Abstract
Prostate cancer frequently metastasizes to the bone, and the treatment outcome for metastatic prostate cancer has been disappointing so far. Dietary genistein, derived primarily from soy product, has been proposed to be partly responsible for the low rate of prostate cancer in Asians. Our previous studies have shown that genistein elicits pleiotropic effects on prostate cancer cells, but there are no studies documenting comprehensive gene expression profiles and antitumor effects of dietary genistein on human prostate cancer grown in human bone environment. In this study, we investigated the effects of genistein on PC3 prostate cancer cells and experimental PC3 bone tumors created by injecting PC3 cells into human bone fragments previously implanted in severe combined immunodeficient (SCID) mice (SCID human model). We found that genistein significantly inhibited PC3 bone tumor growth using both prevention and intervention strategies. By using microarray and real-time polymerase chain reaction technology, we found that genistein regulated the expression of multiple genes involved in the control of cell growth, apoptosis, and metastasis both in vitro and in vivo. For example, the expression of various metalloproteinases (MMPs) in PC3 bone tumors was inhibited by genistein treatment, whereas osteoprotegerin was upregulated. MMP immunostaining and transfection experiments also demonstrated that MMP-9 expression was inhibited in PC3 cells in vitro and PC3 bone tumors in vivo after genistein treatment. These results, particularly the in vivo results, demonstrate that dietary genistein may inhibit prostate cancer bone metastasis by regulating metastasis-related genes. Genistein may thus be a promising agent for the prevention and/or treatment of prostate cancer.
Keywords: Genistein, prostate cancer, bone metastasis, microarray, gene expression
Introduction
Prostate cancer is the second leading cause of cancerrelated deaths in men in the United States with an estimated 220,900 new cases and 28,900 deaths in 2003 [1]. Up to 30% of patients with prostate cancer undergoing radical prostatectomy will relapse, often as a result of micrometastatic disease present at the time of surgery [2,3]. Bone metastasis is common in advanced prostate cancer and causes considerable morbidity including pain, pathologic fractures, spinal cord compression, and disability. Systemic androgen deprivation is initially effective in treating metastasis; however, the metastatic deposits ultimately become refractory to hormonal or chemotherapeutic manipulations and continue to grow. Therefore, there is a tremendous need for the development of mechanismbased strategies for the treatment of prostate cancer. To discover new strategies, it is important to explore the precise mechanisms of cancer cell metastasis to the bone and the molecular mechanism(s) by which new agents exert their inhibitory effects on cancer metastasis.
Because of the suitable microenvironment of the bone for colonization and growth of metastatic tumors, the bone has long been recognized as a common target organ for prostate cancer [4]. The preference of prostate cancer for the bone is due to the results of cancer cell interactions with multiple other cells and molecules in the local microenvironment. Among these molecules, matrix metalloproteinases (MMPs), receptor activator of NF-κB (RANK), receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG) are more evidently involved in the cancer cell bone metastases [5–9]. The matrix MMPs are a family of proteases that play important roles in the degradation of extracellular matrix and the release or activation of growth factors [10,11]. MMPs are upregulated in virtually all human and animal tumors as well as in most tumor cell lines [11,12]. In several cases, the stage of tumor progression is positively correlated with the expression of MMP family members. Changes in MMP levels can markedly affect the invasive behavior of tumor cells and their ability to metastasize in experimental animal models [13].With regard to bone metastasis, we and others have demonstrated that inhibition of MMP activity can disrupt the “vicious cycle” between bone tumor growth and bone matrix remodeling in experimental prostate and breast cancer bone metastasis [14,15]. Because bone matrix remodeling is important in bone metastasis, much attention has been focused on the osteoclasts [16]. Osteoclastic activity is believed to be a critical target for therapy against bone metastasis, and RANK, RANKL, and OPG are known to be the major molecules that modulate osteoclast differentiation and maturation. Therefore, these molecules have received much attention in the area of bone metastasis research [5].
In contrast to the population in western countries, Asian men who consume a traditional diet high in soy products have a relatively low incidence and mortality of prostate cancer, suggesting that a high intake of soy products may protect men against prostate cancer [17]. Genistein is a prominent isoflavonoid found in soy products, and has been proposed to be partly responsible for the low rate of prostate cancer in Asian men [17,18]. Genistein has been identified as an inhibitor of protein tyrosine kinases, which play key roles in cell growth and apoptosis. Studies from our laboratory and others have found that genistein can inhibit cancer cell growth, induce apoptosis, modulate the expression of genes related to the apoptotic pathway, and inhibit NF-κB and Akt activation in cancer cells [18–21]. In the TRAMP model, dietary genistein supplementation, yielding serum levels of genistein comparable with those found in Asian men on a regular soy diet, reduced the incidence of poorly differentiated prostate carcinoma [22]. Similarly, dietary soy significantly reduced tumor cell proliferation, increased apoptosis, and reduced microvessel density in PC3 xenograft tumors in severe combined immunodeficient (SCID) mice [23]. Genistein also can inhibit the in vitro invasive potential of human prostate cancer cell lines, suggesting that genistein could inhibit the metastatic growth of prostate cancer [24]. Moreover, our studies using microarray have shown that genistein can inhibit prostate cancer cells in vitro by regulating the expression of genes, which are critically involved in cell growth, cell cycle, cell signal transduction, angiogenesis, tumor cell invasion, and metastasis [25,26]. However, the role of genistein in the inhibition of invasion and metastasis and the comprehensive gene expression profiles of human prostate cancer grown in the human bone environment in vivo have not been documented. In this study, we have utilized the SCID human (SCID-hu) model of prostate cancer bone metastasis [27] to determine the effect of genistein in vivo and investigated the gene expression profiles of SCID-hu prostate cancer bone tumors altered by dietary genistein using microarray, real-time polymerase chain reaction (PCR), and other techniques. The purpose of our current investigation: was 1) to determine the effects of genistein on prostate cancer cells in vitro; 2) to determine the effects of genistein on prostate cancer bone tumor growth in vivo; 3) to determine the alterations in gene expression profiles by genistein treatment in both in vitro and in vivo studies; and 4) to compare the gene expression profiles of PC3 bone tumors and PC3 subcutaneous tumors in SCID mice so as to better understand the molecular mechanism(s) by which genistein exerts its antimetastatic effect on prostate cancer cells.
