Introduction
‘All these years that I’m painfully counting
The few, the many, the unknown
Wandering alone
Shining my light in front of a magical mirror’
(The Guard and the King, Socratis Malamas)
How is the ‘identity’ of the nuclear envelope established and what does this structure do, apart from separating the cytoplasm from the nucleoplasm? Touching on these questions, I discuss here the molecular features and the nearest-neighbour relationships of five integral proteins that represent major components of the inner nuclear membrane. As it turns out, these proteins are organized in large, multi-subunit complexes, which anchor at the nuclear lamina and ‘trap’ in situ a variety of regulatory factors. It is these factors that impart domain specificity and give the nuclear envelope its unique characteristics.
Substructure of the nuclear envelope and relationship with the endoplasmic reticulum
The nuclear envelope is a modular structure, consisting of discrete, flattened cisternae. These cisternae comprise two different ‘unit membranes’: the outer nuclear membrane, which faces the cytoplasm and is continuous with the rough endoplasmic reticulum (ER); and the inner nuclear membrane, which faces the nucleoplasm and is covered by the nuclear lamina. The inner and outer membranes are separated by a narrow lumen (perinuclear space) and join periodically at the pore membrane. This is a highly specialized membrane domain, exposed on both sides of the cytoplasm–nucleoplasm interface and accommodating the nuclear pore complex.
The physico-chemical properties of the nuclear membranes have not been directly determined. Nevertheless, several studies have been made using intact nuclei and nuclei lacking large pieces of the outer nuclear membrane (e.g. Schindler et al., 1985). From these experiments it has been inferred that the inner and outer membranes do not differ significantly, having essentially the same phospholipid mobility and lipid composition. A structural relatedness between the nuclear envelope and the ER membrane has been suggested by recent observations, showing that nuclear envelope assembly in Xenopus extracts involves ‘rough’ vesicles and tubular intermediates that are morphologically similar to the cisternae of the ER (Wiese et al., 1997; Dreier and Rapoport, 2000).
Although the ER and the nuclear envelope represent a continuum, the major proteins of the inner nuclear membrane and the pore membrane do not ‘leak’ to the outer nuclear membrane or the peripheral ER and remain stably localized in the corresponding territories (reviewed in Gant and Wilson, 1997). Moreover, despite the similarities of the nuclear envelope and the other endomembranes, ER vesicles do not possess any lamin or chromatin-binding activity (Foisner and Gerace, 1993; Maison et al., 1995; Pyrpasopoulou et al., 1996). Finally, in higher eukaryotic organisms the nuclear envelope is universally disassembled during mitosis, while the ER is sometimes fragmented and sometimes not (for an illustrative example see Zeligs and Wollman, 1979).
Common features of inner nuclear membrane proteins
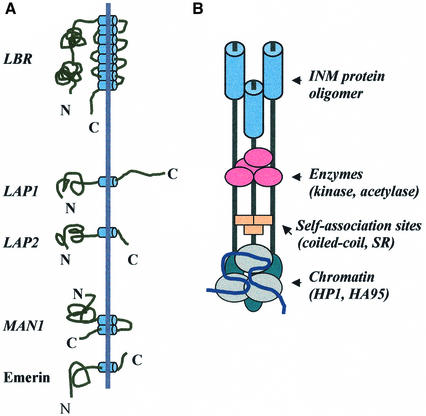
So far, five integral proteins have been characterized as permanent residents of the inner nuclear membrane. These proteins include the lamin B receptor (LBR), the lamina-associated polypeptide-1 (LAP1), the lamina-associated polypeptide-2 (LAP2), emerin and MAN1 (Figure 1). The inner nuclear membrane is also thought to contain IP3/IP4 receptors (reviewed in Rogue and Malviya, 1999), Ire1, a multifunctional protein involved in chaperoning (Sidrauski and Walter, 1997), and nurim, a recently identified protein of unknown function (Rolls et al., 1999); however, definitive morphological evidence supporting an exclusively inner membrane localization of these proteins is not available.
