Abstract
Aberrant nuclear factor-κB (NF-κB) activation has been implicated in the pathogenesis of several human malignancies. In this study, we determined whether NF-κB is constitutively activated in human prostate adenocarcinoma, and, if so, whether increased NF-κB activation and its binding to DNA influence tumor progression. Using tissue samples obtained during transurethral prostatic resection and paraffin-embedded sections of benign and cancer specimens, we determined the nuclear expression of NF-κB/p65 and NF-κB/p50, cytoplasmic expression of IκBα, its phosphorylation, and expression of NF-κB-regulated genes, specifically Bcl2, cyclin D1, matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor (VEGF). A progressive increase in the expression of NF-τB/p65 (but not of p50) was observed in cancer specimens compared to benign tissue, which correlated with increasing levels of IτBα and its phosphorylation. NF-τB DNA-binding activity increased with increasing tumor grade and the binding complex mainly consisted of NF-τB/p65-p50 heterodimers. Immunohistochemical analysis showed enhanced nuclear staining for NF-τB/p65 in both high-grade (P < .0001) and low-grade (P < .003) cancer specimens, compared to benign tissue. The nuclear levels of NF-τB/p65 correlated with concurrent increase in cytosolic levels of IτBα along with NF-τB-dependent expression of Bcl2, cyclin D1, MMP-9, and VEGF. These results demonstrate that NF-τB/p65 is constitutively activated in human prostate adenocarcinoma and is related to tumor progression due to transcriptional regulation of NF-τB-responsive genes.
Keywords: Nuclear factor-κB, NF-κB-responsive genes, prostate cancer, IκB, Rel A
Introduction
Prostate cancer often progresses from an androgendependent, organ-confined disease to a highly invasive, androgen-independent malignancy with metastatic growth properties [1,2]. Although localized forms of prostate cancer can be effectively managed with surgery and other modalities, no effective treatment is currently available for hormone-refractory prostate cancer [3]. Considerable progress has been made in the early detection and treatment of prostate cancer; however, it has been difficult to distinguish between cancers that will remain indolent and those that will behave aggressively. Identification of predictive markers for prostate cancer, especially those that are indicative of disease aggressiveness, is important for improving the clinical management and survival of these patients.
Rel/nuclear factor-κB (NF-κB) is a dimeric transcription factor that plays important roles in the control of growth, differentiation, and apoptosis [4,5]. It is also involved in immune and adaptive responses to changes in cellular redox balance [6–8]. Rel/NF-κB consists of homodimers and heterodimers formed by several subunits: NF-κB1 (p50/p105), NF-κB2 (p52/100), Rel A (p65), Rel B, and c-Rel proteins [9]. These subunits have a high level of sequence homology within the NH2-terminal 300 amino acids in the Rel homology domain [9,10]. The inactive form of NF-κB is localized in the cytoplasm and consists of three subunits: DNA-binding p50 and p65 subunits and an inhibitory subunit, called IκB, which is bound to p65 [10]. IκB masks the nuclear localization sequence and its release initiates activation of NF-κB and its subsequent translocation to the nucleus, where it can bind to target sites in DNA [10]. Activation of NF-κB results in the induction of a large number of genes that influence cellular proliferation, inflammation, and cellular adhesion [11]. A number of critical genes that are regulated by NF-κB include: anti-apoptotic genes (cIAP, survivin, Bcl2, and BClxL), cell cycle-regulatory genes (cyclin D1), genes encoding adhesion molecules, chemokines, inflammatory cytokines, and genes involved in tumor metastases (matrix metalloproteinase-9 [MMP-9], cyclooxygenase-2 [COX-2], nitric oxide synthase-2 [NOS-2], and vascular endothelial growth factor [VEGF]) [4–7,11]. Inappropriate regulation of NF-κB and its dependent genes has been associated with various pathologic conditions including toxic/septic shock, graft-versus-host disease, acute inflammatory conditions, acute-phase response, viral replication, radiation damage, atherosclerosis, and cancer [4–8]. Aberrant NF-κB activation has been implicated in the pathogenesis of several human malignancies including cancer of the breast [12], colon [13], esophagus [14], gastrointestines [15], liver [16], lungs [17], pancreas [18], skin [19], and uterine cervix [20]. Studies have reported that NF-κB is constitutively activated in human prostate cancer and prostate cancer xenografts [21–24]. This activation occurs through signal transduction pathways involving tyrosine kinases, NF-κB-inducing kinase (NIK), and IKK, which ultimately leads to phosphorylation and faster turnover of IκBα, the superrepressor of NF-κB activation [21,22]. NF-κB has been shown to activate a transcription-regulatory element of the prostatespecific antigen (PSA)-encoding gene, a marker of prostate cancer development and progression [25]. Increased NF-κB activity in androgen-insensitive human prostate carcinoma PC-3 cells contributes directly to its aggressive behavior [26]. Conversely, blockade of NF-κB activity in human prostate carcinoma cells is associated with suppression of angiogenesis, invasion, and metastasis [27]. These findings suggest that incessant activation of NF-κB in androgen-insensitive human prostate carcinoma cells may contribute to aggressive behavior; however, a definitive correlation between NF-κB activation and tumor aggressiveness is lacking. We undertook the present study to determine whether increased NF-κB activation and its binding to DNA enhance tumor aggressiveness and could predict the clinical outcome of the disease. Using several techniques including immunohistochemistry, Western blot analysis, and electrophoretic mobility shift assay (EMSA), we found evidence of constitutive activation of NF-κB/p65, its nuclear localization, increased DNA binding, along with upregulation of NF-κB-regulated gene increases with tumor grade. These results further suggest that the NF-κB signaling pathway is a potential target for therapeutic intervention.