Materials and Methods
Cell Culture and Cell Growth Inhibition
PC3 human prostate cancer cells (ATCC, Manassas, VA) were cultured in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in a 5% CO2 atmosphere at 37°C. Genistein (Toronto Research Chemicals, North York, Ontario, Canada) was dissolved in 0.1 M Na2CO3 to make a 10-mM stock solution and was added directly to the culture media at different concentrations. The PC3 cells were seeded at a density of 1 x 103/well in 96-well culture dishes. After 24 hours, the cells were treated with 5, 15, 30, and 50 µm genistein or 0.5 mM Na2CO3 (vehicle control). Cells treated with genistein or Na2CO3 for 1 to 3 days were incubated with MTT (0.5 mg/ml; Sigma, St. Louis, MO) at 37°C for 4 hours and then with DMSO at room temperature for 1 hour. The spectrophotometric absorbance of the samples was determined by using ULTRA Multifunctional Microplate Reader (TECAN, Durham, NC) at 495 nm.
Animal Care and Human Bone Implantation
Male homozygous CB-17 scid/scid mice, aged 4 weeks, were purchased from Taconic Farms (Germantown, NY). The mice were maintained according to the National Institutes of Health standards established in the “Guidelines for the Care and Use of Experimental Animals,” and all experimental protocols were approved by the Animal Investigation Committee of Wayne State University (Detroit, MI). Human male fetal bone tissue was obtained by a third-party, nonprofit organization (Advanced Bioscience Resources, Alameda, CA) and written informed consent was obtained from the donor, consistent with regulations issued by each state involved and the federal government. Isoflurane anesthesia was used during all surgical procedures. After 1 week of acclimatization, the mice were implanted with a single human fetal bone fragment as described previously [27].
Production of Prostate Cancer Bone Tumors and Genistein Treatment
Suspensions of PC3 cells (1 x 105 cells in a volume of 20 µl of RPMI 1640 medium) were injected intraosseously by insertion of a 27-gauge needle through the mouse skin directly into the marrow surface of the previously implanted bone. The mice were divided into three groups: prevention (n = 7), intervention (n = 7), and control (n = 6) groups. In the prevention group, the mice were fed a genistein-containing diet (1 g/kg diet) beginning on the day of intraosseous tumor cell injection. The mice in the intervention group were given the genistein-containing diet as soon as the majority of the bone implants began to enlarge (now called a “bone tumor”) as determined by caliper measurements (23rd day after cancer cell injection). The control mice received the exact same diet (AIN76A; Purina Test Mills, Richmond, IN) but without genistein. The volume of the bone tumor in each group was determined by twice-weekly caliper measurements according to the formula ab2 / 2, where a = length and b = cross-sectional diameter. For statistical analysis, log transformation and linear mixed effect regression model were used to evaluate the significance of difference in tumor volume and rate of tumor volume growth between groups. The procedure of Holm [28] was used to adjust for multiple comparisons.
The mice were sacrificed on the 59th day after cancer cell injection. Blood samples collected at sacrifice were subjected to quantification of plasma genistein. Bone tumors were removed and subjected to ex vivo imaging on a Lo-Rad M-IV mammography unit (Lorad, Danbury, CT) using a magnified specimen technique. Images were developed using a Kodak 2000 screen and radiography film (Kodak, Rochester, NY). Two bone tumors from each group were subjected to microarray analysis and immunostaining. As a microenvironment control, PC3 subcutaneous tumors were created in SCID mice by injecting 5 x 106 cells. The PC3 subcutaneous tumors were harvested after 30 days.
Quantification of Plasma Genistein Using LC/ES-MS
Genistein in the plasma from mice was quantified using the method published previously [29]. Solid-phase extraction of total plasma isoflavones was performed following enzymatic deconjugation. Genistein in the samples was measured using isotope dilution LC-ES/MS with d4-genistein internal standards. The method detection limit was approximately 0.02 µM and the interassay and intra-assay precision and accuracy was ±5% to ±10%. Quality control procedures included concurrent analysis of isoflavone-fortified human plasma and blank plasma.
