Abstract
Male germ cell tumors (GCTs) are extremely sensitive to platinum-containing chemotherapy, with only 10% of patients showing therapy resistance. However, the biological basis of the high curability of disseminated GCTs by chemotherapy is still unknown. Recently, we demonstrated that the mammalian serine/arginine-rich protein-specific kinase 1 (SRPK1) is a cisplatin-sensitive gene, inactivation of which leads to cisplatin resistance. Because, in mammalians, the expression of SRPK1 is preferentially high in testicular tissues, cisplatin responsiveness of male GCTs might be associated with SRPK1 levels. In the present study, we monitored SRPK1 protein expression in a unique series of nonseminomatous GCTs by immunohistochemistry. Randomly selected GCTs (n = 70) and tumors from patients responding to standard chemotherapy (n = 20) generally showed strong SRPK1 staining. In contrast, expression in refractory GCTs (n = 20) as well as in GCTs from poor-prognosis patients responding to high-dose chemotherapy only (n = 11) was significantly lower (two-sided Wilcoxon rank sum test: P < .001). In conclusion, our data suggest that SRPK1 expression might be an important prognostic indicator for the chemoresponsiveness of nonseminomatous GCTs.
Keywords: Chemotherapy resistance, germ cell tumors, chemotherapy sensitivity, protein kinase SRPK1, immunohistochemistry
Introduction
Cisplatin is one of the most active and widely appreciated anticancer drugs with clinical use in the treatment of a variety of solid tumors, including tumors of the head and neck, and testicular and ovarian cancers. Except in the case of germ cell tumors (GCTs), cellular resistance, either intrinsic or acquired, is frequently encountered and severely limits the therapeutic potential of the drug [1]. In vitro studies have revealed numerous resistance mechanisms, including reduced intracellular accumulation, increased detoxification and DNA repair, increased tolerance to DNA damage, and aberrations in pathways modulating programmed cell death [2,3]. However, in the clinical setting, the reason why a particular tumor does indeed react to cisplatin-containing chemotherapy, whereas other tumors fail to respond, is still completely unknown. In previous studies, we have used yeast as a model system to elucidate cellular mechanisms underlying cisplatin sensitivity and resistance [4–7], and identified Saccharomyces cerevisiae SKY1 (serine/arginine-rich proteins-pecific kinase from budding yeast) as a novel drug-sensitive gene, whose disruption conferred resistance to cisplatin, carboplatin, and anthracycline [6]. Heterologous expression of the human homologue SRPK1 (serine/arginine-rich protein-specific kinase 1) in sky1Δ disruption mutant yeast cells restored cisplatin sensitivity [5]. Analogous to the original finding in yeast, downregulation of the SRPK1 protein in a human ovarian carcinoma cell line also conferred resistance to cisplatin [5]. Overexpression of Sky1p in yeast and SRPK1 in mammalian cells induced hypersensitivity to cisplatin [6] (unpublished results). These data indicate that SRPK1 is a cisplatin-sensitive gene, which might potentially play a role in clinical drug response.
GCTs are extremely sensitive to platinum-based chemotherapy, with cure rates of about 90%, even in the presence of metastatic disease [8]. However, the biological basis of the high curability of disseminated nonseminomas (NS) by chemotherapy is still obscure [9,10]. Because it has been documented that mammalian testicular parenchyma has high SRPK1 expression [11], we explored a possible role of SRPK1 in the sensitivity and resistance of nonseminomatous GCTs toward platinum-containing chemotherapy. Using a unique collection of chemorefractory NS, we were able to compare SRPK1 expression in chemosensitive and chemorefractory GCTs.
Materials and Methods
Patients
SRPK1 expression was analyzed in a total of 121 different GCT samples. Four groups of GCTs were studied (see also Table 1).
Table 1.
Patient and Tumor Characteristics by Patient Group.
| Unselected (n = 70) | Standard Chemoresponsive (n = 20) | High-Dose Chemoresponsive (n = 11) | Chemorefractory (n = 20) | |
| Median age in years (range) | 33 (15–63) | 32 (17–49) | 27 (21–47) | 28 (16–56) |
| Histology | ||||
| Mixed NS | 43 | 9 | 6 | 9 |
| Embryonal carcinoma | 20 | 3 | 1 | 3 |
| Yolk sac tumor | 1 | 3 | 2 | 7 |
| Mature teratoma | 6 | 5 | 1 | 0 |
| Choriocarcinoma | 0 | 0 | 1 | 1 |
| Treatment | Not applicable | Standard first line | High-dose chemotherapy | Standard first line, high-dose chemotherapy, salvage chemotherapy |
| Median progression-free survival | Not applicable | > 5 years | 129 weeks | 29 weeks, all DOD |
Randomly selected NS (n = 70; 27 pure subtypes and 43 tumors consisting of a mixture of embryonal carcinoma, yolk sac tumor, teratoma, and/or choriocarcinoma subtypes). No data on the clinical course were available for these patients.
