublished data). Introduction of recombinant β1 integrin in both N-type cell lines increases their attachment to fibronectin (Figure 4B; SH-SY5Y, P = .02; IMR32, P = .007), whereas there is a trend to increase attachment on collagen IV. Although the untransfected N-type NBL cell lines attach relatively equally to fibronectin (Figure 3A), the difference in the amount of recombinant β1 integrin expressed correlates with the amount of attachment on fibronectin and collagen IV. SH-SY5Y cells express less recombinant β1 integrin than IMR32 cells, and the transfected SH-SY5Y cells attach less to fibronectin than the transfected IMR32 cells.
Figure 4.
Recombinant β1 integrin expression in N-type NBL cell lines. (A) Western blot of recombinant β1 integrin (anti-V5) (top blot) and total β1 integrin (bottom blot) in SHEP, SH-SY5Y, SH-SY5Y pcDNA3.1 zeo, SH-SY5Y β1 integrin, IMR32, IMR32 pcDNA3.1 zeo, and IMR32 β1 integrin. Blots are one representative example of three separate experiments. (B) Attachment assay of β1 integrin-transfected SH-SY5Y and IMR32 cells on fibronectin and collagen IV. Optical density at 540 nm is plotted against cell line and condition. Error bars show SEM. The graphs are one representative example of two separate experiments, plated in triplicate.
The α5β1 Integrin-Blocking Antibody and Decreased β1 Integrin Expression Promote Migration in SHEP Cells
Migration refers to the ability of a cell to move in a specific direction, usually toward a chemoattractant or away from a chemorepellent. SHEP cell movement toward 10% CS was examined to investigate the role of β1 in cell migration. SHEP cells were plated in a transwell membrane and allowed to migrate toward a solution of DMEM with 10% CS for 18 hours. Addition of the α5β1 integrin-blocking antibody increased migration of SHEP cells toward the 10% CS (Figure 5). These data imply that the α5β1 integrin-blocking antibody, which causes SHEP cells to adhere less to fibronectin, also allows them to move toward the chemoattractant. Intact β1 integrin signaling in the control-treated SHEP cells causes them to adhere securely to the ECM, thus impeding their migration toward 10% CS.
Figure 5.
Migration of SHEP cells. SHEP cells were plated in duplicate in DMEM on transwells in the presence or absence of the α5β1 integrin-blocking antibody and allowed to migrate toward 10% CS forx 18 hours. Optical density is plotted against condition. Error bars show SEM.
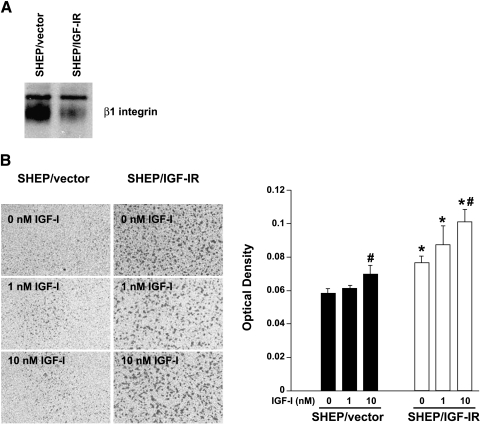
Our laboratory has previously shown that NBL cells with increased tumorigenic potential express higher levels of the IGF-IR [18,19]. This increase in IGF-IR levels promotes NBL cell proliferation, survival, anchorage-independent growth, invasion, and tumor formation in nude mice [11,14,18]. In the current study, we find that SHEP cells expressing increased IGF-IR have decreased expression of β1 integrin compared with control SHEP cells (Figure 6A). SHEP/IGF-IR cells are significantly more migratory than control cells with or without IGF-I ligand present (Figure 6B; P < .01), suggesting that increased IGF-IR expression alone is enough to increase migratory potential. Both cell types respond to IGF-I as a chemoattractant in a dose-dependent fashion, with significantly increased migration in the presence of 10 nM IGF-I (Figure 6B; SHEP/vector cells, P = .02; SHEP/IGF-IR cells, P = .0062). These data support our hypothesis that decreases in β1 integrin protein expression promote a more migratory phenotype in human NBL cell lines.