Microarray Analysis for Gene Expression Profiles
PC3 cells treated with 50 µM genistein or 0.5 mM Na2CO3 for 6, 36, and 72 hours; PC3 bone tumors from each group; and PC3 subcutaneous tumors were subjected to microarray analysis. Total RNA from each sample was isolated by Trizol (Invitrogen) and purified by RNeasy Mini Kit and RNase-free DNase Set (QIAGEN, Valencia, CA) according to the manufacturer's protocol. cDNA for each sample was synthesized by using Superscript cDNA Synthesis Kit (Invitrogen) with T7-(dT)24 primer in place of the oligo(dT) provided in the kit. Then, the biotin-labeled cRNA was transcripted in vitro (IVT) from cDNA by using BioArray HighYield RNA Transcript Labeling Kit (ENZO Biochem, New York, NY), and purified by RNeasy Mini Kit (QIAGEN, Valencia, CA). The purified cRNA was fragmented and applied to Human Genome U95 or U133A Array (Affymetrix, Santa Clara, CA). After hybridization, washing, and staining, the arrays were scanned. The gene expression levels of samples were normalized and analyzed by using Microarray Suite, MicroDB, and Data Mining Tool software (Affymetrix). Clustering and annotation of the gene expression were analyzed by using Cluster, TreeView [30], Onto-Express [31], and GenMAPP (www.genmapp.org).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis for Gene Expression
The total RNA prepared for microarray was also subjected to real-time PCR using the method published previously [26]. Briefly, 2 µg of total RNA from each sample was subjected to reverse transcription using Superscript firststrand cDNA synthesis kit (Invitrogen). Real-time PCR reactions were then carried out in a total of 25 µl of reaction mixture (2 µl of cDNA, 12.5 µl of 2 x SYBR Green PCR Master Mix, 1.5 µl of 5 µm of each specific gene primer, and 7.5 µl of H2O) in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The sequences of primers used in RT-PCR reaction have been described previously [25]. The PCR program was initiated by 10 minutes at 95°C before 40 thermal cycles, each of 15 seconds at 95°C and 1 minute at 60°C. Data were analyzed according to the comparative Ct method and were normalized by GAPDH expression in each sample. Melting curves for each PCR reaction were generated to ensure the purity of the amplification product.
Reporter Gene Constructs and Transfection
Matrix metalloproteinase-9-chloramphenicol acetyltransferase (MMP-9-CAT) containing a nuclear factor-kappa B (NF-κB) binding site in the sequence of MMP-9 promoter was generously provided by Dr. Douglas Boyd (MD Anderson Cancer Center, Houston, TX). The MMP-9-CAT or empty vector was transiently cotransfected with CMV-β-galactosidase into PC3 cells using the LipofectAMINE method (Invitrogen). After incubation for 5 hours, the transfected cells were washed and incubated overnight with RPMI 1640 media (Invitrogen) supplemented with 10% fetal bovine serum followed by treatment with genistein for 36 hours. Subsequently, the CAT activities in the samples were measured by using CAT ELISA system (Roche, Palo Alto, CA) and β-Galactosidase Enzyme Assay System (Promega, Madison, WI) in an ULTRA Multifunctional Microplate Reader (TECAN).
Immunohistochemical Staining for MMP-9
Freshly harvested tumors grown in the implanted bones were fixed in 10% buffered formalin, decalcified, embedded, and sectioned. The paraffin sections of tumor were deparaffinized, then rehydrated through a graded alcohol series. Slides were placed in 10 mM citrate buffer (pH 6.0) and boiled by microwave heating for 3 minutes. Nonspecific sites were blocked by incubation with Superbloc (ScyTek, Logan, UT). Sections were incubated with anti-MMP-9 (5 µg/ml in a mixture of PBS and 2% bovine serum albumin; Calbiochem, La Jolla, CA) monoclonal antibodies for 45 minutes at room temperature. Control slides received no primary antibody. Sections were then incubated with rabbit anti-mouse immunoglobulin G (1:40 dilution), followed by alkaline phosphatase/anti-alkaline phosphatase monoclonal antibody (APAAP) (1:40 dilution). Positive immunoreactive sites were visualized with the Sigma Fast Red (Sigma) substrate. Sections were briefly counterstained with hematoxylin and mounted in aqueous medium.
Results
Cell Growth Inhibition by Genistein
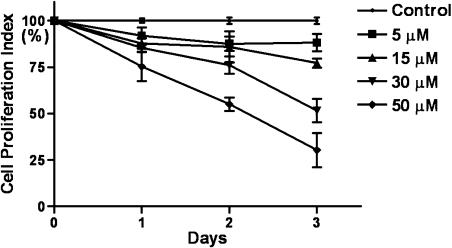
PC3 prostate cancer cells were treated with 0 to 50 µM genistein over 3 days. The effect of genistein on the proliferation of PC3 cells is depicted in Figure 1. Treatment of PC3 cells with genistein resulted in a dose- and time-dependent inhibition of cell proliferation as previously observed [26], demonstrating an inhibitory effect of genistein on PC3 prostate cancer cell growth in vitro. We further investigated whether or nor genistein could inhibit PC3 cell growth in vivo in the SCID-hu model of human prostate cancer bone metastasis.
Figure 1.
Inhibitory effects of genistein on the growth of PC3 cells in culture. PC3 cells were treated with 5, 15, 30, and 50 µM genistein for 24, 48, and 72 hours. (*P < .05, n = 3, cell proliferation index: the percentage of sample absorbance versus control absorbance).
Inhibition of Bone Tumor Growth and Osteolysis by Genistein
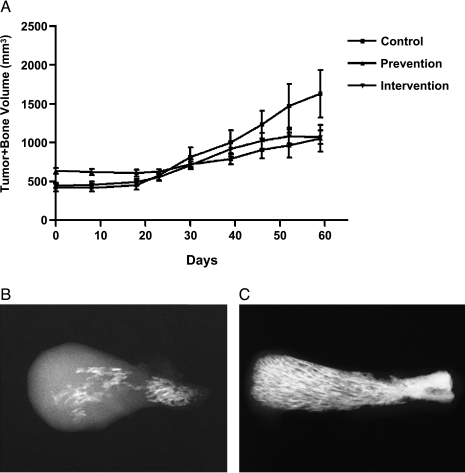
The PC3 cells were injected into a human bone fragment previously implanted in SCID mice, and expansion of the bone implant into a bone tumor was followed by serial caliper measurements. The concentration of plasma genistein in the mice from both the prevention and intervention groups was significantly higher compared to control (Table 1), suggesting the bioavailability of genistein from the diet in these mice. It is important to note that the mean concentration of plasma genistein in the mice fed genistein diet is in the range (1.4 ± 0.7 to 4.09 ± 0.94 µm) of plasma genistein found in populations consuming foods rich in isoflavones [32,33]. We found that dietary genistein significantly inhibited prostate cancer bone tumor growth and osteolysis in SCID-hu mice (Figure 2), demonstrating an inhibitory effect of genistein in an in vivo model of bone metastasis. The statistical analysis indicated that compared to the control group, bone tumor growth rate was significantly lower in both the prevention (P ≤ .0001) and intervention (P =.0003) groups, and the rate in the prevention group was lower than in the intervention group. Log tumor volume growth rate in the prevention group was 34% of the growth rate in the control group, whereas the rate in the intervention group was 70% of that in the controls. To examine the alteration of gene expression induced by dietary genistein, we determined the gene expression profiles of PC3 bone tumors and PC3 subcutaneous tumors in the dietary genistein and control groups.