Fig. 1. Topology and organization of inner nuclear membrane proteins. (A) Schematic diagram depicting the structural and topological features of the currently known integral proteins of the inner nuclear membrane. The hydrophilic end-domains (irregular lines) project to the nucleoplasm (left) and the perinuclear space (right), while the membrane-spanning regions (barrels) cross the inner nuclear membrane (straight line). (B) A highly hypothetical model of inner nuclear membrane complexes. The aggregate is postulated to contain oligomers of membrane proteins (held in place via coiled-coiled or SR–SR interactions), modulating enzymes and chromatin-associated elements.
All inner membrane proteins are arranged with their N-termini facing the nucleoplasm and are modified by various kinases. In addition, nearly all of them associate with the nuclear lamina and/or chromatin and possess hydrophilic end-domains that can drive non-nuclear proteins to the nuclear envelope. Finally, four (out of five) of these proteins (LAP1, LAP2, MAN1 and emerin) possess coiled-coil motifs (Martin et al., 1995; A.S.Politou and S.D.Georgatos, unpublished observations) and may form homo- or hetero-oligomeric structures in vivo.
Topology and nearest-neighbour relationships
The most extensively characterized inner nuclear membrane protein is LBR. This is a ubiquitous component, expressed in all metazoans and consisting of a hydrophilic N-terminal domain and a hydrophobic C-terminal part that includes eight (predicted) transmembrane segments (Worman et al., 1988, 1990). The N-terminal domain, which projects into the nucleoplasm, is extremely basic and contains multiple serine/arginine (SR) motifs. These motifs provide a platform for protein–protein interactions and are the targets of site-specific phosphorylation (see below).
The hydrophobic core of human LBR shows significant sequence similarity to vertebrate, yeast and plant sterol reductases, suggesting an evolutionary kinship with these enzymes. This structural relationship can be better understood in terms of genomic data (Holmer et al., 1998 and references therein). The human LBR gene is located on chromosome 1q42.1 and contains 13 exons. The first four of these encode the N-terminal domain, and the remainder the C-terminal domain. Since a large intron separates exons 4 and 5, it is likely that the present-day LBR gene has evolved from recombination of two primordial genes, one of which encoded a histone-like protein and the other an ER enzyme (Schuler et al., 1994).
LBR is part of a large protein complex (Simos and Georgatos, 1992, 1994). The LBR complex, which has been isolated in a native form from nucleated erythrocytes, includes A- and B-type lamins, a specific kinase (LBR kinase), another integral protein termed p18 and a low molecular weight component called p32/p34. The LBR kinase is similar to the SRPK1 kinase (Nikolakaki et al., 1996; Papoutsopoulou et al., 1999), an enzyme that modifies SR motifs. SR-specific phosphorylation releases p32/p34 from the LBR complex, but does not affect lamin association (Nikolakaki et al., 1996). p18 is structurally related to the peripheral-type benzodiazepine receptors. It binds B-type lamins and LBR in vitro and is expressed in a limited number of well differentiated cells (Simos et al., 1996).
Whether the LBR complex dissociates during mitosis is controversial. LBR and B-type lamins co-localize and are co-immunoprecipitated from lysates of mitotic chicken hepatoma cells (Meier and Georgatos, 1994), but this does not seem to happen in other systems (e.g. Chaudhary and Courvalin, 1993; Collas et al., 1996). Since cell-specific factors may account for these contradictory results, it would be interesting to study the stability of the archetypical LBR complex, isolated from avian erythrocytes, upon in vitro modification by mitotic kinases.
Overexpression of green fluorescent protein (GFP) fusion constructs in Cos-7 cells yields a pattern of partitioning that strongly suggests ‘re-absorption’ of LBR to the ER during mitosis (Ellenberg et al., 1997). However, this observation should be interpreted with caution, because under certain conditions the distribution of recombinant LBR might be aberrant. Since the nuclear membranes (and their mitotic derivatives) represent just 5% of the ER surface, ‘back-flow’ of GFP–LBR to the ER will inevitably occur at high levels of expression, unless the cells develop a hypertrophic nuclear envelope or increase the surface area of the nuclear membranes by producing ‘crypts’ and ‘projections’. This is exactly what happens in a fraction of the transfected cells, where the protein accumulates in deep, finger-like invaginations that do not occur to such an extent under normal circumstances (Ellenberg et al., 1997).