Figure 6.
SHEP/IGF-IR cells downregulate β1 integrin. (A) Western blot of β1 integrin in SHEP/vector and SHEP/IGF-IR cells. (B) SHEP/vector and SHEP/IGF-IR cells were plated on uncoated 3-µm transwell filters in DMEM + 0.1% serum until cells attached. Medium was then changed to DMEM in the upper chamber and to DMEM + 0, 1, or 10 nM IGF-I in the lower chamber. Cells were allowed to migrate for 24 hours, nonmigrating cells were removed from the upper surface of the membrane, migrating cells on the bottom surface were stained, and quantitation of the experiment was performed as previously described [18]. Values are represented as mean ± SEM for each condition. An asterisk (*) represents statistical significance (P < .02) compared to 0 nM control, and a delta (Δ) represents statistical significance (P < .01) compared to SHEP/vector control.
αvβ3 Integrin Enhances NBL Cell Migration
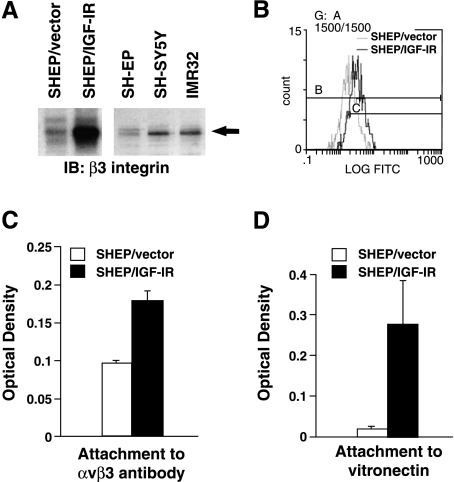
Recent reports suggest that αvβ3 integrin occupancy is required for IGF-IR-mediated migration [35–38]. Given the increase in IGF-IR in tumorigenic NBL cells, we investigated αvβ3 integrin expression in SHEP cells overexpressing the IGF-IR. In SHEP/IGF-IR cells, β3 integrin protein levels are increasedµboth overall expression levels (Figure 7A, left panel) and expression on the cell surface (Figure 7B). To confirm that δvβ3 expression is also increased in NBL cells endogenously expressing higher IGF-IR levels, Western immunoblotting for â3 integrin was performed in SHEP, SH-SY5Y, and IMR32 cells. β3 Integrin levels are low in SHEP cells, but are increased in SH-SY5Y and IMR32 cells (Figure 7A, right panel). Cell binding to immobilized αvβ3 integrin antibody is also increased in SHEP/IGF-IR cells, suggesting that functional αvβ3 is increased (Figure 7C). These data suggest that the high levels of migration in SHEP/IGF-IR cells may be, in part, due to the expression of αvβ3 integrin.
Figure 7.
IGF-IR overexpression increases αvβ3 integrin in SHEP NBL cells. (A) SHEP/vector, SHEP/IGF-IR, SHEP, SH-SY5Y, and IMR32 cells were grown to near confluence and whole cell lysates were collected. Lysates were run on a SDS-PAGE gel and Western-immunoblotted for the β3 integrin subunit using a β3 integrin polyclonal antibody. (B) αvβ3 Integrin surface expression measured by flow cytometry. The average relative log fluorescence for SHEP/vector is 17.5 compared with 50.1 for SHEP/IGF-IR. (C) αvβ3 Integrin binding assay. Error bars show SEM (P = .029). (D) SHEP/vector and SHEP/IGF-IR cells were plated on vitronectin. Cells were allowed to attach for 2 hours and rinsed, then adherent cells were stained and read on a fluorimeter [59] (P = .002).