Table 1.
The Concentration of Plasma Genistein in the SCID-hu Mice.
| Group | Concentration of Genistein (µm) | n | P | |
| Control | 0.0065 ± 0.0159 | 6 | ||
| Prevention | 0.8261 ± 0.3684 | 7 | <.01 | |
| Intervention | 1.6976 ± 1.2469 | 7 | <.01 | |
Figure 2.
(A) Inhibitory effects of genistein on the growth of bone tumors formed by PC3 cells in SCID-hu mice (Control, n = 6; Prevention, n = 7; Intervention, n = 7). (B and C) Ex vivo bone tumor X-ray showing more osteolysis and tumor growth in the control group (B) than in the prevention group (C).
Regulation of mRNA Expression by Genistein Treatment
In PC3 cells grown in culture, genistein regulated the expression of genes that are critically involved in the control of cell growth, cell cycle, apoptosis, cell signaling transduction, angiogenesis, tumor cell invasion, and metastasis [26]. In this in vivo study, microarray analysis also showed that the gene expression in PC3 bone tumors was dramatically altered by genistein treatment (Table 2A). It appears that genistein altered gene expression profiles more significantly in the prevention group than in the intervention group, corresponding with more inhibition of tumor growth in the prevention group. The genes with altered expression in PC3 bone tumors were subjected to cluster analysis according to their biologic function using Onto-Express and GenMAPP computerized annotation. It is important to note that the data analysis software does not allow finding out how many genes there are in each categorized cluster in a whole gene chip. We found that genistein in vivo-regulated some genes that are involved in the regulation of cell cycle, apoptosis, signal transduction, oncogenesis, chemotaxis, transcription, and protein biosynthesis (Tables 2B and 3, Figure 3). This is the first report regarding gene expression profiles altered by genistein in vivo. Interestingly, we also found that genistein regulated the expression of bone metastasis-related genes with downregulation of MMPs and upregulation of OPG (Tables 3 and 4, Figure 3). However, the significance of many of these genes in the cause-and-effect relationships between deregulated genes and genistein-induced biologic effects in prostate cancer needs further in-depth investigation.
Table 2A.
Numbers of Genes Showing ≥2-Fold Changes in PC3 Bone Tumors After Genistein Treatment.
| Prevention | Intervention | |||
| P1 | P2 | I1 | I2 | |
| Up | 582 | 327 | 242 | 211 |
| Down | 66 | 49 | 46 | 31 |
| Total | 648 | 376 | 288 | 242 |
P1, P2: PC3 bone tumors in mice in the prevention group; I1, I2: PC3 bone tumors in mice in the intervention group.
Table 2B.
Numbers of Altered Genes in Different Categories in PC3 Bone Tumors After Genistein Treatment.
| Up | Down | |
| Apoptosis | 12 | 1 |
| Cell cycle arrest, negative regulation of cell proliferation, and transcription | 13 | 0 |
| Signal transduction, chemotaxis | 10 | 7 |
| Regulation of transcription and protein biosynthesis | 11 | 10 |
| Oncogenesis | 8 | 4 |
Table 3.
Fold Changes of Specific Genes in PC3 Bone Tumors in SCID-hu Mice Treated with Genistein.