LAP1 is a type II membrane protein consisting of a nucleoplasmic N-terminal domain, a single transmembrane segment and a C-terminal piece that protrudes into the perinuclear space. In rodents, where it was originally identified, this protein is expressed in three different forms (LAP1A, B and C), arising from alternative splicing of the same primary transcript. LAP1C occurs in all cell types, whereas LAP1A and LAP1B are expressed only in well differentiated cells (Foisner and Gerace, 1993; Martin et al., 1995). Whether LAP1 occurs in all metazoans is not clear.
Like LBR, LAP1 seems to be part of a large protein assembly. Co-immunoprecipitation experiments suggest that it is tightly associated with B-type lamins and a specific, calcium-dependent kinase (Maison et al., 1997). Consistent with this, sequence analysis indicates that LAP1A and LAP1B possess a 27-residue coiled-coil motif that may be operative in LAP1–LAP1 interactions (Martin et al., 1995). Finally, immunoelectron microscopy shows that LAP1 forms sizable clusters, which are periodically arranged along the nuclear envelope (Maison et al., 1997). The LAP1 complex must be distinct from other inner nuclear membrane complexes, because the various proteins involved are not cross-precipitated with the corresponding antibodies.
The association of LAP1 with the nuclear lamins is disrupted by mitotic phosphorylation (Foisner and Gerace, 1993). The modified protein is then released from its lamina anchor and partitions with mitotic membranes. Whether these membranes are part of the ER or represent discrete vesicles is debatable (Maison et al., 1997; Yang et al., 1997).
LAP2 is a polymorphic protein that comprises at least three isotypes in humans (α, β, γ) and seven isotypes in mice (α, β, β′, γ, ε, δ, ζ). Of these proteins (which are produced by differential splicing), the β, γ, δ and ε forms represent type II membrane proteins, consisting of a nucleoplasmic N-terminal piece, a single transmembrane domain, and a short C-terminal segment that extends into the perinuclear space. LAP2α and LAP2ζ lack a transmembrane domain and are, therefore, nucleoplasmic. The N-terminal piece of LAP2β is long (∼400 amino acids), while the corresponding domains of γ, δ and ε are shorter. LAP2α is the most distantly related member of the family, sharing only a part with the N-terminal domain of LAP2β and containing an isotype-specific region (Dechat et al., 2000 and references therein).
Following the example, LAP2β seems to participate in the formation of a multi-subunit complex. This complex, recently identified by co-immunoprecipitation and chemical cross-linking, contains at least four other nuclear proteins and should have a large size (see below). Consistent with this interpretation, biophysical studies and cross-linking experiments indicate that the N-terminal region of LAP2β is capable of forming oligomers (A.S.Politou, P.A.Theodoropoulos and S.D.Georgatos, unpublished observations).
LAP2β also interacts with B-type lamins (Foisner and Gerace, 1993; Maison et al., 1997). This association is abolished upon mitotic phosphorylation. Like LAP1, the hyperphosphorylated LAP2β protein is mobilized and partitions with mitotic membranes. The LAP2β-containing membranes are not enriched in LAP1 and the two proteins do not co-isolate by absorption in magnetic immunobeads, suggesting segregation in different compartments (Maison et al., 1997).
Emerin possesses a nucleoplasmic, serine-rich N-terminal domain, a single transmembrane segment and a very short C-terminal tail extending into the perinuclear space. This protein is the product of the human STA gene and also exists in rodents. Human STA is located at the X chromosome (Xq28) and, if mutated, gives rise to a form of muscular dystrophy called the Emery–Dreifuss disease (Nagano et al., 1996 and references therein; Cartegni et al., 1997). Immunohistochemical studies indicate a nuclear envelope-specific localization, except in cardiac myocytes where emerin-related material is also detected at the intercalated disks. The existence of non-nuclear emerin has been questioned by more recent studies (Manilal et al., 1999); however, the protein seems to be present in platelets, which are anucleate and do not possess membrane specializations (Squarzoni et al., 2000).