Materials and Methods
Tissue Samples
Discarded benign and malignant prostate tissues from patients undergoing surgery were obtained from the Tissue Procurement Facility of Case Research Institute and the Midwestern Division of the Cooperative Human Tissue Network (Columbus, OH). The Gleason grade and score of adenocarcinoma specimens were assigned by a surgical pathologist experienced in genitourinary pathology. Immediately after procurement, samples were snap-frozen in liquid nitrogen and stored at -80°C until further use. In addition, 4-µm tissue sections were obtained from 15 benign specimens of the prostate and 49 other prostate cancer specimens. Preoperative serum PSA levels were determined by review of medical records. These studies were approved by the Institutional Review Board at Case Western Reserve University.
Western Blot Analysis
Frozen tissues (benign or cancer) were processed for cytosolic and nuclear lysate, and the protein content was determined using the DC Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA) as previously described [28]. For Western blot analysis, 25 µg of protein was resolved using 4% to 20% polyacrylamide gels (Novex, Carlsbad, CA) and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% nonfat dry milk/1% Tween-20 in 20 mM TBS, pH 7.6) for 2 hours at room temperature; incubated with mouse monoclonal antibodies of NF-κB/p65, NF-κB/p50, IκBα, Bcl2 (Santa Cruz Biotechnology, Santa Cruz, CA), p-IκBα (Cell Signaling Technology, Beverly, MA), cyclin D1, MMP-9, and VEGF (Lab Vision Corp., Fremont, CA) in blocking buffer for 2 hours at room temperature or overnight at 4°C; followed by incubation with anti-mouse IgG secondary antibody conjugated with horseradish peroxidase (HRP; Amersham-Pharmacia, Piscataway, NJ) and detected by ECL chemiluminescence and autoradiography using XAR-5 film (Eastman Kodak, Rochester, NY). It is important to emphasize here that for detection of NF-κB/Rel members, we used monoclonal antibodies that could detect the activated form of NF-κB/Rel proteins by recognizing an epitope overlapping the nuclear localization signal and IκBa-binding site of NF-κB/p65 and NF-κB/p50, and are therefore selective and specific for the activated forms.
EMSA
EMSA for NF-κB was performed in the nuclear fraction of prostate tissue using Lightshift Chemiluminiscent EMSA kit (Pierce Biotechnology, Rockford, IL) following manufacturer's protocol. Briefly, DNA was biotin-labeled using the biotin 3′ end labeling kit (Pierce Biotechnology) in a 50-µl reaction buffer, 5 pmol of double-stranded NF-κB oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 3′-TCA ACTCCCCTGAAAGGGTCCG-5′) incubated in a microfuge tube with 10 µl of 5 x terminal deoxynucleotidyl transferase (TdT) buffer, 5 µl of 5 µM biotin-N4-CTP, 10 U of diluted TdT, and 25 µl of ultrapure water at 37°C for 30 minutes. The reaction was stopped with 2.5 µl of 0.2 M EDTA. To extract labeled DNA, 50 µl of chloroform:isoamyl alcohol (24:1) was added to each tube and centrifuged at 13,000g. The top aqueous phase containing the labeled DNA was further used for binding reactions. Each binding reaction contained 1 x binding buffer (100 mM Tris, 500 mM KCl, 10 mM dithiothreitol, pH 7.5), 2.5% glycerol, 5 mM MgCl2, 50 ng/µl poly(dI-dC), 0.05% NP-40, 2.5 µg of nuclear extract, and 20 to 50 fmol of biotin end-labeled target DNA. The contents were incubated at room temperature for 20 minutes. To this reaction mixture, 5 µl of 5 x loading buffer was added, subjected to gel electrophoresis on a native polyacrylamide gel, and transferred to a nylon membrane. After transfer was completed, DNA was crosslinked to the membrane at 120 mJ/cm2 using a UV crosslinker equipped with 254-nm bulb. The biotin end-labeled DNA was detected using streptavidin-HRP conjugate and a chemiluminiscent substrate. The membrane was exposed to X-ray film (XAR-5; Amersham Life Science, Inc., Arlington Height, IL) and developed using a Kodak film processor (Eastman Kodak). For supershift assay, antibodies against NF-κB/p65 and NF-κB/p50 were added 30 minutes after the beginning of the reaction, and incubation was continued for an additional 30 to 45 minutes. DNA protein complexes were analyzed as described earlier.
Immunohistochemistry
Immunohistochemical staining of paraffin-embedded sections was performed with a sensitive Dako EnVision+ System (Dako Cytomation, Carpentaria, CA) according to the manufacturer's protocol. This is an extremely sensitive system and offers an enhanced signal generation for the detection of antigen. Briefly, 4-µm-thick paraffin-embedded sections from benign and cancer tissues were deparaffinized, rehydrated, immersed in target retrieval solution, and blocked for endogenous peroxidase activity. The sections were permeabilized in TNB-BB (100 mM Tris, pH 7.5, 150 mM NaCl, 0.5% blocking agent. 0.3% Triton-X, and 0.2% saponin) and incubated in primary monoclonal antibodies of NF-κB/p65, NF-κB/p50, and IκBα (Santa Cruz Biotechnology) at 1:200 dilution, respectively, overnight at 4°C. Control sections were incubated with antisera in the presence of 10-fold excess of these antibodies or with isotype-matched IgG normal goat serum. After washing three times in TBS, sections were incubated for 2 hours at room temperature with HRP-labeled polymer conjugated with secondary antibody. Immunoreactive complexes were detected using AEC+ substrate chromagen consisting of 3-amino-9-ethylcarbazole. Slides were then counterstained in Mayer's hematoxylin, mounted in crystal mount media, and dried overnight on a level surface.