Finally, to further support that the αvβ3 integrin expressed in the SHEP/IGF-IR cells is functional, these cells were plated in an attachment assay. Attachment to vitronectin was tested, as this is the primary ECM substrate for αvβ3. SHEP cell binding to vitronectin increases by nearly 10-fold when IGF-IR is overexpressed (Figure 7D). Interestingly, the N-cells used in the first part of this study, which express higher levels of endogenous IGF-IR (Figure 1A) and δvβ3 (Figure 7A), also attach more strongly to vitronectin (Figure 3A). Therefore, results of this study, coupled with previous reports from our laboratory, indicate that highly tumorigenic NBL cells express increased IGF-IR, decreased β1 integrin, and increased δvβ3, leading to decreased attachment to their primary ECM and increased migration.
Discussion
Integrins and growth factors both play a role in tumor progression and are involved in tumor cell growth, migration, and invasion, ultimately leading to metastasis [39]. Our laboratory is interested in how these two classes of proteins interact to control NBL migration and invasion. In the current study, we compared the tumorigenicity of NBL cell lines with integrin expression levels. The most striking result demonstrates a near loss of β1 integrin expression in highly tumorigenic NBL cells. SHEP cells, the least tumorigenic of the NBL cell lines tested [31,32], have the highest β1 integrin expression (Figure 1). Conversely, SH-SY5Y and IMR32 cells are the most tumorigenic NBL cell lines [31,32], and express the least β1 integrin (Figure 1). Our expression data support previous flow-activated cell sorting analysis data showing that α5β1 integrin is expressed in S-type, but not N-type, NBL cell lines [40]. The most common genetic anomaly detected in NBL tumors is amplification of the MYCN gene [41], which results in highly aggressive tumors. We and others have shown that expression of N-myc, the gene product of MYCN, in NBL cell lines decreases β1 integrin expression and increases tumorigenicity [25,42]. Furthermore, highly malignant NBL tumors with MYCN amplification do not express most β1 integrin heterodimers [24]. These results suggest that there may be an inverse relationship between N-myc overexpression and loss of β1 integrin expression, and that high β1 integrin expression may promote a less aggressive phenotype in NBL cell lines.
Changes in β1 integrin may be due to differences in transcription, translation, or protein turnover. Our data suggest that NBL cell lines have similar levels of β1 integrin mRNA expression, but differences in β1 integrin protein turnover (Figure 2). The mechanism for increased β1 integrin turnover in N-type cells is unknown. In fibroblasts, integrins are continually synthesized, processed, and transported to the cell surface. Once on the cell surface, β1 integrin is rapidly internalized and degraded within lysosomes [43]. Alternatively, β1 integrin may be posttranslationally modified, leading to its degradation in less adherent cells. For instance, many proteins become targets for the 26S proteasome when they are phosphorylated. As cytoplasmic β1 integrin is phosphorylated and β1 integrin localized to focal adhesions is dephosphorylated [44], β1 integrin not localized at the cell membrane may be targeted to the 26S proteasome for degradation. Another possibility lies in the idea that N-cells may synthesize but not express β1 integrin. For example, cells grown in agar (i.e., anchorage-independent conditions) are able to synthesize β1 integrin but do not express it at the cell surface [43]. Our results do suggest that a positive feedback cycle may exist between NBL attachment and β1 integrin expression. Decreased adhesion in N-type cells may lower β1 integrin levels, preventing attachment to the ECM. This decreased attachment to ECM then further lowers β1 integrin levels, promoting a feed-forward cycle that contributes to the malignant properties of high-grade NBL tumors.