Emerin, LBR, LAP2β and B-type lamins co-immunoisolate and are probably parts of a multimeric complex (see below). Furthermore, direct binding of emerin to nuclear lamin A has been detected recently employing biomolecular interaction analysis (BIA) (Clements et al., 2000). Since mutations in the lamin A gene also cause Emery–Dreifuss muscular dystrophy (Bonne et al., 1999), this strongly suggests that lamins and associated proteins act as an integrated unit and serve a unique function in muscle.
Finally, MAN1, the newest member of the inner nuclear membrane protein family, contains two transmembrane segments, a large N-terminal domain and a relatively short C-terminal domain projecting into the nucleoplasm (Lin et al., 2000). Protein sequence analysis reveals that this protein shares a 40-residue motif with emerin and LAP2β, the so-called LEM module. This module is also present in two Caenorhabditis elegans proteins, indicating that the MAN1/LAP2/emerin family may include more than three members. The human MAN1 gene contains 10 exons, of which seven are protein coding, and has been assigned to chromosome 12q14. A single mRNA species has been identified and the protein seems to be present in all tissues examined.
Association with chromatin
The molecular interactions between inner nuclear membrane proteins and chromatin have a dual significance. First, as indicated by numerous morphological and biochemical studies, binding of these proteins to condensed chromatin provides the driving force for nuclear envelope reassembly at the end of mitosis (reviewed in Georgatos and Theodoropoulos, 1999). Secondly, as suggested by genetic and cell biological experiments, coupling of chromatin domains to the nuclear envelope results in their transcriptional inactivation (e.g. Andrulis et al., 1998). This probably means that the inner nuclear membrane attracts and ‘concentrates’ specific silencers and remodelling factors that operate locally to alter chromatin structure.
Three different polypeptides have been proposed to connect the inner nuclear membrane to the underlying chromatin network: heterochromatin protein 1 (HP1), barrier-to autointegration factor (BAF) and HA95. HP1 is a component of heterochromatin and has been implicated in gene silencing. It consists of two structural domains, the so-called chromodomain and chromo shadow domain, and comprises three distinct variants, HP1α, HP1β and HP1γ, which are the products of distinct genes (Jones et al., 2000). Two-hybrid screens and biochemical studies suggest that the α and γ forms associate specifically with LBR (Ye and Worman, 1996). In line with this, HP1α, β and γ have been reported to bind specifically to nuclear envelope vesicles stripped of peripheral membrane components (Kourmouli et al., 2000).
The idea of a direct interaction between LBR and heterochromatin-specific proteins, although appealing, does not fit with the fact that HP1 resides in internal sites of the cell nucleus and does not exhibit a peripheral, nuclear envelope-specific distribution (Wreggett et al., 1994; Horsley et al., 1996). However, a clue that might explain this discrepancy is provided by recent microinjection experiments (Kourmouli et al., 2000). When recombinant (i.e. unmodified) mouse HP1 proteins are injected into the cytoplasm of living cells, they accumulate first in the area of the nuclear envelope and only then translocate to nucleoplasmic foci. Since mobilization of peripherally localized HP1 is strongly promoted by acetylation, it could be that association with the nuclear envelope involves a small subpopulation of non-modified molecules or occurs in a very dynamic fashion (for further comments see Wolffe and Hansen, 2001).
HP1 seems to participate in post-mitotic nuclear envelope reassembly, facilitating the recruitment of inner nuclear membrane proteins to the surfaces of chromosomes. Peptides containing the nuclear envelope-binding site of mouse HP1β (M31) inhibit targeting of LAP2β and B-type lamins around chromosomes behaving as dominant-negative mutants. Furthermore, HP1 proteins segregate at a polar, peri-chromosomal ‘cap’, at about the same time that inner membrane proteins begin accumulating in this territory (Kourmouli et al., 2000; for relevant data see also Haraguchi et al., 2000).