Evaluation of Immunostaining
The immunostained sections were examined independently by three of the authors (S.S., S.G., and G.T.M.) using light microscopy. Positive nuclear staining in high-grade cancer cells was used as positive control for NF-κB staining. Sections were examined with an inverted Olympus BH2 microscope (Olympus America Inc., Melville, NY) and images were acquired with Image Proplus software (Media Cybernetics, Carlsbad, CA), digitally scored on a computer. The intensity of staining was graded semiquantitatively and each specimen was assigned a score on a scale from 0 to 3, designated as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The immunoreactive score was designated by the percentage of positive cells and the staining intensity as previously described [29]. The percent of nuclear staining for NF-κB/p65 was scored by counting the positive-stained cells and the total number of cells quantified in random microscopic fields (magnification, x400) with the assistance of the software program.
Statistical Analysis
All measures were summarized as mean ± SD. Graphical summaries of the distributions of measure were made using boxplots. Kruskal-Wallis test, a nonparametric test based on Wilcoxon scores, was used to examine the mean or median difference of NF-κB/p65, NF-κB/p50, and IκBα (using actual values instead of levels) between two tissue groups. Differences in nuclear staining of NF-κB/p65 between benign tissue and cancers of differing Gleason score (2 + 2, 3 + 3, 4 + 4, and 5 + 5) were also tested using Kruskal-Wallis test. All tests were two-sided and P values less than .05 were considered significant. All analyses were performed with the SAS (Statistical Analysis System, version 6.12) software (Cary, NC).
Results
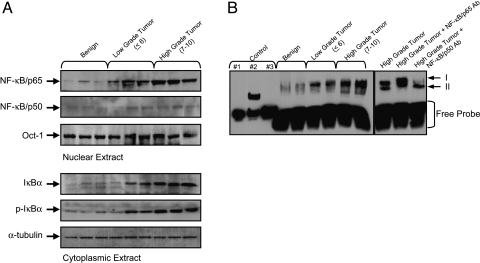
To ascertain whether NF-κB is activated in human prostate adenocarcinoma, we performed immunoblot analysis for NF-κB/p65 and NF-κB/p50 in the nuclear fraction of benign prostate tissue and prostate cancer specimens. As shown in Figure 1A, immunoblot analysis for NF-κB/p65 in the nuclear fraction exhibited significantly higher levels of protein expression in high-grade cancer tissue (Gleason score 5 + 4 and 5 + 5) compared with low-grade (Gleason score 3 + 3) and benign tissue. However, no significant variation in the protein expression of NF-κB/p50 was observed in these tissues.
Figure 1.
(A) Protein expression of NF-κB/p65, NF-κB/p50, IκBα, and its phosphorylation in benign prostate tissue and prostate cancer specimens. Cytosolic and nuclear extracts from tissues were prepared and electrophoresed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting with anti-p65 and anti-p50 antibodies in the nuclear fraction, and anti-IκBα and anti-p-IκBα in the cytosolic fraction. Cancer tissue is subdivided into lowgrade cancer (Gleason score ≤6) and high-grade cancer (Gleason score 7–10). A progressive increase in the protein expression of NF-κB/p65 in the nuclear fraction, and IκBα and p-IκBα in the cytosol, was observed in prostate cancer specimens. To ensure equal protein loading in the nuclear and cytosolic fractions, the membrane was stripped and reprobed with anti-Oct-1 and anti-α-tubulin antibody. (B) NF-κB DNA-binding activity in benign and prostate cancer specimens by EMSA. EMSA was performed to identify nuclear translocation of NF-κB dimers and their binding to DNA. An increase in the nuclear translocation of NF-κB/p65 was observed in cancer tissue compared to benign tissue. NF-κB complexes I and II are indicated with arrows to the right of the panel. Supershift assay was performed in high-grade tumor tissue with antibodies specific for NF-κB/p65 and NF-κB/p50. A shift in NF-κB/p65 was observed in high-grade tumor tissue. The details are described in Materials and Methods section. Controls: (1) biotin-EBNA control DNA; (2) biotin-EBNA control DNA + EBNA extract; and (3) biotin-EBNA control DNA + EBNA extract + 20-fold molar excess of unlabeled EBNA DNA.
Next we analyzed the expression of NF-κB-inhibitory protein, IκBα, in the cytosolic fraction of benign prostate tissue and prostate cancer specimens. As shown in Figure 1A, immunoblot analysis for IκBα exhibited a similar pattern of protein expression as previously observed for NF-κB/p65 with significantly high levels in high-grade cancer tissue compared with low-grade cancer and benign tissue. Because the rapid turnover of IκBα requires phosphorylation at its N-terminal serine residues as a signal for ubiquitination that eventually leads to its degradation, we determined the phosphorylation of IκBα in benign prostate tissue and prostate cancer specimens. For this, we used an antibody specific to phosphorylation sites at the N-terminal Ser 32/36 of IκBα. Compared to benign tissue, a progressive increase in p-IκBα with increasing tumor grade was observed in prostate cancer specimens similar to the previously noted increase in IκBα (Figure 1A).
We next performed EMSA in the nuclear fractions obtained from benign prostate tissue and prostate cancer specimens. EMSA is a technique used to confirm the increased nuclear translocation and DNA-binding activity of NF-κB. As shown in Figure 1B, increased DNA-binding activity was observed in prostate cancer tissue, compared to benign tissue. The increase in DNA-binding activity was more significant in high-grade cancer tissue compared with low-grade cancer or benign tissue. To further confirm the specificity of NF-κB DNA binding, we performed supershift assay with antibodies specific for NF-κB/p65 and NF-κB/p50 and a competitive study with a 20-fold excess of unlabeled oligonucleotide. As shown in Figure 1B, antibodies specific for NF-κB/p65 (which recognizes Rel A homodimer) and NF-κB/p50 supershifted complex I but were unable to shift complex II, consisting of NF-κB/p50. Unlabeled oligonucleotide diminished the intensity of NF-κB/p65, indicating that the shifted complex was a NF-κB-specific band (data not shown) (Figure 1B).