| Gene | Prevention | Intervention |
| Increase | ||
| Apoptosis | ||
| NM 004849.1 Homo sapiens APG5 (autophagy 5, Staphylococcus cerevisiae)-like (APG5L) | 2.6 | NC |
| NM 014456.1 H. sapiens programmed cell death 4 (PDCD4) | 2.3 | NC |
| BF433902 tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | 2.1 | NC |
| NM 002546.1 tumor necrosis factor receptor superfamily, osteoprotegerin | 2.0 | NC |
| NM 006144.2 H. sapiens granzyme A | 2.6 | NC |
| NM 004402.1 H. sapiens DNA fragmentation factor (caspase-activated DNase) | 2.5 | 2.5 |
| AF001294.1 H. sapiens IPL (IPL) mRNA | 2.1 | NC |
| AF293841.1 H. sapiens apoptosis-related protein (APG5L) | 2.8 | NC |
| NM 022037.1 H. sapiens TIA1 cytotoxic granule-associated RNA-binding protein | 2.1 | NC |
| AF091627.1 H. sapiens CUSP mRNA | 2.1 | NC |
| AB037736.1 H. sapiens mRNA for KIAA1315 protein | 2.1 | 1.7 |
| NM 013229.1 H. sapiens apoptotic protease-activating factor (APAF1) NC | NC | 2.6 |
| Cell cycle arrest, negative regulation of cell proliferation, and transcription | ||
| N23018 C-terminal binding protein 2 | 2.5 | 2.3 |
| NM 003591.1 H. sapiens cullin 2 | 2.0 | NC |
| NM 004585.2 H. sapiens retinoic acid receptor responder 3 | 2.5 | NC |
| NM 020310.1 H. sapiens MAX-binding protein (MNT) | 2.3 | NC |
| BF673013 spectrin SH3 domain-binding protein 1 | 2.1 | NC |
| NM 015895.1 H. sapiens geminin (LOC51053) | 2.0 | 1.7 |
| NM 003451.1 H. sapiens zinc finger protein 177 (ZNF177) | 2.1 | NC |
| BE046521 cut (Drosophila)-like 1 (CCAAT displacement protein) | 2.0 | NC |
| NM 004992.2 H. sapiens methyl CpG-binding protein 2 (MECP2) | NC | 3.7 |
| Decrease | ||
| Signal transduction, chemotaxis | ||
| AW089415 SFRP4 | -2.1 | -1.1 |
| NM 003014.2 H. sapiens SFRP4 | -2.8 | -1.6 |
| BF304996 regulator of G-protein signalling 16 | -2.5 | NC |
| NM 004887.1 H. sapiens small inducible cytokine subfamily B, member 14 | -1.4 | -2.3 |
| NM 002984.1 H. sapiens small inducible cytokine A4 | -2.0 | -1.4 |
| L35594.1 human autotaxin mRNA | -2.1 | -1.1 |
| Regulation of transcription and protein biosynthesis | ||
| NM 014660.1 H. sapiens KIAA0783 gene product (KIAA0783) | -2.5 | -3.7 |
| AF022654.1 H. sapiens homeodomain protein (OG12) mRNA | -2.0 | -2.0 |
| NM 012082.2 H. sapiens friend of GATA2 (FOG2) | -8.0 | NC |
| AK026674.1 H. sapiens cDNA: FLJ23021 fis, similar to HUMSEF21B | -2.0 | -1.4 |
| BC000023.1 H. sapiens, ribosomal protein S19 | -2.3 | -2.1 |
| BF680255 ribosomal protein S11 | -2.3 | -4.0 |
| AW302047 ribosomal protein S10 | -2.1 | -2.6 |
| Oncogenesis | ||
| BC000023.1 H. sapiens, ribosomal protein S19 | -2.3 | -2.1 |
| AW089415 SFRP4 | -2.1 | -1.1 |
| NM 003014.2 H. sapiens | -2.8 | -1.6 |
| BF673699 v-Ki-ras2 Kirsten rat sarcoma 2 viral oncogene | -1.3 | -2.0 |
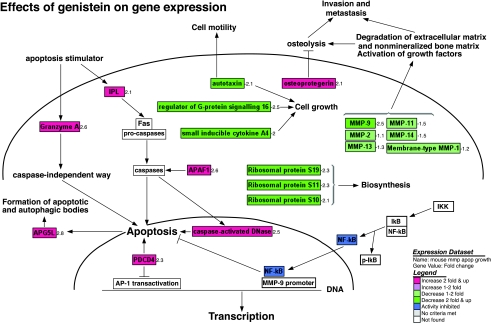
Figure 3.
Effects of genistein on pathway-related gene expression analyzed and visualized by GenMAPP software integrated with cDNA microarray data (positive value: increase in fold change; negative value: decrease in fold change).
Table 4.
Fold Changes of MMP gene Expression in PC3 Bone Tumors in Mice Fed Genistein Diet Compared to Mice Fed Control Diet.
| Genes | P1/C | P2/C | I1/C | I2/C |
| Z48481 mRNA for membrane-type matrix MMP-1 | -1.2 | -1.2 | -1.1 | 0 |
| NM 004530.1 H. sapiens matrix MMP-2 | -1.1 | -1.1 | -1.1 | 0 |
| NM 004995.2 H. sapiens matrix MMP-14 | -1.5 | -1.2 | -1.1 | 0 |
| NM 005940.2 H. sapiens matrix MMP-11 | -1.5 | -1.3 | 0 | -1.1 |
| NM 004994.1 H. sapiens matrix MMP-9 | -2.5 | -1.7 | -1.2 | -1.1 |
| NM 002421.2 H. sapiens matrix MMP-1 | 0 | 0 | 0 | 0 |
| NM 002426.1 H. sapiens matrix MMP-12 | 0 | 0 | 0 | 0 |
| NM 002427.2 H. sapiens matrix MMP-13 | -1.3 | -2.5 | -1.5 | -2.6 |
| NM 004142.1 H. sapiens matrix MMP-like 1 | 0 | 0 | 0 | 0 |
P1, P2: bone tumors in the prevention group; I1, I2: bone tumors in the intervention group; C: bone tumor in the control group.