NS (n = 20; 11 pure subtypes and 9 mixtures; follow-up > 5 years) obtained from patients who were cured by standard first-line platinum-based chemotherapy (cisplatin/etoposide/bleomycin/vinblastine/ifosfamide).
NS (n = 11; five pure subtypes and six mixtures; median follow-up, 129 weeks; range, 26–287) from poor-prognosis patients (according to the International Germ Cell Cancer Cooperative Group classification) who managed long-term survival after initial high-dose chemotherapy (cisplatin/etoposide/ifosfamide, including bone marrow support) as first-line treatment [12].
Chemorefractory NS (n = 20; 11 pure subtypes and 9 mixtures) of which the patients died despite first-line and salvage chemotherapy including high-dose platinum-containing therapy (all death of disease [DOD]; median time to progression, 29 weeks; range, 0–174). First-line treatment consisted of combinations of cisplatin with etoposide, ifosfamide, vinblastine, and/or bleomycin (n = 18), or of first-line high-dose cisplatin combined with vinblastine and ifosfamide (n = 2) as described previously [12]. Salvage high-dose chemotherapy was given to 15 patients and, finally, all patients received further palliative treatment for refractory disease, containing etoposide, oxaliplatin, bendamustine, or gemcitabine [13].
Formalin-fixed paraffin-embedded tissue blocks from the randomly selected NS and the NS obtained from patients cured by standard chemotherapy were collected between 1991 and 2001 in the southwestern part of The Netherlands. The tissue samples from these patients were derived from orchiectomy. The group of standard chemosensitive patients was treated at the University Hospital Rotterdam between 1991 and 1994. After initial therapy, all 20 patients had complete remission of the tumor and are still disease-free. The 20 chemotherapy-refractory patients and the poor-prognosis patients responding to high-dose chemotherapy were diagnosed between 1991 and 1998, and treated within various clinical trials led by Tuebingen University (Tuebingen, Germany) [12,13]. Patients were considered refractory when progression or relapse occurred despite adequate and salvage treatment, including high-dose chemotherapy with autologous stem cell transplantation. The specimens of 12 patients were obtained at initial diagnosis; in eight cases, the specimens were sampled after exposure to chemotherapy. Table 1 summarizes the relevant patient and tumor characteristics of the responding and refractory patients. All cases were diagnosed according to the World Heath Organization classification.
Immunohistochemical Detection of SRPK1
SRPK1 expression was estimated by immunohistochemistry (IHC) on paraffin-embedded tissue sections according to standard procedures. Paraffin sections (3 µm) were mounted on 3-aminopropyl-triethoxysilane-coated slides, deparaffinized in xylene, and rehydrated. Pressure cooking (1.2 bar) in citrate buffer (0.01 mol/L, pH 6.0) was used for antigen retrieval. The slides were immunostained using the horseradish-labeled streptavidin-biotin complex (DAKO A/S, Glostrup, Denmark). After blocking endogenous peroxidase, the sections were incubated overnight (4°C, 16 hours) with a SRPK1-specific monoclonal antibody (dilution 1/400 in 1% bovine serum albumin) (BD Transduction Laboratories, Lexington, KY). Peroxidase was visualized with 3,3-diaminobenzidine tetrahydrochloride. Sections were counterstained with Mayer's hematoxylin. The IHC staining was assessed and blinded for clinical outcome independently by three observers (L.H.J.L., K.N., and P.W.S.), and, in case of discordance, slides were reevaluated and discussed to obtain consensus. The IHC staining of the tumor cells was scored as “negative” (-), “weak” (-/+), “positive” (+), “strong” (++), or “very strong” (+++).