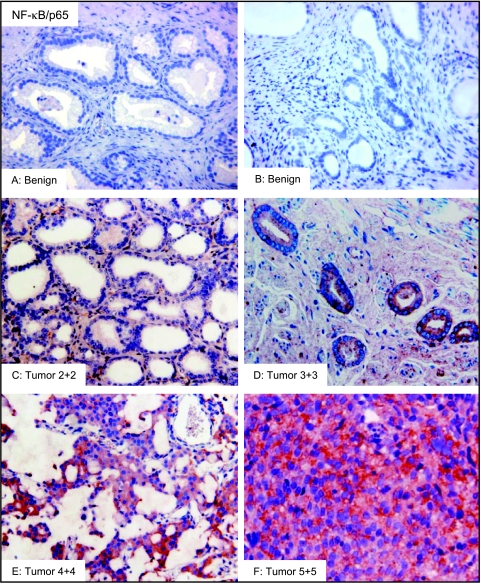
We next examined the protein expression of NF-κB/p65, NF-κB/p50, and IκBα by immunohistochemical analysis in benign prostate tissue and prostate cancer specimens. Cancers were analyzed according to Gleason grade and tissue specimens were assigned to three subgroups consisting of benign tissue, low-grade cancer (Gleason score ≤ 6), and high-grade cancer (Gleason score 7–10). The staining intensity was based on the scoring pattern of positive-stained cells in the tissue specimens. Benign tissues did not exhibit any significant NF-κB/p65 expression. In 15 specimens analyzed, two exhibited moderate expression, six exhibited weak expression, and seven were negative for NF-κB/p65 (Table 1). Most of the stainings observed in benign tissue were nonspecific and localized in areas of inflammation (Figure 3, A and B). A progressive increase in the staining intensity of NF-κB/p65 with increasing tumor grade was observed in the prostate cancer specimens. In 21 low-grade tumors, 4 exhibited strong NF-κB/p65 expression, 10 exhibited moderate staining, 5 exhibited weak staining, and 2 showed no NF-κB/p65 staining. In 28 high-grade tumors, 8 exhibited strong staining, 14 exhibited moderate staining, 3 exhibited weak staining, and 3 were negative for NF-κB/p65 staining (Table 1). The boxplots of NF-κB/p65 protein exhibited a wide interindividual variation in cancer specimens compared to benign tissue. The average scoring patterns were as follows: benign tissue 0.72 ± 0.6 (mean ± SD); lowgrade tumor 1.66 ± 0.6 (mean ± SD); and high-grade tumor 1.96 ± 0.8 (mean ± SD), respectively (Figure 2A). A significant increase in NF-κB/p65 score was observed in cancer tissue compared with benign tissue, representing a 2.3-fold increase in low-grade tumor (P < .003) and 2.7-fold increase in high-grade tumor specimens (P < .0001) (Figure 2A). Compared to benign tissue where negative to weak staining was observed in most of the specimens, moderate to strong staining was observed in the majority of cancers (Figure 3, A and F). Staining for NF-κB/p65 was observed both in the cytoplasm and nucleus of tumor cells in low-grade tumor (Gleason score 2 + 2), with a progressive increase in the number of positive-stained cells with increasing tumor grade (Gleason score 3 + 3) (Figure 3, C and D). Intense and distinctly granular staining with increasing numbers of positive stained nuclei was observed in the higher-grade tumor specimens (Gleason score 4 + 4 and 5 + 5) (Figure 3, E and F).
Table 1.
Expression of NF-κB/p65, NF-κB/p50, and IκBα in Benign and Prostate Cancer Tissue Specimens.
| NF-κB/p65 | NF-κB/p50 | IκBα | ||||||||||||||
| Specimen Type | Number | None | Weak | Moderate | Strong | P | None | Weak | Moderate | Strong | P | None | Weak | Moderate | Strong | P |
| Benign | 15 | 7 | 6 | 2 | 0 | 13 | 2 | 0 | 0 | 8 | 4 | 3 | 0 | |||
| Low-grade (≤6) | 21 | 2 | 5 | 10 | 4 | 18 | 3 | 0 | 0 | 4 | 7 | 6 | 4 | |||
| High-grade (7–10) | 28 | 3 | 3 | 14 | 8 | .003* | 16 | 6 | 4 | 2 | .185 | 2 | 3 | 16 | 6 | .002* |
The expression of NF-κB/p65, NF-κB/p50, and IκBα was evaluated on staining intensity of the tissues as: none (0), weak (1), moderate (2), and strong (3). Fischer exact test was used to test the association between staining intensity and tissue type, or staining intensity and tumor grade.
P value less than .05 was considered as significant.
Figure 3.
Immunostaining for NF-κB/p65 in representative samples of benign prostate tissue and prostate cancer specimens of various Gleason grades. Benign tissues showed no significant NF-κB/p65 staining (A and B). A progressive increase of NF-κB/p65 protein was observed in cancer specimens. Low-grade cancer exhibited weak to moderate NF-κB/p65 protein expression (C and D), whereas strong staining was observed in high-grade cancer specimens with more granular nuclear staining (E and F) (magnification, x200). The details are described in Materials and Methods section.
Figure 2.