Inhibition of MMP Expression by Genistein Treatment
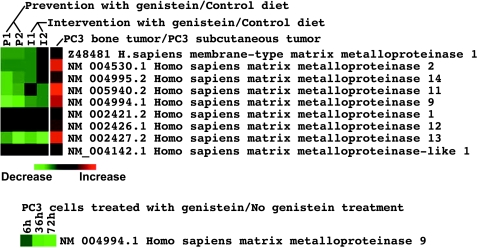
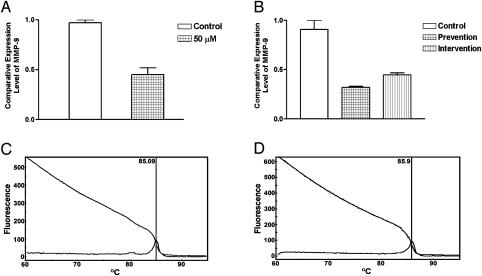
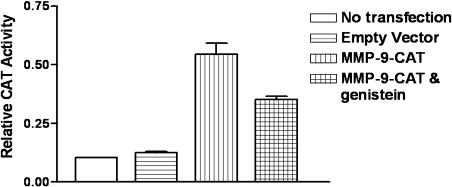
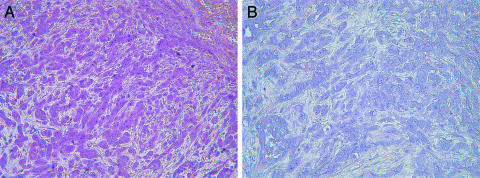
Microarray analysis showed that genistein inhibited the expression of MMP-9 in PC3 cells in culture [25] and the expression of various MMPs in PC3 bone tumors (Figure 4, Table 4). Real-time RT-PCR was conducted to confirm the alteration in the expression of MMP-9. The results of RT-PCR analysis for MMP-9 mRNA expression were in agreement with the microarray data in general (Figure 5), although the fold changes in expression level were not exactly equal. Our gene transfection experiments showed that CAT activity of the MMP-9 promoter decreased after genistein treatment (Figure 6). Together, these results suggest that genistein treatment downregulates the transcription of several MMPs, particularly MMP-9. In order to verify whether the alteration of MMP-9 at the level of transcription ultimately resulted in alterations in protein levels or nor, we conducted immunohistochemical analysis for MMP-9 protein. The MMP-9 immunostaining showed that MMP-9 protein was decreased in PC3 bone tumors in SCID-hu mice receiving the genistein-containing diet (Figure 7). Zymographic analysis also showed a significant decrease in pro-MMP-9 in the conditioned medium from genistein-treated PC3 cells compared to control [25]. These results clearly suggest that genistein inhibited mRNA transcription and protein levels of MMPs, especially MMP-9, in vitro and in vivo.
Figure 4.
Cluster map of MMP gene expression analyzed by cDNA microarray. (A) Different levels of MMP mRNA expression in PC3 cells grown in culture, PC3 bone tumors, and PC3 subcutaneous tumors. (B and C) The expression of MMP mRNA was inhibited by genistein treatment both in vivo (B) and in vitro (C) (P1, P2: PC3 bone tumors grown in mice in the prevention group; I1, I2: PC3 bone tumors grown in mice in the intervention group; 6 h, 36 h, 72 h: PC3 cells treated with 50 µM genistein for 6, 36, and 72 hours).
Figure 5.
Real-time RT-PCR analysis of MMP-9 genes. Comparative analysis shows the downregulation of MMP-9 mRNA expression in PC3 cells grown in culture (n = 3, P =.0199) (A) and in PC3 bone tumor in SCID-hu mice (n = 3, P < .01) (B) after genistein treatment compared to control. Real-time RT-PCR melting curve shows that the PCR products of MMP-9 (C) and GAPDH (D) are pure (only one peak).
Figure 6.
MMP-9 promoter transfection and CAT assay show that the activity of MMP-9 promoter was inhibited by genistein treatment (n = 2, P =.0035).
Figure 7.
MMP-9 immunostaining shows that MMP-9 protein was reduced in PC3 bone tumors after genistein treatment (B) compared to control (A). Immunoreactivity for MMP-9 is indicated by the red color.
Discussion
Genistein has been shown to inhibit cell growth in a wide variety of cancer cells through regulation of several cell signal transduction pathways [18–21,25]. Here, we demonstrated that genistein significantly inhibits the growth of PC3 prostate cancer cells in culture, and also the growth of PC3 bone tumors in the SCID-hu model of prostate cancer bone metastasis. These preclinical data suggest that genistein may have beneficial effects in patients with prostate cancer bone metastasis.
We have found that the inhibitory effects of genistein in vitro were mediated by a large number of genes that are related to the control of carcinogenesis, cell survival, and physiological behaviors; these results have been published previously [26]. However, little is known regarding the gene expression profiles of prostate cancer cells after in vivo genistein treatment. In order to investigate the molecular effects of genistein on human prostate cancer bone metastasis, in this study, we utilized the high-throughput gene chip to determine gene expression profiles in experimental prostate cancer bone tumors created by injecting PC3 cells, and to analyze the alteration of these profiles after exposing tumor-bearing animals to dietary genistein.
From microarray analysis with pathway-related annotation (Figure 3, Tables 2B and 3), we found that the molecular response to genistein in PC3 bone tumors involved inhibition of expression of some genes that are related to signal transduction, chemotaxis, transcription, protein biosynthesis, oncogenesis, and metastasis. However, genistein upregulated some genes critical to the induction of apoptosis, cell cycle arrest, and negative regulation of cell proliferation and transcription. For example, DNA fragment factor is a caspase-activated DNase and has been found to cleave DNA in the apoptotic processes [34]. Granzyme A, programmed cell death 4 (PDCD4), and apoptosis-related protein (APG) also play important roles in the induction of apoptosis [35–37]. Secreted frizzled-related protein 4 (SFRP4) has been found to be involved in oncogenesis by regulating Wnt signaling pathway [38]. In vivo, genistein upregulated the expression of DNA fragment factor, Granzyme A, PDCD4, and APG, and downregulated SFRP4, suggesting the effect of genistein on the induction of apoptotic processes in vivo. Our results demonstrated that the inhibition of tumor growth in vivo by genistein was associated with alterations in expression of a large number of genes critically involved in the control of carcinogenesis, cell survival, and physiological behaviors. However, genistein also upregulated several genes related to signal transduction, transcription, protein biosynthesis, and oncogenesis, some of which may promote cell survival. More studies are needed and are ongoing in our laboratory to address this variation in vivo.