Results
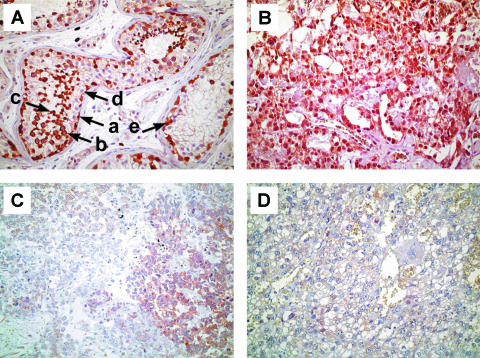
SRPK1 expression was estimated by IHC on paraffin-embedded tissue sections using a SRPK1-specific monoclonal antibody. In normal testicular parenchyma, we observed high SRPK1 expression (Figure 1A) as anticipated [11]. Positive nuclear and cytoplasmic staining of SRPK1 was found in spermatogonia, spermatocytes, and spermatids (Figure 1A), whereas no staining was observed in mature spermatozoa, Sertoli cells, or blood vasculature. In carcinoma in situ (the precursor of testicular GCTs), we also found SRPK1 (Figure 1A). Strong SRPK1 staining was found in the majority of randomly selected and standard chemotherapy-sensitive NS (Figure 1B). In contrast, in most tumors from patients with treatment-refractory disease and patients with advanced disease responding to high-dose chemotherapy only, SRPK1 expression was considerably lower or even absent. A large number of the treatment-refractory DOD cases were negative or stained only weakly for SRPK1 (Figure 1, C and D). We did not see obvious differences in staining between embryonal carcinoma, yolk sac tumor, teratoma, and choriocarcinoma components.
Figure 1.
Representative examples of immunohistochemical staining for SRPK1. (A) Normal spermatogenesis (left) and carcinoma in situ (right). (a) Spermatogonium; (b) spermatocyte; (c) spermatid; (d) Sertoli cell; and (e) carcinoma in situ cell. (B) Chemotherapy-sensitive yolk sac tumor showing “very strong” SRPK1 staining. (C and D) Chemotherapy-resistant germ cell tumors (C, yolk sac tumor; D, choriocarcinoma) scored as “negative” to “weak” (heterogeneous), and “negative” (homogeneous), respectively. Original magnification, x 200.
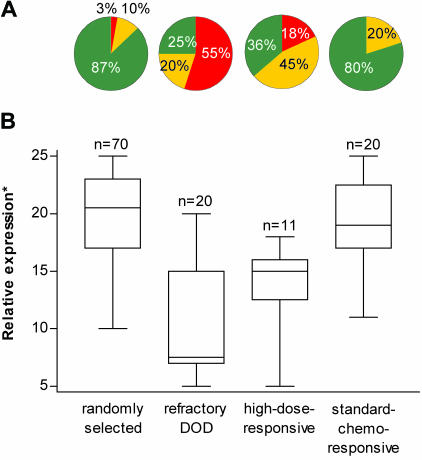
Regardless of their histological subclassification, for several samples examined, a more or less heterogeneous SRPK1 staining was noticed among the tumor cells. Therefore, a total of five randomly chosen microscopic tumor fields per IHC slide was scored in order to get a representative estimate of SRPK1 expression. The semiquantitative score of (-) to (+++) was assigned the value 1, 2, 3, 4, or 5, respectively, and the cumulative scores of five different tumor fields were calculated. Although most randomly selected and standard chemotherapy-sensitive GCTs showed overall scores > 15 corresponding to strong or very strong SRPK1 staining, the majority of treatment-refractory and high-dose-responsive GCTs displayed scores ≤ 15 for negative, weak, or intermediate staining (Figure 2A). The median cumulative IHC scores for the randomly selected, standard chemotherapy-responsive, refractory, and high-dose-responsive NS were 20.5 (range 10–25), 19 (11–25), 7.5 (5–20), and 15 (5–18), respectively (Figure 2B). SRPK1 expression in the randomly selected versus refractory and high-dose-responsive groups was significantly different at P < .0001 and P < .0001 (two-sided Wilcoxon rank sum test), respectively. The relative expression in the standard chemotherapy-responsive versus the refractory and high-dose-responsive group was significantly different at P < .0001 and P < .001 (two-sided Wilcoxon rank sum test), respectively. We conclude that high SRPK1 expression might be an important prognostic indicator for the responsiveness of nonseminomatous GCTs toward platinum-containing chemotherapy, whereas its absence or low expression might predict resistance.
Figure 2.
Relative SRPK1 expression was compared between randomly selected germ cell tumors, chemorefractory germ cell tumors (DOD), poorprognosis high-dose-responsive, and standard chemotherapy-responsive germ cell tumors. (A) Pie chart showing the percentages of tumors with “negative” to “weak” SRPK1 staining (red), “intermediate” staining (yellow), and “strong” to “very strong” staining (green). (B) Box Whisker plots with cumulative SRPK1 scores. *Boxes encompass 25th to 75th percentiles, and the center lines within boxes show medians. Whiskers extend to the extreme values because all data points fell within 1.5 x the interquartile range. Median expression was compared by the two-sided Wilcoxon rank sum test in the randomly selected versus refractory (P < .0001) and high-dose-responsive group (P < .0001), and the standard chemotherapy-responsive versus the refractory (P < .0001) and high-dose-responsive group (P < .001).