BAF is a LAP2β-binding protein (Furukawa, 1999), originally identified for its ability to protect retroviral DNA against autointegration. It forms a dodecamer with DNA (Zheng et al., 2000 and references therein), consistent with the idea that it may bind to a LAP2β oligomer (see above). The interaction of BAF with nuclear envelope proteins and DNA suggests a role in chromatin organization and chromosome dynamics. This is more than a theoretical possibility, because RNA interference experiments with BAF-specific probes yield incomplete chromosome segregation in C.elegans.
Finally, HA95, also referred to as HAP95 or NAKA95, is a recently identified nuclear protein homologous to the nuclear A-kinase anchoring protein AKAP95 (Orstavik et al., 2000; Seki et al., 2000). It contains two zinc finger motifs and associates with interphase chromatin and metaphase chromosomes. HA95 associates with B-type lamins and forms a complex with two, and potentially three, inner nuclear membrane proteins: LBR (tight binding), LAP2β (tight binding) and emerin (looser binding) (Martins et al., 2000). The relationship of this complex with previously characterized inner nuclear membrane complexes is unclear.
The ‘affinity trapping’ hypothesis
Although the picture is still incomplete, the unifying concept that emerges from the literature is that all inner nuclear membrane proteins organize as large, multi-subunit complexes. These complexes mediate nuclear envelope reassembly at the end of mitosis, tether the nuclear envelope to underlying structures (nuclear lamina, chromatin) during interphase, and probably serve as ‘affinity traps’ that capture and immobilize in situ a variety of nuclear components.
It is no accident that two of the already characterized inner membrane complexes contain protein kinases (LBR and LAP1 kinase). The LBR kinase is likely to play an important regulatory role because, apart from LBR, it can modify SR motifs found in splicing factors and associated proteins (Nikolakaki et al., 1996; Papoutsopoulou et al., 1999). Also of note is that LBR, which has structural similarity to sterol reductases, functions as an enzyme and complements an ERG24 mutant in yeast (Silve et al., 1998). Finally, it is very revealing that a hormonally regulated atypical P-type ATPase (RFBP) that binds to the SWI/SNF-related protein RUSH has been recently identified in the inner nuclear membrane (Mansharamani et al., 2001). Based on these observations, it would be reasonable to expect that a number of remodelling factors and nuclear chaperones will soon be discovered as parts of multi-subunit complexes that localize at specific sites of the inner nuclear membrane.
How are the core elements of these complexes ‘trapped’ in the inner nuclear membrane? One possibility is that newly synthesized integral proteins engage in coiled-coil (or SR–SR) interactions with pre-existing inner membrane proteins, or form homo- or hetero-oligomers as soon as they enter the nucleus. These oligomeric complexes could be further stabilized by anchorage at subjacent multimeric assemblies, such as the nuclear lamina or elements of the chromatin network (e.g. BAF dodecamers).
The establishment of nuclear envelope micro-domains is expected to be a ‘nucleated’, highly cooperative process. With regard to this, it is interesting that one single protein, the small GTPase Ran, can recruit all downstream components and mediate nuclear envelope assembly around chromatin-free matrices in Xenopus extracts (Hetzer et al., 2000; Zhang and Clarke, 2000). At the present time, the mechanism of this recruitment remains unknown. However, future studies will undoubtedly shed light on whether or not the Ran-associated protein RCC1 and other constituents of chromatin (e.g. HP1, HA95) participate in the initial reassembly reactions, attracting inner nuclear membrane proteins on the surfaces of chromosomes and facilitating the formation of a functional nucleus at the end of mitosis.
Acknowledgments
Acknowledgements
I thank Takis Theodoropoulos, Niki Kourmouli and Anastasia Politou for their critical comments on the manuscript. Research in my laboratory is funded by the Greek Secretariat of Research and Technology, AFM (France) and the European Union (Fifth Framework).