Boxplots for (A) NF-κB/p65, (B) NF-κB/p50, and (C) IκBα based on the staining pattern in benign prostate tissue and prostate cancer specimens. The intensity of staining was graded semiquantitatively by assigning a score to each tissue. Compared to benign tissue, staining was prominent for NF-κB/p65 in low-grade (P < .003) and high-grade (P < .0001) cancer specimens. Similar results were observed for IκBα staining in low-grade (P < .01) and high-grade (P α .0001) specimens, compared to benign tissue. No significant difference in staining pattern between benign and cancer tissue was observed for NF-κB/p50. NS, nonsignificant; (I) Min-Max, (□), 25% to 75%; ( ) median value. The details are described in Materials and Methods section.
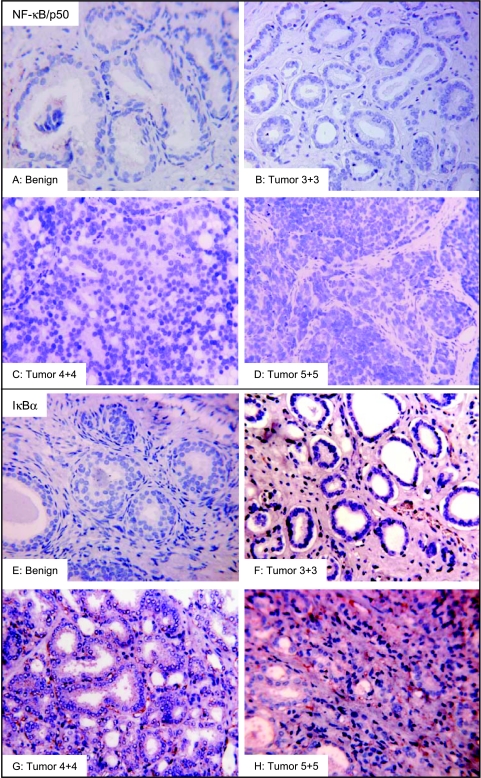
We next examined the NF-κB/p50 protein expression in benign prostate tissue and prostate cancer specimens. Staining patterns for NF-κB/p50 in benign and cancerous tissues were nonspecific; staining was limited to muscle cells and scattered epithelial cells (Figure 4, A–D). In 15 benign specimens, 2 exhibited weak expression, whereas 13 were negative for NF-κB/p50 expression. The staining pattern for NF-κB/p50 in cancer specimens was predominantly weak to moderate and nonspecific. Weak expression was noted in 3 of 21 specimens in low-grade tumor tumors and 18 tumors were negative for NF-κB/p50 expression. A similar staining pattern was observed in high-grade tumors. Strong staining was observed in 2 of 28 specimens, 4 exhibited moderate staining, 6 exhibited weak staining, and 16 were negative for NF-κB/p50 expression (Table 1). The boxplots of NF-κB/p50 protein exhibited low scores, implying that NF-κB/p50 does not participate in prostate carcinogenesis. Average scoring patterns were as follows: benign tissue 0.19 ± 0.2 (mean ± SD); low-grade tumor 0.22 ± 0.2 (mean ± SD); and high-grade tumor 0.66 ± 0.7 (mean ± SD), respectively (Figure 2B).
Figure 4.
Immunostaining for NF-κB/p50 and IκBα in representative samples of benign prostate tissue and prostate cancer specimens of various Gleason grade. Benign and cancer specimens were negative for NF-κB/p50 staining (A–D). IκBα expression was weak to negative in benign tissue (E). A progressive increase in IκBα protein was observed in cancer specimens. Low-grade cancer exhibited weak to moderate IκBα protein expression, (F) whereas strong staining pattern was observed in high-grade cancer specimens, mostly in the cytoplasm of epithelial cells (G and H) (magnification, x200). The details are described in Materials and Methods section.
Next we examined the levels of cytosolic IκBα protein expression in the benign prostate tissue and prostate cancer specimens. IκBα is bound to the NF-κB/p65 in the cytoplasm and inhibits its nuclear translocation. Benign tissue did not exhibit any significant IκBα expression. Moderate staining was noted in 3 of 15 specimens, 4 exhibited weak staining, and 8 were negative for IκBα expression. Prostate cancer specimens showed significant staining for IκBα, which progressively increased as tumor grade increased. In 21 lowgrade tumor specimens, strong IκBα expression was observed in four specimens, six specimens exhibited moderate staining, seven specimens exhibited weak expression, and four were negative for IκBα expression. Strong staining was noted in 6 of 28 high-grade tumors, 16 exhibited moderate staining, 3 exhibited weak staining, and 2 samples were negative for IκBα expression (Table 1). The boxplots of IκBα protein displayed a wide interindividual variation in tumor specimens compared to benign tissue. Average scoring patterns were as follows: benign tissue 0.61 ± 0.6 (mean ± SD); low-grade tumor 1.31 ± 0.8 (mean ± SD); and high-grade tumor 1.88 ± 0.6 (mean ± SD), respectively (Figure 2C). A significant increase in IκBα score was observed in tumor tissue compared with benign tissue, representing a 2.1-fold increase in low-grade tumor (P < .01) and 3.1-fold increase in high-grade tumor specimens (P < .0001) (Figure 2C). Compared to benign tissue (Figure 4E), the staining for IκBα was predominantly observed in the cytoplasmic fraction in cancer specimens. Staining intensity progressively increased with tumor grade (Figure 4, F–H).