A functional role for decreased β1 integrin in NBL cells may be decreased attachment to matrix, allowing increased migration. S-type cells have high levels of β1 integrin and attach well to fibronectin and collagen IV (Figure 3). N-type cells, in contrast, express low levels of β1 integrin and are only loosely attached to fibronectin and collagen IV. Additionally, blockade of α5β1 integrin using a blocking antibody interferes with attachment of S-type cells, but not N-type cells, to fibronectin, indicating that α5β1 integrin is important in S-type cell attachment, but not N-type cell attachment, to the ECM (Figure 3). Lastly, introduction of recombinant β1 integrin into N-type cells increased their attachment to fibronectin (Figure 4). β1 Integrin is involved in ECM attachment in other tumors as well, including breast carcinoma [45], invasive melanoma cells [46], and chronic myelogenous leukemia [47]. In some breast cancers, a decrease in α2β1 integrin causes a loss of contact with myoepithelial cells and the basement membrane, which is accompanied by increased cell proliferation, thus initiating tumor growth and metastasis [45]. We therefore propose that decreased attachment and increased migration in tumorigenic NBL cells through downregulation of β1 integrin may be factors that promote metastasis. Blocking α5β1 integrin binding to the ECM and downregulation of β1 integrin cause increased migration in SHEP cells (Figures 5 and 6). These results indicate that the low rate of migration in untreated SHEP cells is related to strong attachment to the ECM by α5β1 integrins (Figures 5 and 6). Similarly, in invasive melanoma tumors, migration and migration-associated ECM reorganization are also dependent on the strength of β1 integrin adhesion [46].
In addition to integrins, growth factors also regulate tumor cell migration, invasion, and metastasis. We previously demonstrated that highly tumorigenic NBL cells express increased levels of the IGF-IR [19,48]. In fact, increased expression of IGF system components is present in a wide range of human cancers [49], including lung, breast, thyroid, prostate, glioblastoma, rhabdomyosarcoma, leukemia, and NBL (reviewed in Ref. [50]). In the current study, SHEP cells, with low IGF-IR, migrate little, although migration increases slightly with addition of IGF-I. SHEP cells overexpressing the IGF-IR have decreased β1 integrin expression and significantly more baseline and IGF-I-stimulated migration. IGF-IR activation enhances migration in several other cell types, including epithelial cells [51,52], cancer cells [51,53,54], and smooth muscle cells [36,37,51]. Furthermore, bone contains high levels of IGF-I [55,56]. NBL metastasizes to the bone, and the presence of bone metastasis in children indicates a stage IV tumor with poor prognosis, resulting in a survival rate of less than 7% [1]. Therefore, IGF-IR-expressing NBL tumor cells with an increased migratory capability respond to IGF-I as a chemoattractant, which potentially explains why bone is the primary site of NBL metastases [55,57].
Recent evidence suggests that αvβ3 integrin occupancy is required for IGF-IR-mediated migration [35–38]. In the current study, IGF-IR overexpression increases the levels of functional αvβ3. Increased αvβ3 promotes attachment to vitronectin, one of the ECM components of bone. In smooth muscle cells, IGF-I-mediated migration only occurs if the αvβ3 integrin is bound to ECM, and unoccupied integrin heterodimers or mutated β3 subunits (but not β1) block IGF-IR-mediated migration [35–38]. In NBL tumors, expression of only one integrin, αvβ3, is increased in undifferentiated NBL and, when overexpressed, results in a highly invasive metastatic phenotype [58]. Taken together, these studies, coupled with our results, suggest that increases in αvβ3 integrin may enhance IGF-IR-mediated NBL cell migration and increase NBL cell attachment to bone ECM components, thereby promoting metastasis.
In summary, less tumorigenic S-type NBL cells have high β1 integrin expression, are securely attached to the ECM through β1 integrin interactions, have low IGF-IR levels and αvβ3 expression, and are less migratory. N-type NBL cells are more tumorigenic, have low β1 integrin expression, are loosely attached to the ECM, have increased IGF-IR and αvβ3, and are more migratory. The differences in β1 integrin expression between the N-type and S-type NBL cell lines are due to variations in β1 integrin protein turnover. Decreased β1 integrin levels, coupled with increased IGF-IR and αvβ3 integrin expression, may therefore represent one mechanism promoting increased NBL metastasis. Future studies will fully explore the interaction between the IGF-IR and αvβ3 integrin during NBL cell migration.