References
- Andrulis E.D., Neiman,A.M., Zappulla,D.C. and Sternglanz,R. (1998) Perinuclear localization of chromatin facilitates transcriptional silencing. Nature, 394, 592–595. [DOI] [PubMed] [Google Scholar]
- Bonne G. et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nature Genet., 21, 285–288. [DOI] [PubMed] [Google Scholar]
- Cartegni L. et al. (1997) Heart-specific localization of emerin: new insights into Emery–Dreifuss muscular dystrophy. Hum. Mol. Genet., 6, 2257–2264. [DOI] [PubMed] [Google Scholar]
- Chaudhary N. and Courvalin,J.C. (1993) Stepwise reassembly of the nuclear envelope at the end of mitosis. J. Cell Biol., 122, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements L., Manilal,S., Love,D.R. and Morris,G.E. (2000) Direct interaction between emerin and lamin A. Biochem. Biophys. Res. Commun., 267, 709–714. [DOI] [PubMed] [Google Scholar]
- Collas P., Courvalin,J.C. and Poccia,D. (1996) Targeting of membranes to sea urchin sperm chromatin is mediated by a lamin B receptor-like integral membrane protein. J. Cell Biol., 135, 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T., Vlcek,S. and Foisner,R. (2000) Lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics. J. Struct. Biol., 129, 335–345. [DOI] [PubMed] [Google Scholar]
- Dreier L. and Rapoport,T.A. (2000) In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J. Cell Biol., 148, 883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg J., Siggia,E.D., Moreira,J.E., Smith,C.L., Presley,J.F., Worman,H.J. and Lippincott-Schwartz,J. (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol., 138, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R. and Gerace,L. (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell, 73, 1267–1279. [DOI] [PubMed] [Google Scholar]
- Furukawa K. (1999) LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2–chromatin interaction. J. Cell Sci., 112, 2485–2492. [DOI] [PubMed] [Google Scholar]
- Gant T.M. and Wilson,K.L. (1997) Nuclear assembly. Annu. Rev. Cell Dev. Biol., 13, 669–695. [DOI] [PubMed] [Google Scholar]
- Georgatos S.D. and Theodoropoulos,P.A. (1999) Rules to remodel by: what drives nuclear envelope disassembly and reassembly during mitosis? Crit. Rev. Eukaryot. Gene Expr., 9, 373–381. [DOI] [PubMed] [Google Scholar]
- Haraguchi T. et al. (2000) Live fluorescence imaging reveals early recruitment of emerin, LBR, RanBP2, and Nup 153 to reforming functional nuclear envelopes. J. Cell Sci. 113, 779–794. [DOI] [PubMed] [Google Scholar]
- Hetzer M., Bilbao-Cortes,D., Walther,T.C., Gruss,O.J. and Mattaj,I.W. (2000) GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol. Cell, 5, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Holmer L., Pezhman,A. and Worman,H.J. (1998) The human lamin B receptor/sterol reductase multigene family. Genomics, 54, 469–476. [DOI] [PubMed] [Google Scholar]
- Horsley D., Hutchings,A., Butcher,G.W. and Singh,P.B. (1996) M32, a murine homologue of Drosophila heterochromatin protein 1 (HP1), localises to euchromatin within interphase nuclei and is largely excluded from constitutive heterochromatin. Cytogenet. Cell Genet., 73, 308–311. [DOI] [PubMed] [Google Scholar]
- Jones D.O., Cowell,I.G. and Singh,P.B. (2000) Mammalian chromodomain proteins: their role in genome organization and expression. BioEssays, 22, 124–137. [DOI] [PubMed] [Google Scholar]
- Kourmouli N., Theodoropoulos,P.A., Dialynas,G., Bakou,A., Politou,A.S., Cowell,I.G., Singh,P.B. and Georgatos,S.D. (2000) Dynamic associations of heterochromatin protein 1 with the nuclear envelope. EMBO J., 19, 6558–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Blake,D.L., Callebaut,I., Skerjanc,I.S., Holmer,L., McBurney,M.W., Paulin-Levasseur,M. and Worman,H.J. (2000) MAN1, an inner nuclear membrane protein that shares the LEM domain with Lamina-associated Polypeptide 2 and Emerin. J. Biol. Chem., 275, 4840–4847. [DOI] [PubMed] [Google Scholar]