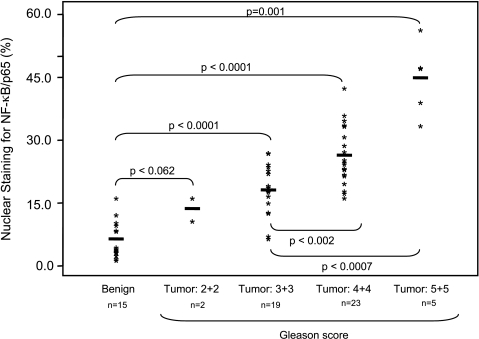
Next we extended our observation of increased constitutive NF-κB/p65 activation in prostate cancer by quantification of nuclear NF-κB/p65 expression in specimens of different cancer grades. As shown in Figure 5, we counted the number of stained cells and calculated the percentage of positive-stained cells in benign and tumor specimens. Compared to benign tissue, a significant increase in the percentage of cells with positive nuclear staining was observed with increasing tumor grade that ranged from 13.5% in Gleason score 2 + 2 to 17.6% in Gleason score 3 + 3, 25.2% in Gleason score 4 + 4, and 44.8% in Gleason score 5 + 5, respectively. Our findings suggest that NF-κB/p65 is activated in prostate cancer and its translocation to the nucleus increases progressively in parallel with increasing tumor grade.
Figure 5.
Score for nuclear staining of NF-κB/p65 in benign prostate tissue and prostate cancer specimens. Nuclear staining was determined by counting the number of NF-κB/p65-positive cells and total number of cells at x400 magnification. A progressive increase in the percentage of NF-κB/p65 cells was observed as tumor grade increased. Compared to benign tissue, there is an increase in percent nuclear expression of NF-κB/p65 in low-grade cancer (Gleason score 2 + 2) (P < .062), and a significantly higher level of percent nuclear expression (P < .0001) in high-grade cancer. The details are described in Materials and Methods section.
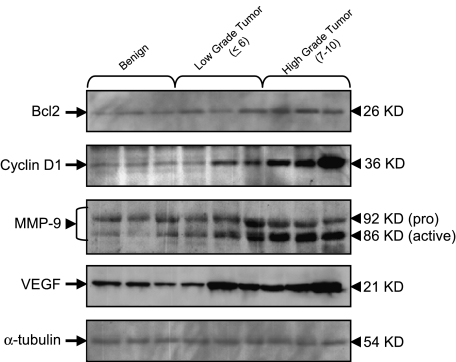
Because NF-κB has been shown to regulate the expression of a number of genes whose products are involved in carcinogenesis, we evaluated the protein expression of Bcl2, cyclin D1, MMP-9, and VEGF, all of which are associated with progression of prostate cancer. As shown in Figure 6, a significant increase in the protein expression of Bcl2, cyclin D1, MMP-9, and VEGF was observed in high-grade cancer tissue compared with low-grade and benign tissue. Patterns of increased expression of NF-κB-regulated proteins were comparable to the observed increases in NF-κB/p65 and IκBα expression.
Figure 6.
Protein expression of NF-κB-regulated genes specifically Bcl2, cyclin D1, MMP-9, and VEGF in benign prostate tissue and prostate cancer specimens. Total cell lysates from tissues were prepared and electrophoresed by SDS-PAGE followed by Western blotting with anti-Bcl2, anti-cyclin D1, anti-MMP-9, and anti-VEGF monoclonal antibodies. Cancer tissue is subdivided into low-grade cancer (Gleason score ≤6) and highgrade cancer (Gleason score 7–10). A progressive increase in the protein expression of Bcl2, cyclin D1, MMP-9, and VEGF was observed in prostate cancer specimens. To ensure equal protein loading, the membrane was stripped and reprobed with anti-α-tubulin antibody. The details are described in Materials and Methods section.
Discussion
One of the many challenges in the effective management of prostate cancer is the identification of molecular marker(s) capable of predicting tumor aggressiveness and disease progression. Compelling evidence suggests that constitutive activation of NF-κB is a hallmark of a number of human malignancies [30,31]. Aberrant NF-κB activation has been implicated in the pathogenesis of many human diseases including toxic/septic shock, graft-versus-host disease, acute inflammatory conditions, viral replication, radiation damage, and atherosclerosis [32,33]. Constitutive NF-κB activation has been detected in several types of carcinoma; mutations and rearrangements of NF-κB/IκB family members have been observed only in some hematologic cancers [34,35]. In vivo activation of NF-κB/Rel members has been definitively demonstrated in some forms of human malignancies that include cancer of the breast [12], colon [13], esophagus [14], gastrointestines [15], liver [16], lungs [17], pancreas [18], skin [19], and uterine cervix [20], implicated as part of the activated NF-κB complex. Studies conducted on human prostate carcinoma cells have shown that NF-κB is constitutively activated in androgen-insensitive DU145 and PC3 cells, and prostate cancer xenografts [21–24]. A previous study has demonstrated NF-κB nuclear localization and its prognostic significance in prostate cancer [23]; however, it is not clear whether NF-κB activation reflects proliferative activity of benign and malignant prostate epithelial cells, or whether the activation that has been observed plays a role in disease progression. We examined a large number of benign and malignant human prostate tissues to evaluate the role of NF-κB/IκB activation in human prostate adenocarcinoma. Our studies suggest that NF-κB/p65 (but not NF-κB/p50) is constitutively activated in human prostate adenocarcinoma, and that increasing levels of activation correlate with increasing Gleason grade of cancer. We observed increased DNAbinding activity of NF-κB/p65 in cancer tissue, as compared with benign tissue, correlated with increased IκBα expression in the cytoplasm. Previous studies have demonstrated that Rel A/p65 exhibited strong transactivation potential as observed by its constitutive activation in some forms of human cancers [34]. Nuclear translocation of Rel A and NF-κB-DNA-binding activity are higher in human tissues from cervical cancer [20], colon adenocarcinoma [13], gastric carcinoma [15], hepatocellular carcinoma [16], and pancreatic adenocarcinoma [18] compared to their normal counterparts. Similarly, nuclear translocation of Rel A-p50 complex occurs in human breast cancer tissues and derived cell lines [12,36]; however, others have found that c-Rel, NF-κB1/p50, NF-κB2/p52, and Bcl3, rather than Rel A, are the major components in human breast cancer tissues [37]. Increased Rel A activity and enhanced nuclear localization of p65-p50 dimer have been observed in melanoma and thyroid cancer cells compared to normal equivalent cell lines [38,39]. Likewise, NF-κB/p50 has been noted to have low transactivation activity and may have a limited role in carcinogenesis [40]. Constitutive activation of NF-κB/p50 has been observed in non small cell lung carcinoma [17] and skin carcinogenesis model [41]. In our studies, we found no significant NF-κB/p50 activation in prostate cancer tissue specimens.