Acknowledgements
The authors thank Judy Boldt for excellent secretarial assistance, Tracy Schwab for assistance with flow cytometry, and Mary Soules for technical assistance.
Footnotes
This work was supported by the National Institutes of Health (NIH NS38849, NS36778, NS42099, and NS07222), the Program for Understanding Neurological Diseases (PFUND), and a Rackham Graduate School Predoctoral Fellowship.
References
- 1.Karayalcin G, Paley C, Redner A, Shende A. Neuroblastoma. In: Lanzkowsky P, editor. Manual of Pediatric Hematology and Oncology. Churchill Livingstone: New York; 1995. pp. 419–436. [Google Scholar]
- 2.Philip T. Overview of current treatment of neuroblastoma. Am J Pediatr Hematol Oncol. 1992;14:97–102. doi: 10.1097/00043426-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe M, Ohnuma N, Iwai J, Yoshida H, Takahashi H, Maie M, Etoh T, Kawamura K. Bone marrow metastasis of neuroblastoma analyzed by MRI and its influence on prognosis. Med Pediatr Oncol. 1995;24:292–299. doi: 10.1002/mpo.2950240505. [DOI] [PubMed] [Google Scholar]
- 4.El-Badry OM, Helman LJ, Chatten J, Steinberg SM, Evans AE, Israel MA. Insulin-like growth factor II-mediated proliferation of human neuroblastoma. J Clin Invest. 1991;87:648–657. doi: 10.1172/JCI115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DM, Feldman EL. Regulation of insulin-like growth factor-II expression and its role in autocrine growth of human neuroblastoma cells. J Cell Physiol. 1993;155:290–300. doi: 10.1002/jcp.1041550210. [DOI] [PubMed] [Google Scholar]
- 6.Meghani MA, Martin DM, Singleton JR, Feldman EL. Effects of serum and insulin-like growth factors on human neuroblastoma cell growth. Regul Pept. 1993;48:217–224. doi: 10.1016/0167-0115(93)90350-h. [DOI] [PubMed] [Google Scholar]
- 7.Martin DM, Singleton JR, Meghani MA, Feldman EL. IGF receptor function and regulation in autocrine human neuroblastoma cell growth. Regul Pept. 1993;48:225–232. doi: 10.1016/0167-0115(93)90351-8. [DOI] [PubMed] [Google Scholar]
- 8.Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank AJ, Feldman EL. Insulin-like growth factor-II as a paracrine growth factor in human neuroblastoma cells. Exp Cell Res. 1995;221:179–186. doi: 10.1006/excr.1995.1365. [DOI] [PubMed] [Google Scholar]
- 9.Kiess W, Koepf G, Christiansen H, Blum WF. Human neuroblastoma cells use either insulin-like growth factor-I or insulin-like growth factor-II in an autocrine pathway via the IGF-I receptor: variability of IGF, IGF binding protein (IGFBP) and IGF receptor gene expression and IGF and IGFBP secretion in human neuroblastoma cells in relation to cellular proliferation. Regul Pept. 1997;72:19–29. doi: 10.1016/s0167-0115(97)01026-4. [DOI] [PubMed] [Google Scholar]
- 10.Singleton JR, Dixit VM, Feldman EL. Type I insulin-like growth factor receptor activation regulates apoptotic proteins. J Biol Chem. 1996;271:31791–31794. doi: 10.1074/jbc.271.50.31791. [DOI] [PubMed] [Google Scholar]
- 11.Singleton JR, Randolph AE, Feldman EL. Insulin-like growth factor I receptor prevents apoptosis and enhances neuroblastoma tumorigenesis. Cancer Res. 1996;56:4522–4529. [PubMed] [Google Scholar]
- 12.Matthews CC, Odeh H, Feldman EL. Insulin-like growth factor-I is an osmoprotectant in human neuroblastoma cells. Neuroscience. 1997;79:525–534. doi: 10.1016/s0306-4522(96)00611-2. [DOI] [PubMed] [Google Scholar]