More importantly, we found that dietary genistein also downregulated the expression of multiple metastasis-related genes, including many MMPs. In particular, we found that dietary genistein inhibited the expression of MMP-2, MMP-9, MMP-11, MMP-13, MMP-14, and MT-MMP. These preclinical data corroborate our findings in vitro as reported previously [25,26]. MMPs have been shown by numerous investigators to play a prominent role in metastasis [11,12]. Classically, MMP-2 and MMP-9, otherwise known as the gelatinases, have been implicated in metastasis because of their role in degrading basement membrane collagen. Increased expression of other MMPs, such as MMP-13 and MMP-14, has been demonstrated in many different human cancers [11,12]. For example, MMP-14, a membrane-type MMP also known as MT1-MMP, has been shown to mediate cell invasion in vitro and in vivo [39].
We and others have shown that MMP activity plays an important role in metastasis specifically to the bone [11,14,15]. MMP activity is known to play a role in both normal and cancer-induced bone remodeling. Bone remodeling results in the release of various bioactive factors embedded in the bone extracellular matrix; these factors stimulate the local proliferation of tumor cells. Thus, enhanced MMP activity in bone tissues associated with the presence of tumor cells may be one of the factors involved in the so-called “vicious cycle” hypothesis [5] in which bone matrix turnover and metastatic tumor growth are linked in a positive feedback loop. The enhanced MMP activity in bone metastasis may emanate from many cell types such as tumor cells, stromal cells, osteoclasts, and osteoblasts. Supporting this hypothesis, we previously found that small molecule pharmaceutical inhibitors of MMP activity reduced osteoclast recruitment, prevented bone matrix degradation, and reduced prostate cancer cell proliferation in the human bone implanted in SCID mice [14]. These data have been replicated in other models of bone metastasis [15].
The effects of dietary genistein on bone tumor growth and associated osteolysis in the current study are strikingly similar to our findings with the MMP inhibitor [14]. Our data suggest that dietary genistein leads to diminished MMP activity by limiting increases in MMP gene expression associated with the presence of cancer cells in the bone. Decrease in overall MMP gene expression (in multiple cell types) ultimately limits tumor-induced bone matrix degradation. Controlling the rate of bone matrix turnover diminishes the expansion of tumor within bone. This mechanism seems more likely than a direct effect of genistein on the proliferation of tumor cells because the serum concentration of genistein achieved in the mice by dietary manipulations appears to be below the levels required for a direct effect on tumor cells. One possible mechanism underlying the modulation of MMP gene expression involves the NF-κB transcription factor. Previously, we showed that genistein inhibited NF-κB DNA-binding activity [21]. Because there is a NF-κB-binding site in the promoter of MMP-9 (Figure 3), the inhibition of NF-κB DNA-binding activity by genistein could be one of the molecular mechanisms by which genistein inhibits MMP-9 gene expression in various types of cells.
Genistein may regulate cancer-induced bone matrix turnover by additional mechanisms. We found that dietary genistein upregulated the expression of OPG in PC3 bone tumors, suggesting an inhibitory effect of genistein on osteoclast formation. Metastatic cancer cells are known to release RANKL and OPG into the bone microenvironment, which act on osteoblastic stromal cells to regulate the production of functioning osteoclasts [5,16]. RANKL stimulates the formation and differentiation of osteoclasts by binding to its receptor, RANK, expressed in osteoblastic stromal cells [5,16]. OPG is a decoy receptor that prevents binding of RANKL to RANK by competitive binding to RANKL, leading ultimately to the inhibition of osteoclast formation, survival, and activity [16,40] in the presence of cancer cells. Supporting the “vicious cycle” hypothesis, OPG shows activity for inhibition of bone tumor growth [41,42]. Zhang et al. [9] reported that OPG inhibited prostate cancer-induced osteoclastogenesis and prevented prostate tumor growth in the bone. A recent study also showed that OPG decreased human prostate cancer burden in human adult bone implanted into SCID mice [8]. Thus, upregulation of OPG may be another mechanism by which genistein limits bone metastasis.
Apart from MMPs, urokinase plasminogen activator (uPA), its receptor (urokinase plasminogen activator receptor, uPAR), proteinase M, and protease-activated receptor-2 (PAR-2) are important genes in the processes of tumor cell invasion and metastasis [43–45]. The results from our in vitro study also showed that genistein downregulated the expression of uPA, uPAR, protease M, and PAR-2, and upregulated the expression of connective tissue growth factor and connective tissue activation peptide [25,26], suggesting that genistein may inhibit invasion and metastasis of PC3 prostate cancer cells by multiple mechanisms. The lower concentration of genistein achieved in vivo and complex tumor tissues with multiple cell types might be the reasons why we did not observe significant effects of genistein on the expression of uPA, uPAR, protease M, and PAR-2 in vivo.
In conclusion, dietary genistein regulated the expression of metastasis-related genes and significantly inhibited the growth of PC3 bone tumors in an animal model of human prostate cancer bone metastasis. These results suggest that genistein could be a promising agent for the prevention and/or treatment of prostate cancer and its metastasis. This information could also be exploited for devising chemopreventive and/or therapeutic strategies against prostate cancer, particularly for metastatic prostate cancer for which there is currently no curative therapy. However, the significance of genistein-induced alterations of many of genes in SCID-hu in vivo model requires further in-depth studies in order to determine their causative role in antitumor and antimetastasis effects of genistein on prostate cancer.
Abbreviations
- SCID-hu
severe combined immunodeficient—human
- MMPs
metalloproteinases
- RANK
receptor activator of NF-κB
- RANKL
receptor activator of NF-κB ligand
- OPG
osteoprotegerin
- PDCD4
programmed cell death 4
- APG
apoptosis-related protein
- SFRP
secreted frizzled-related protein
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
- PAR-2
protease-activated receptor-2
Footnotes
This work was partly funded by grants from the National Cancer Institute, National Institute of Health (CA83695-O1A2 to F.H.S. and CA-88028 to M.L.C.).