Discussion
A better understanding of the cellular mechanisms of cisplatin sensitivity and resistance could lead to effective, specific biological and pharmacological intervention, and thus to better treatment results with regard to long-term survival. In the present study, we determined SRPK1 expression in a unique series of human drug-sensitive and drug-resistant male nonseminomatous GCTs and found a clear statistically significant correlation between chemosensitivity and elevated SRPK1 expression. We concluded that high SRPK1 expression might be of importance for the cisplatin sensitivity of NS, whereas the absence or low expression of SRPK1 might determine unresponsiveness. Interestingly, it was recently shown by another group that expression of a dominant-negative inhibitor of SRPK1 also renders mammalian cell lines resistant to bleomycin [14], which is part of standard chemotherapy for GCTs. Downregulation of SRPK1 might thus not only affect the clinical effectiveness of platinum-based drugs, but also that of bleomycin.
SR protein-specific kinases, like Sky1p and SRPK1, and their substrates (the SR proteins) are thought to be key regulators of RNA maturation by regulating splicing or mRNA transport from the nucleus to the cytoplasm [15]. In that way, these constitutively active kinases probably have a broad regulatory role in cellular physiology. For the SRPKs (Sky1p and SRPK1), specific substrates and involvement in cellular functions have been identified. SRPK1 is predominantly found in the testis, where it phosphorylates protamine 1 as well as a cytoplasmic pool of other SR proteins [11]. Protamines are small highly basic proteins that replace histones during spermatogenesis, resulting in extreme chromatin condensation [16]. It was recently shown that Sky1p is a key regulator of inward transport of polyamines such as putrescine, spermine, and spermidine [17]. Along that line, SRPK1 might have a role in spermatogenesis by direct or indirect regulation of intracellular concentrations of polyamines. Although the precise molecular mechanisms, by which the SRPKs promote cellular responsiveness to cytotoxic stress, have not been established yet [5–7], the available data suggest that hyperphosphorylation of the normal cellular substrates might be part of a cellular stress response [18]. Deletion of SKY1/SRPK1 would then mean that cisplatin treatment can no longer efficiently trigger a stress response involved in cell death, resulting in drug resistance. Recently, Mayer et al. [19] found a positive correlation between microsatellite instability and treatment resistance in GCTs. Here, we show that refractory GCTs also have the protein kinase SRPK1 downregulated. Interestingly, our yeast sky1Δ knockout strains display a so-called mutator phenotype with an increased incidence of mutations that is generally associated with microsatellite instability [20]. It is, therefore, tempting to speculate that both observations (downregulation of SRPK1 and MSI in chemotherapy-resistant GCTs) have a common molecular basis.
Acknowledgement
We thank Maxime Look for statistical analysis.
Abbreviations
- IHC
immunohistochemistry
- SRPK1
serine/arginine-rich protein-specific kinase 1
- GCT
germ cell tumor
- NS
nonseminoma
Footnotes
This work was supported, in part, by grants from the Dutch Cancer Society (grants DDHK97-1397 and DDHK01-2560).