Altered expression of IκBα in cancer tissue has been linked to constitutive NF-κB activation through phosphorylation of IκBα at Ser32/36, resulting in the release and nuclear translocation of active NF-κB [9,10]. We observed a progressive increase in the protein expression of IκBα and its phosphorylation in cancer specimens compared with benign tissue, and the level of protein expression of IκBα increased in parallel with cancer grade. This marked increase in IκBα protein expression and its phosphorylation in cancer tissue may be the consequence of functional activation of NF-κB in prostate cancer, which is known to cause strong transcriptional upregulation of IκBα as a feedback mechanism possibly operative in other cancers as well. Our results are in agreement with previous observations that IκBα protein levels were increased in androgen-insensitive human prostate carcinoma cells due to phosphorylation and faster turnover of IκBα in the cytosolic fraction [21,22]. It has been shown that the upstream events associated with the constitutive activation of NF-κB in prostate carcinoma cells involve activation of tyrosine kinases NIK and IKK [21,22]. Studies have further demonstrated that the tumor-suppressor PTEN inhibits NF-κB activation and has been implicated in prostate cancer [42]. The role of NF-κB in prostate cancer cells and tissues has been previously reported, but no correlation has been established between nuclear localization of NF-κB and disease progression. In the present study, we observed that nuclear staining of NF-κB/p65 is more pronounced in cancers of higher Gleason grade.
Accumulating evidence suggests that NF-κB has an important role in carcinogenesis and tumor progression [9,10]. NF-κB may be activated by cytokines, epidermal growth factor, receptor expression, and Ras activation, or through oxidative stress and cell damage [11]. Importantly, chronic inflammation and release of proinflammatory cytokines, known to induce NF-κB, could provide selective growth advantage of neoplastic cells [6,7]. Furthermore, NF-κB is involved in the regulation of several gene products that may be important for cell motility and metastasis [11]. The expression of both MMP-9 and urokinase-like plasminogen activator (u-PA) is upregulated by NF-κB [42,43]. The expression of cytokines IL-8 and IL-6, which are important for cell migration, is also regulated by NF-κB [43]. Studies have shown that the constitutive activation of NF-κB/p50-Rel A and family members of activator protein-1 (AP-1) family, Fra-1 and JunD, are essential for deregulated expression of IL-6 in prostate cancer [44]. Constitutive NF-κB activation in human prostate carcinoma PC-3 cells has been shown to be associated with invasive behavior [26]. Further, blockade of NF-κB activity in these cell lines by transfection with mutated IκBα leads to inhibition of proangiogenic markers: (VEGF, IL-8, and MMP-9) tumor invasion and metastasis [27]. Our study, demonstrating positive correlation between increasing Gleason grade, NF-κB/p65 protein expression, and DNA binding/nuclear colocalization of NF-κB/p65, suggests that NF-κB may be a useful predictive marker for prostate cancer aggressiveness. This may be further correlated with increase in protein expression of MMP-9 and VEGF in cancer specimens observed in our studies.
Prostate cancer progression is often characterized by aggressive locally invasive growth, metastasis, and, eventually, androgen independence [1,2]. The serum level of PSA has been widely recognized as a clinical marker for prostate cancer progression and has proven useful in monitoring effectiveness of treatment [45]. In patients managed by hormone ablation therapy, rising serum PSA levels typically signify prostate cancer progression [45]. Recently, NF-κB has been shown to bind a κB response element in the promoter of the PSA gene that may be responsible for increased serum PSA levels during prostate cancer progression [25]. However, in the present study, we did not observe any significant correlation with serum PSA levels and NF-κB expression (data not shown). Additional studies with a large sample size of various tumor grades are required to validate these findings.
NF-κB is an important regulator of cell proliferation through its direct role in cell cycle progression [46]. Studies have shown that NF-κB can stimulate transcription of cyclin D1, a key regulator of G1 checkpoint control, mediated by direct binding of NF-κB to multiple sites in the cyclin D1 promoter [47,48]. Amplification and/or overexpression of cyclin D1 has been shown in several types of human cancers [49]. Further studies have shown prognostic significance of cyclin D1 in prostate adenocarcinoma and in the development of androgen-independent disease [49,50]. Our studies have shown a positive correlation between cyclin D1 expression during constitutive NF-κB activation in prostate cancer specimens and suggest as an additional predictive marker for prostate cancer progression.