References
- 1.American Cancer Society, author. Cancer Facts and Figures 2003. Atlanta, GA: American Cancer Society, Inc.; 2003. (6 pp.) [Google Scholar]
- 2.Gopalkrishnan RV, Kang DC, Fisher PB. Molecular markers and determinants of prostate cancer metastasis. J Cell Physiol. 2001;189:245–256. doi: 10.1002/jcp.10023. [DOI] [PubMed] [Google Scholar]
- 3.Bianco FJ, Jr, Wood DP, Jr, Gomes DO, Nemeth JA, Beaman AA, Cher ML. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate. 2001;49:235–242. doi: 10.1002/pros.10018. [DOI] [PubMed] [Google Scholar]
- 4.Goltzman D. Mechanisms of the development of osteoblastic metastases. Cancer. 1997;80:1581–1587. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1581::aid-cncr8>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 6.Chung LW. Prostate carcinoma bone-stroma interaction and its biologic and therapeutic implications. Cancer. 2003;97:772–778. doi: 10.1002/cncr.11140. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, McCauley LK, Keller ET, Pienta KJ. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97:739–747. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- 8.Yonou H, Kanomata N, Goya M, Kamijo T, Yokose T, Hasebe T, Nagai K, Hatano T, Ogawa Y, Ochiai A. Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2003;63:2096–2102. [PubMed] [Google Scholar]
- 9.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 12.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 13.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R, Cher ML. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Winding B, NicAmhlaoibh R, Misander H, Hoegh-Andersen P, Andersen TL, Holst-Hansen C, Heegaard AM, Foged NT, Brunner N, Delaisse JM. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res. 2002;8:1932–1939. [PubMed] [Google Scholar]
- 16.Goltzman D. Osteolysis and cancer. J Clin Invest. 2001;107:1219–1220. doi: 10.1172/JCI13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, Hamalainen EK, Markkanen MH, Makela TH, Wahala KT, Adlercreutz T. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125:757S–770S. doi: 10.1093/jn/125.3_Suppl.757S. [DOI] [PubMed] [Google Scholar]
- 18.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125:777S–783S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 20.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35:167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 22.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice TRAMP. Cancer Res. 2001;61:6777–6782. [PubMed] [Google Scholar]
- 23.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 24.Santibanez JF, Navarro A, Martinez J. Genistein inhibits proliferation and in vitro invasive potential of human prostatic cancer cell lines. Anticancer Res. 1997;17:1199–1204. [PubMed] [Google Scholar]
- 25.Li Y, Sarkar FH. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186:157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Sarkar FH. Gene expression profiles of genisteintreated PC3 prostate cancer cells. J Nutr. 2002;132:3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 27.Nemeth JA, Harb JF, Barroso U, Jr, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. [PubMed] [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 29.Holder CL, Churchwell MI, Doerge DR. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ES-MS. J Agric Food Chem. 1999;47:3764–3770. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- 30.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri P, Draghici S, Ostermeier GC, Krawetz SA. Profiling gene expression using Onto-Express. Genomics. 2002;79:266–270. doi: 10.1006/geno.2002.6698. [DOI] [PubMed] [Google Scholar]
- 32.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Wang HJ, Murphy PA, Hendrich S. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J Nutr. 2000;130:798–801. doi: 10.1093/jn/130.4.798. [DOI] [PubMed] [Google Scholar]
- 34.Ben Yehudah A, Aqeilan R, Robashkevich D, Lorberboum-Galski H. Using apoptosis for targeted cancer therapy by a new gonadotropin releasing hormone-DNA fragmentation factor 40 chimeric protein. Clin Cancer Res. 2003;9:1179–1190. [PubMed] [Google Scholar]
- 35.Nakajima H, Park HL, Henkart PA. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med. 1995;181:1037–1046. doi: 10.1084/jem.181.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond EM, Brunet CL, Johnson GD, Parkhill J, Milner AE, Brady G, Gregory CD, Grand RJ. Homology between a human apoptosis specific protein and the product of APG5, a gene involved in autophagy in yeast. FEBS Lett. 1998;425:391–395. doi: 10.1016/s0014-5793(98)00266-x. [DOI] [PubMed] [Google Scholar]
- 37.Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, Matsuhashi S, Colburn NH. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci USA. 1999;96:14037–14042. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar R. New insights into phosphate homeostasis: fibroblast growth factor 23 and frizzled-related protein-4 are phosphaturic factors derived from tumors associated with osteomalacia. Curr Opin Nephrol Hypertens. 2002;11:547–553. doi: 10.1097/00041552-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 40.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 41.Clohisy DR, Ramnaraine ML, Scully S, Qi M, Van G, Tan HL, Lacey DL. Osteoprotegerin inhibits tumor-induced osteoclastogenesis and bone tumor growth in osteopetrotic mice. J Orthop Res. 2000;18:967–976. doi: 10.1002/jor.1100180617. [DOI] [PubMed] [Google Scholar]
- 42.Croucher PI, Shipman CM, Van Camp B, Vanderkerken K. Bisphosphonates and osteoprotegerin as inhibitors of myeloma bone disease. Cancer. 2003;97:818–824. doi: 10.1002/cncr.11125. [DOI] [PubMed] [Google Scholar]
- 43.Rabbani SA, Mazar AP. The role of the plasminogen activation system in angiogenesis and metastasis. Surg Oncol Clin North Am. 2001;10:393–415. [PubMed] [Google Scholar]
- 44.Tanimoto H, Underwood LJ, Shigemasa K, Parmley TH, O'Brien TJ. Increased expression of protease M in ovarian tumors. Tumour Biol. 2001;22:11–18. doi: 10.1159/000030150. [DOI] [PubMed] [Google Scholar]
- 45.D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–2041. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]