- Maison C., Pyrpasopoulou,A. and Georgatos,S.D. (1995) Vimentin-associated mitotic vesicles interact with chromosomes in a lamin B- and phosphorylation-dependent manner. EMBO J., 14, 3311–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C., Pyrpasopoulou,A., Theodoropoulos,P.A. and Georgatos,S.D. (1997) The inner nuclear membrane protein LAP1 forms a native complex with B-type lamins and partitions with spindle-associated mitotic vesicles. EMBO J., 16, 4839–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilal S., Sewry,C.A., Pereboev,A., Man,N., Gobbi,P., Hawkes,S., Love,D.R. and Morris,G.E. (1999) Distribution of emerin and lamins in the heart and implications for Emery–Dreifuss muscular dystrophy. Hum. Mol. Genet., 8, 353–359. [DOI] [PubMed] [Google Scholar]
- Mansharamani M., Hewetson,A. and Chilton,B.S. (2001) Cloning and characterization of an atypical type IV P-type ATPase that binds to the RING motif of RUSH transcription factors. J. Biol. Chem., 276, 3641–3649. [DOI] [PubMed] [Google Scholar]
- Martin L., Crimaudo,C. and Gerace,L. (1995) cDNA cloning and characterization of lamina associated polypeptide 1C (LAP1C), an integral protein of the inner nuclear membrane. J. Biol. Chem., 270, 8822–8828. [DOI] [PubMed] [Google Scholar]
- Martins S., Eide,T., Steen,R.L., Jahnsen,T., Skalhegg,B.S. and Collas,P. (2000) HA95 is a protein of the chromatin and nuclear matrix regulating nuclear envelope dynamics. J. Cell Sci., 113, 3703–3713. [DOI] [PubMed] [Google Scholar]
- Meier J. and Georgatos,S.D.(1994) Type B lamins remain associated with the integral nuclear envelope protein p58 during mitosis: implications for nuclear reassembly. EMBO J., 13, 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano A. et al. (1996) Emerin deficiency at the nuclear membrane in patients with Emery–Dreifuss muscular dystrophy. Nature Genet., 12, 254–259. [DOI] [PubMed] [Google Scholar]
- Nikolakaki E., Simos,G., Georgatos,S.D. and Giannakouros T. (1996) A nuclear envelope-associated kinase phosphorylates arginine-serine motifs and modulates interactions between the lamin B receptor and other nuclear proteins. J. Biol. Chem., 271, 8365–8373. [DOI] [PubMed] [Google Scholar]
- Orstavik S., Eide,T., Collas,P., Han,I.O., Tasken,K., Kieff,E., Jahnsen,T. and Skalhegg,B.S. (2000) Identification, cloning and characterization of a novel nuclear protein, HA95, homologous to A-kinase anchoring protein 95. Biol. Cell, 92, 27–37. [DOI] [PubMed] [Google Scholar]
- Papoutsopoulou S., Nikolakaki,E. and Giannakouros,T. (1999) SRPK1 and LBR protein kinases show identical substrate specificities. Biochem. Biophys. Res. Commun., 255, 602–607. [DOI] [PubMed] [Google Scholar]
- Pyrpasopoulou A., Meier,J., Maison,C., Simos,G. and Georgatos,S.D. (1996) The lamin B receptor (LBR) provides essential chromatin docking sites at the nuclear envelope. EMBO J., 15, 7108–7119. [PMC free article] [PubMed] [Google Scholar]
- Rogue P. and Malviya,A.N. (1999) Calcium signals in the cell nucleus. EMBO J., 18, 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M.M., Stein,P.A., Taylor,S.S., Ha,E., McKeon,F. and Rapoport,T.A. (1999) A visual screen of a GFP-fusion library identifies a new type of nuclear envelope membrane protein. J. Cell Biol., 146, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Holland,J.F. and Hogan,M. (1985) Lateral diffusion in nuclear membranes. J. Cell Biol., 100, 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler E., Lin,F. and Worman,H.J. (1994) Characterization of the human gene encoding LBR, an integral protein of the nuclear envelope inner membrane. J. Biol. Chem., 269, 11312–11317. [PubMed] [Google Scholar]