Constitutive NF-κB activation is a mechanism of protection against diverse apoptotic stimuli, including chemotherapy and radiation therapy [51,52]. Numerous anti-apoptotic genes are regulated by NF-κB, including genes encoding Bcl2-like proteins and inhibitors of apoptosis proteins [4,8]. Activation of NF-κB and its nuclear translocation leads to the transcription of these anti-apoptotic genes [4,8]. Studies have shown that transcriptional regulation of Bcl2 by NF-κB protects human prostate carcinoma cells from many different apoptotic stimuli such as hormone ablation, radiotherapy, chemotherapy, and immunotherapy [53]. Studies have further demonstrated that blockade of IκBα phoshorylation inhibits NF-κB translocation and activation, and IL-6 production, and induces TNF-α-mediated apoptosis in both androgen-dependent LNCaP and androgen-independent PC-3 cells [27]. Our studies suggest that increase in Bcl2 expression correlates with DNA binding and nuclear localization of NF-κB/p65 in prostate cancer specimens, and, as such, suggest that Bcl2 expression along with NF-κB/p65 and IκBα levels in prostate cancer may be useful as predictive markers of disease progression. Studies are required to identify mechanisms of NF-κB activation and to further evaluate the distinct roles of NF-κB/IκB family members, to assess their usefulness in devising strategies for the effective management of prostate cancer.
Abbreviations
- NF-κB
nuclear factor-κB
- NIK
NF-κB-inducing kinase
- EMSA
electrophoretic mobility shift assay
- MMP-9
matrix metalloproteinase-9
- u-PA
urokinase-like plasminogen activator
- AP-1
activator protein-1
- IL-6
interleukin-6
- VEGF
vascular endothelial growth factor
- PSA
prostate-specific antigen
- IAP
inhibitor of apoptosis
- COX-2
cyclooxygenase-2
- NOS-2
nitric oxide synthase-2
Footnotes
This work was supported by grants CA 94248 and CA 99049 from the United States Public Health Service and funds from the Cancer Research and Prevention Foundation.
This work was conducted in The James and Eilleen Dicke Research Laboratory, Department of Urology, Case Western Reserve University, Cleveland, OH.
References
- 1.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 2.De La Taille A, Vacherot F, Salomon L, Druel C, Gil Diez De Medina S, Abbou C, Buttyan R, Chopin D. Hormone-refractory prostate cancer: a multi-step and multi-event process. Prostate Cancer Prostatic Dis. 2001;4:204–212. doi: 10.1038/sj.pcan.4500534. [DOI] [PubMed] [Google Scholar]
- 3.Martel CL, Gumerlock PH, Meyers FJ, Lara PN. Current strategies in the management of hormone-refractory prostate cancer. Cancer Treat Rev. 2003;29:171–187. doi: 10.1016/s0305-7372(02)00090-7. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 7.Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore TD. The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–265. [PubMed] [Google Scholar]
- 10.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 11.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 12.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM, III, Macky S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 14.Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, McConkey C, Stuart RC, Wright N, Harrison R, Jankowski JA. Tumour necrosis factor-alpha in Barrett's oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–6081. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M, Katano M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136–4142. [PubMed] [Google Scholar]
- 16.Tai DI, Tsai SL, Chang YH, Huang SN, Chen TC, Chang KS, Liaw YF. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer. 2000;89:2274–2281. [PubMed] [Google Scholar]
- 17.Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- 18.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 19.Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003;15:1–7. doi: 10.1016/s0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 20.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- 21.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 22.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 23.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91:417–420. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 24.Levine L, Lucci JA, III, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr, Hellmich MR. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63:3495–3502. [PubMed] [Google Scholar]
- 25.Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22:2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindholm PF, Bub J, Kaul S, Shidham VB, Kajdacsy-Balla A. The role of constitutive NF-kappaB activity in PC-3 human prostate cancer cell invasive behavior. Clin Exp Metastasis. 2000;18:471–479. doi: 10.1023/a:1011845725394. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappaB and induction of apoptosis. Oncogene. 2003;23:2507–2522. doi: 10.1038/sj.onc.1207353. (December 15 (E-publication ahead of print publication)). [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Hussain T, MacLennan GT, Fu P, Patel J, Mukhtar H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol. 2003;21:106–112. doi: 10.1200/JCO.2003.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Haefner B. NF-kappa B: arresting a major culprit in cancer. Drug Discov Today. 2002;7:653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Gaynor RB. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- 33.Aradhya S, Nelson DL. NF-kappaB signaling and human disease. Curr Opin Genet Dev. 2001;11:300–306. doi: 10.1016/s0959-437x(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 34.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 35.Cheson BD. Hematologic malignancies: new developments and future treatments. Semin Oncol. 2002;29:33–45. doi: 10.1053/sonc.2002.34878. [DOI] [PubMed] [Google Scholar]
- 36.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 39.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F, Santoro M, Fusco A. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 40.Phi van L. Transcriptional activation of the chicken lysozyme gene by NF-kappa Bp65 (RelA) and c-Rel, but not by NF-kappa Bp50. Biochem J. 1996;313:39–44. doi: 10.1042/bj3130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budunova IV, Perez P, Vaden VR, Spiegelman VS, Slaga TJ, Jorcano JL. Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene. 1999;18:7423–7431. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 43.Andela VB, Schwarz EM, Puzas JE, O'Keefe RJ, Rosier RN. Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res. 2000;60:6557–6562. [PubMed] [Google Scholar]
- 44.Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–2215. [PubMed] [Google Scholar]
- 45.D'Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 46.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 47.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han EK, Lim JT, Arber N, Rubin MA, Xing WQ, Weinstein IB. Cyclin D1 expression in human prostate carcinoma cell lines and primary tumors. Prostate. 1998;35:95–101. doi: 10.1002/(sici)1097-0045(19980501)35:2<95::aid-pros2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 50.Kallakury BV, Sheehan CE, Ambros RA, Fisher HA, Kaufman RP, Jr, Ross JS. The prognostic significance of p34cdc2 and cyclin D1 protein expression in prostate adenocarcinoma. Cancer. 1997;80:753–763. doi: 10.1002/(sici)1097-0142(19970815)80:4<753::aid-cncr15>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 51.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 52.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 53.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]