- 13.van Golen CM, Feldman EL. Insulin-like growth factor I is the key growth factor in serum that protects neuroblastoma cells from hyperosmotic-induced apoptosis. J Cell Physiol. 2000;182:24–32. doi: 10.1002/(SICI)1097-4652(200001)182:1<24::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.van Golen CM, Castle VP, Feldman EL. IGF-I receptor activation and Bcl-2 overexpression prevent early apoptotic events in human neuroblastoma. Cell Death Differ. 2000;7:654–665. doi: 10.1038/sj.cdd.4400693. [DOI] [PubMed] [Google Scholar]
- 15.van Golen CM, Schwab TS, Woods Ignatoski KM, Ethier SP, Feldman EL. PTEN/MMAC1 overexpression decreases insulin-like growth factor-I-mediated protection from apoptosis in neuroblastoma cells. Cell Growth Differ. 2001;12:371–378. [PubMed] [Google Scholar]
- 16.Meyer GE, Shelden E, Kim B, Feldman EL. Insulin-like growth factor I stimulates motility in human neuroblastoma cells. Oncogene. 2001;20:7542–7550. doi: 10.1038/sj.onc.1204927. [DOI] [PubMed] [Google Scholar]
- 17.Meyer G, Feldman EL. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem. 2002;83:490–503. doi: 10.1046/j.1471-4159.2002.01185.x. [DOI] [PubMed] [Google Scholar]
- 18.Noujaim D, van Golen CM, van Golen KL, Grauman A, Feldman EL. N-myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene. 2002;21:4549–4557. doi: 10.1038/sj.onc.1205552. [DOI] [PubMed] [Google Scholar]
- 19.Kim B, van Golen C, Feldman EL. Insulin-like growth factor-I signaling in human neuroblastoma cells. Oncogene. 2003;23:130–141. doi: 10.1038/sj.onc.1206924. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Turbyville T, Fritz A, Whitesell L. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res. 1998;58:5432–5438. [PubMed] [Google Scholar]
- 21.Meyer AL, van Golen CM, Feldman EL. Overexpression of Integrin-linked kinase in SHEP human neuroblastoma cells causes increased expression of β1 integrin and differentiation. Mol Biol Cell. 2002;13(Suppl):486a. [Google Scholar]
- 22.Previtali SC, Feltri ML, Archelos JJ, Quattrini A, Wrabetz L, Hartung H. Role of integrins in the peripheral nervous system. Prog Neurobiol. 2001;64:35–49. doi: 10.1016/s0301-0082(00)00045-9. [DOI] [PubMed] [Google Scholar]
- 23.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favrot MC, Combaret V, Goillot E, Lutz P, Frappaz D, Thiesse P, Thyss A, Dolbeau D, Bouffet E, Tabone E. Expression of integrin receptors on 45 clinical neuroblastoma specimens. Int J Cancer. 1991;49:347–355. doi: 10.1002/ijc.2910490306. [DOI] [PubMed] [Google Scholar]
- 25.Judware R, Culp LA. Over-expression of transfected N-myc oncogene in human SKNSH neuroblastoma cells down-regulates expression of β1 integrin subunit. Oncogene. 1995;11:2599–2607. [PubMed] [Google Scholar]
- 26.Rossino P, Defilippi P, Silengo L, Tarone G. Up-regulation of the integrin alpha 1/beta 1 in human neuroblastoma cells differentiated by retinoic acid: correlation with increased neurite outgrowth response to laminin. Cell Regul. 1991;2:1021–1033. doi: 10.1091/mbc.2.12.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozzo C, Ratti P, Ponzoni M, Cornaglia-Ferraris P. Modulation of α1β1, α2β1, and α3β1 integrin heterodimers during human neuroblastoma cell differentiation. FEBS Lett. 1993;332:263–267. doi: 10.1016/0014-5793(93)80646-c. [DOI] [PubMed] [Google Scholar]