References
- 1.Johnson SW, Ferry KV, Hamilton TC. Recent insight into platinum drug resistance in cancer. Drug Resist Updates. 1998;1:243–254. doi: 10.1016/s1368-7646(98)80005-8. [DOI] [PubMed] [Google Scholar]
- 2.Perez RP. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 3.Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–1160. [PubMed] [Google Scholar]
- 4.Burger H, Capello A, Schenk PW, Stoter G, Brouwer J, Nooter K. A genome-wide screening in Saccharomyces cerevisiae for genes that confer resistance to the anticancer agent cisplatin. Biochem Biophys Res Commun. 2000;269:767–774. doi: 10.1006/bbrc.2000.2361. [DOI] [PubMed] [Google Scholar]
- 5.Schenk PW, Boersma AWM, Brandsma JA, den Dulk H, Burger H, Stoter G, Brouwer J, Nooter K. SKY1 is involved in cisplatin-induced cell kill in Saccharomyces cerevisiae and inactivation of its human homologue, SRPK1, induces cisplatin resistance in a human ovarian carcinoma cell line. Cancer Res. 2001;61:6982–6986. [PubMed] [Google Scholar]
- 6.Schenk PW, Boersma AWM, Brok M, Burger H, Stoter G, Nooter K. Inactivation of the Saccharomyces cerevisiae SKY1 gene induces a specific modification of the yeast anticancer drug sensitivity profile accompanied by a mutator phenotype. Mol Pharmacol. 2002;61:659–666. doi: 10.1124/mol.61.3.659. [DOI] [PubMed] [Google Scholar]
- 7.Schenk PW, Brok M, Boersma AWM, Brandsma JA, den Dulk H, Burger H, Stoter G, Brouwer J, Nooter K. Anticancer drug resistance induced by disruption of the Saccharomyces cerevisiae NPR2 gene: a novel component involved in cisplatin- and doxorubicin-provoked cell kill. Mol Pharmacol. 2003;64:259–268. doi: 10.1124/mol.64.2.259. [DOI] [PubMed] [Google Scholar]
- 8.Bosl GJ, Motzer RJ. Testicular germ cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 9.Mayer F, Honecker F, Looijenga LHJ, Bokemeyer C. Towards an understanding of the biological basis of response to cisplatin-based chemotherapy in germ-cell tumours. Ann Oncol. 2003;14:825–832. doi: 10.1093/annonc/mdg242. [DOI] [PubMed] [Google Scholar]
- 10.Kóberle B, Masters JRW, Hartley JA, Wood RD. Defective repair of cisplatin-induced damage caused by reduced XPA protein in testicular germ cell tumours. Curr Biol. 1999;9:273–276. doi: 10.1016/s0960-9822(99)80118-3. [DOI] [PubMed] [Google Scholar]
- 11.Papoutsopoulou S, Nikolakaki E, Chalepakis G, Kruft V, Chevaillier P, Giannakouros T. SR protein-specific kinase 1 is highly expressed in testis and phosphorylates protamine 1. Nucleic Acids Res. 1999;15:2972–2980. doi: 10.1093/nar/27.14.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bokemeyer C, Kollmansberger C, Meisner C, Harstrick A, Beyer J, Metzner B, Hartmann JT, Schmoll HJ, Einhorn L, Kanz L, Nichols C. First-line high-dose chemotherapy compared to standard-dose PEB/VIP chemotherapy in patients with advanced germ cell tumours: a multivariate and matched-pair analysis. J Clin Oncol. 1999;17:3450–3456. doi: 10.1200/JCO.1999.17.11.3450. [DOI] [PubMed] [Google Scholar]
- 13.Bokemeyer C, Gerl A, Schoffski P, Harstrick A, Niederle N, Beyer J, Casper J, Schmoll HJ, Kanz L. Gemcitabine in patients with relapsed or cisplatin-refractory testicular cancer. J Clin Oncol. 1999;17:512–516. doi: 10.1200/JCO.1999.17.2.512. [DOI] [PubMed] [Google Scholar]
- 14.Sanz G, Mir L, Jacquemin-Sablon A. Bleomycin resistance in mammalian cells expressing a genetic suppressor element derived from the SRPK1 gene. Cancer Res. 2002;62:4453–4456. [PubMed] [Google Scholar]
- 15.Siebel CW, Feng L, Guthrie C, Fu XD. Conservation in budding yeast of a kinase specific for SR splicing factors. Proc Natl Acad Sci USA. 1999;96:5440–5445. doi: 10.1073/pnas.96.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliva R, Dixon GH. Vertebrate protamine genes and the histone-to-protamine replacement reaction. Prog Nucleic Acid Res Mol Biol. 1991;40:25–94. doi: 10.1016/s0079-6603(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 17.Erez O, Kahana C. Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis. Mol Cell Biol. 2001;21:175–184. doi: 10.1128/MCB.21.1.175-184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamachi M, Le TM, Kim SJ, Geiger ME, Anderson P, Utz PJ. Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J Exp Med. 2002;196:1213–1225. doi: 10.1084/jem.20021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer F, Gillis AJM, Dinjens W, Oosterhuis JW, Bokemeyer C, Looijenga LHJ. Microsatellite instability of germ cell tumors is associated with resistance to systemic treatment. Cancer Res. 2002;62:2758–7560. [PubMed] [Google Scholar]
- 20.Branch P, Hampson R, Karran P. DNA mismatch binding defects, DNA damage tolerance, and mutator phenotypes in human colorectal carcinoma cell lines. Cancer Res. 1995;55:2304–2309. [PubMed] [Google Scholar]