- Seki N., Ueki,N., Yano,K., Saito,T., Masuho,Y. and Muramatsu,M. (2000) cDNA cloning of a novel human gene NAKAP95, neighbor of A-kinase anchoring protein 95 (AKAP95) on chromosome 19p13.11–p13.12 region. J. Hum. Genet. 45, 31–37. [DOI] [PubMed] [Google Scholar]
- Sidrauski C. and Walter,P. (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell, 90, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Silve S., Dupuy,P.H., Ferrara,P. and Loison,G. (1998) Human lamin B receptor exhibits sterol C14 reductase activity in Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1392, 233–244. [DOI] [PubMed] [Google Scholar]
- Simos G. and Georgatos,S.D. (1992) The inner nuclear membrane protein p58 associates in vivo with a p58 kinase and the nuclear lamins. EMBO J., 11, 4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos G. and Georgatos,S.D. (1994) The lamin B receptor associated protein p34 shares sequence homology and antigenic determinants with the splicing factor 2-associated protein p32. FEBS Lett., 346, 225–228. [DOI] [PubMed] [Google Scholar]
- Simos G., Maison,C. and Georgatos,S.D. (1996) Characterization of p18, a component of the lamin B receptor complex and a new integral membrane protein of the avian erythrocyte nuclear envelope. J. Biol. Chem., 271, 12617–12625. [DOI] [PubMed] [Google Scholar]
- Squarzoni S. et al. (2000) Emerin presence in platelets. Acta Neuropathol. (Berl.), 100, 291–298. [DOI] [PubMed] [Google Scholar]
- Wiese C., Goldberg,M.W., Allen,T.D. and Wilson,K. (1997) Nuclear envelope assembly in Xenopus extracts visualized by scanning EM reveals a transport-dependent ‘envelope-smoothing’ event. J. Cell Sci., 110, 1489–1502. [DOI] [PubMed] [Google Scholar]
- Wolffe A. and Hansen,J.C. (2001) Nuclear visions: functional flexibility from structural instability. Cell, 104, 631–634. [DOI] [PubMed] [Google Scholar]
- Worman H.J., Yuan,J., Blobel,G. and Georgatos,S.D. (1988) A lamin B receptor in the nuclear envelope. Proc. Natl Acad. Sci. USA, 85, 8531–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H.J., Evans,C. and Blobel,G. (1990) The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J. Cell Biol., 111, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett K.A., Hill,F., James,P.S., Hutchings,A., Butcher,G.W. and Singh,P.B. (1994) A mammalian homologue of Drosophila heterochromatin protein 1 (HP1) is a component of constitutive heterochromatin. Cytogenet. Cell Genet., 66, 99–103. [DOI] [PubMed] [Google Scholar]
- Yang L., Guan,T. and Gerace,L. (1997) Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J. Cell Biol., 137, 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q.A. and Worman,H.J. (1996) Interactions between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J. Biol. Chem., 271, 14653–14656. [DOI] [PubMed] [Google Scholar]
- Zeligs J.D. and Wollman,S.H. (1979) Mitosis in rat thyroid epithelial cells in vivo. I. Ultrastructural changes in cytoplasmic organelles during the mitotic cycle. J. Ultrastruct. Res., 66, 53–77. [DOI] [PubMed] [Google Scholar]
- Zhang C. and Clarke,P.R. (2000) Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science, 288, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Zheng R., Ghirlando,R., Lee,M.S., Mizuuchi,K., Krause,M. and Craigie,R. (2000) Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc. Natl Acad. Sci. USA, 97, 8997–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]