- 28.Hotchin NA, Gandarillas A, Watt FM. Regulation of cell surface beta 1 integrin levels during keratinocyte terminal differentiation. J Cell Biol. 1995;128:1209–1219. doi: 10.1083/jcb.128.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng H-L, Randolph A, Yee D, Delafontaine P, Tennekoon G, Feldman EL. Characterization of insulin-like growth factor-I (IGF-I), IGF-I receptor and binding proteins in transected nerves and cultured Schwann cells. J Neurochem. 1996;66:525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- 30.Ignatoski KM, Maehama T, Markwart SM, Dixon JE, Livant DL, Ethier SP. ERBB-2 overexpression confers PI 3′ kinase-dependent invasion capacity on human mammary epithelial cells. Br J Cancer. 2000;82:666–674. doi: 10.1054/bjoc.1999.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biedler JL, Spengler BA, Tien-Ding C, Ross RA. Transdifferentiation of human neuroblastoma cells results in coordinate loss of neuronal and malignant properties. Adv Neuroblastoma Res. 1988;2:265–276. [PubMed] [Google Scholar]
- 32.Flickinger KS, Judware R, Lechner R, Carter WG, Culp LA. Integrin expression in human neuroblastoma cells with or without N-myc amplification and in ectopic/orthotopic nude mouse tumors. Exp Cell Res. 1994;213:156–163. doi: 10.1006/excr.1994.1185. [DOI] [PubMed] [Google Scholar]
- 33.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 34.Mould AP, Garratt AN, Puzon-McLaughlin W, Takada Y, Humphries MJ. Regulation of integrin function: evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha5 beta1. Biochem J. 1998;331(Part 3):821–828. doi: 10.1042/bj3310821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng B, Clemmons DR. Blocking ligand occupancy of the alphaVbeta3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:11217–11222. doi: 10.1073/pnas.95.19.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995;136:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y, Clemmons DR. Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyribonucleic acid synthesis by insulin-like growth factor-I. Endocrinology. 1999;140:4228–4235. doi: 10.1210/endo.140.9.6980. [DOI] [PubMed] [Google Scholar]
- 38.Maile LA, Badley-Clarke J, Clemmons DR. Structural analysis of the role of the beta 3 subunit of the alpha V beta 3 integrin in IGF-I signaling. J Cell Sci. 2001;114:1417–1425. doi: 10.1242/jcs.114.7.1417. [DOI] [PubMed] [Google Scholar]
- 39.Bogenrieder T, Herlyn M. Cell-surface proteolysis, growth factor activation and intercellular communication in the progression of melanoma. Crit Rev Oncol Hematol. 2002;44:1–15. doi: 10.1016/S1040-8428(01)00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshihara T, Esumi N, Humphries MJ, Imashuku S. Unique expression of integrin fibronectin receptors in human neuroblastoma cell lines. Int J Cancer. 1992;51:620–626. doi: 10.1002/ijc.2910510419. [DOI] [PubMed] [Google Scholar]
- 41.Corvi R, Savelyeva L, Schwab M. Patterns of oncogene activation in human neuroblastoma cells. J Neuro-Oncol. 1997;31:25–31. doi: 10.1023/a:1005721027709. [DOI] [PubMed] [Google Scholar]
- 42.van Golen CM, Soules ME, Grauman AR, Feldman EL. N-myc overexpression leads to decreased β1 integrin expression and increased apoptosis in human neuroblastoma cells. Oncogene. 2003;22:2664–2673. doi: 10.1038/sj.onc.1206362. [DOI] [PubMed] [Google Scholar]
- 43.Dalton SL, Scharf E, Briesewitz R, Marcantonio EE, Assoian RK. Cell adhesion to extracellular matrix regulates the life cycle of integrins. Mol Biol Cell. 1995;6:1781–1791. doi: 10.1091/mbc.6.12.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barreuther MF, Grabel LB. The role of phosphorylation in modulating beta 1 integrin localization. Exp Cell Res. 1996;222:10–15. doi: 10.1006/excr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 45.Alford D, Pitha-Rowe P, Taylor-Papadimitriou J. Adhesion molecules in breast cancer: role of alpha 2 beta 1 integrin. Biochem Soc Symp. 1998;63:245–259. [PubMed] [Google Scholar]
- 46.Friedl P, Brocker EB, Zanker KS. Integrins, cell matrix interactions and cell migration strategies: fundamental differences in leukocytes and tumor cells. Cell Adhes Commun. 1998;6:225–236. doi: 10.3109/15419069809004478. [DOI] [PubMed] [Google Scholar]
- 47.Bhatia R, Verfaillie CM. The effect of interferon-alpha on beta-1 integrin mediated adhesion and growth regulation in chronic myelogenous leukemia. Leuk Lymphoma. 1998;28:241–254. doi: 10.3109/10428199809092680. [DOI] [PubMed] [Google Scholar]
- 48.Jasty R, van Golen C, Lin HJ, Solomon G, Heidelberger K, Polverini P, Opipari A, Feldman E, Castle VP. Bcl-2 and N-myc coexpression increases IGF-IR and features of malignant growth in neuroblastoma cell lines. Neoplasia. 2001;3:304–313. doi: 10.1038/sj.neo.7900171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones JI, Doerr ME, Clemmons DR. Cell migration: interactions among integrins, IGFs and IGFBPs. Prog Growth Factor Res. 1995;6:319–327. doi: 10.1016/0955-2235(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 52.Andre F, Rigot V, Thimonier J, Montixi C, Parat F, Pommier G, Marvaldi J, Luis J. Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int J Cancer. 1999;83:497–505. doi: 10.1002/(sici)1097-0215(19991112)83:4<497::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 53.Mira E, Manes S, Lacalle RA, Marquez G, Martinez-A C. Insulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9. Endocrinology. 1999;140:1657–1664. doi: 10.1210/endo.140.4.6623. [DOI] [PubMed] [Google Scholar]
- 54.Bartucci M, Morelli C, Mauro L, Ando' S, Surmacz E. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61:6747–6754. [PubMed] [Google Scholar]
- 55.Abboud SL, Bethel CR, Aron DC. Secretion of insulin-like growth factor I and insulin-like growth factor-binding proteins by murine bone marrow stromal cells. J Clin Invest. 1991;88:470–475. doi: 10.1172/JCI115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Middleton J, Arnott N, Walsh S, Beresford J. Osteoblasts and osteoclasts in adult human osteophyte tissue express the mRNAs for insulin-like growth factors I and II and the type 1 IGF receptor. Bone. 1995;16:287–293. doi: 10.1016/8756-3282(94)00040-9. [DOI] [PubMed] [Google Scholar]
- 57.Mohan S, Baylink DJ. Bone growth factors. Clin Orthop. 1991;263:30–48. [PubMed] [Google Scholar]
- 58.Gladson CL, Hancock S, Arnold MM, Faye-Petersen OM, Castleberry RP, Kelly DR. Stage-specific expression of integrin alphaV-beta3 in neuroblastic tumors. Am J Pathol. 1996;148:1423–1434. [PMC free article] [PubMed] [Google Scholar]
- 59.Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Physical activity, trauma, and ALS: a case-control study. Acta Neurol Scand. 1996;94:45–50. doi: 10.1111/j.1600-0404.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]