Abstract
The American Cancer Society has estimated that in 2003, there will be approximately 239,600 new cases of urologic cancer diagnosed and 54,600 urologic cancer-related deaths in the United States. To date, the majority of research and therapy design have focused on the microenvironment of the primary tumor site, as well as the microenvironment of the metastatic or secondary (target) tumor site. Little attention has been placed on the interactions of the circulating tumor cells and the microenvironment of the circulation (i.e., the third microenvironment). The purpose of this review is to present the methods for the detection and isolation of circulating tumor cells and to discuss the importance of circulating tumor cells in the biology and treatment of urologic cancers.
Keywords: Cancer, circulating tumor cells, prostate, renal, bladder
Introduction to Circulating Tumor Cells and Metastasis
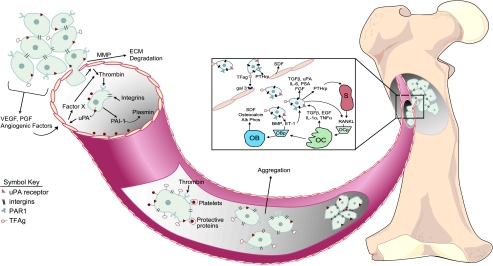
The progression of metastasis involves a complex series of chemical, molecular, and physical events, resulting in the ultimate deposition and proliferation of cancer cells at distant targeted sites. The current paradigm of metastasis describes the progression of cancer as the dissemination of neoplastic tumor cells from the primary tumor to distant target organs. The majority of cancer research over the past century have focused on two predominant environments: 1) the tissue of origin where the neoplasm develops, known as the primary microenvironment; and 2) the target tissue where cancer metastases occur, known as the secondary microenvironment. The environment of the circulatory system from the perspective of cancer metastasis has been underappreciated until recently. The importance of understanding how tumor cells derived from solid tissues survive in transit through the mechanical hardships of the circulation and avoid destruction by the immune system is an essential step in cancer metastases and, therefore, the circulatory system must be regarded as the third microenvironment (Figure 1).
Figure 1.
Diagrammatic representation of cancer metastasis. Initial localized tumorigenesis (first microenvironment) promotes angiogenesis by the release of a variety of angiogenic factors (e.g., vascular endothelial growth factor [VEGF], platelet-derived growth factor [PDGF]). Tumor cells secrete proteases matrix metalloproteinases (MMP) to degrade the extracellular matrices (ECM) and allow the migration of tumor cells into the circulation (third microenvironment). Several factors are released in response to tumor cell intravasation, including urokinase plasminogen activator (uPA), plasminogen activator inhibitor type-1 (PAI1), and thrombin, which promote tumor cell survival and metastasis. The aggregation of tumor cells and platelets during transit promotes survival and ultimately extravasation at the secondary tumor site (second microenvironment). The mechanisms of tumor cell extravasation into the secondary target site of bone are illustrated in the expanded box. Invading tumor cells have been shown to release factors that stimulate both osteoblastic and osteoclastic activity. OBp, osteoblastic progenitor cells; OCp, osteoclastic progenitor cells; OB, osteoblast; OC, osteoclast; S, stromal cell; TFAg, Thomas Friedrich antigen; gal 3, galectin 3 receptor; PTHrp, parathyroid hormone-related protein; SDF, stromal-derived factor.
The impetus to explore the microenvironment of the circulatory system for invading tumor cells actually stems back as far as the 19th century. In 1869, Ashworth [1] described the presence of tumor-like cells in the peripheral blood of a cancer patient at autopsy. Ashworth's observations incited interest in the process of metastasis and the mechanisms of tumor cell dissemination. Paget [2], in 1889, was the first to hypothesize a nonrandom pattern of neoplastic tumorigenesis and, as a result, developed the “seed and soil” theory of cancer metastases, which stated that distinct subpopulations of tumor cells were attracted to specific end-target organs resulting in metastasis. Later, the “seed and soil” hypothesis was redefined based on evidence suggesting that neoplastic tumors contained a high degree of heterogeneity with subpopulations of cells possessing different angiogenic, invasive, and metastatic properties. Furthermore, the metastatic process was shown to be selective for cells that survived in the circulatory system and migrated to distant end-target organs [3].
The predominant focus of cancer research has been to target the neoplastic development and tumorigenesis within the primary organ, as well as the affinity of tumor cells for the metastatic site. However, accumulating evidence suggested that localized tumors begin shedding neoplastic tumor cells into the circulation during early stages of the disease, with distinct cell populations having the potential to develop into metastatic disease [4–6]. Thus, there has been growing interest in identifying tumor cells in the circulation and using the detection of circulating tumor cells to monitor the progression of various types of urologic metastatic cancers, including prostate cancer, renal cancer, and bladder cancer [7–9].
These studies mentioned above outline the importance of understanding the biology of cancer cells that are released into the circulation and the survival mechanism inherent to this population of tumor cells. The number of patients with poor prognosis and clinical outcome from metastatic disease affirms the importance of understanding the progression of a tumor cell from the site of origin into the circulatory system. The ability to detect circulating tumor cells during early-stage disease may provide potential prognostic value and may aid in designing more appropriate therapeutic regimens. The lack of quality detection methods, however, limits the ability to consistently detect, quantify, and characterize these neoplastic cells. Thus, the focus of this review is to outline the concepts and methods of detection and isolation specifically in urologic cancers and to discuss the prevailing evidence regarding the limited techniques employed in studying circulating tumor cells.
Emerging Concepts of Tumor Cell Survival in the Third Microenvironment and Metastasis
Tumorigenesis and the process of metastasis are comprised of several intermediate steps [10–14]. The initial phase of cancer development is organ-confined, localized tumorigenesis. Transformation of cells to an oncogenic phenotype occurs in a defined organ (i.e., prostate, bladder, and so on) and leads to dysregulated localized cellular proliferation resulting in tumor development. Multiple factors, both endogenous and exogenous, induce changes in the phenotype and genotype of oncogenic cells, resulting in aberrant growth of a subpopulation of tumor cells with increased metastatic potential. This subpopulation of tumor cells induces angiogenesis at the site of the primary tumor supplementing the increasing metabolic needs of the growing tumor mass. Localized tumor cells secrete factors (e.g., MMP) that change and breakdown the extracellular matrix (ECM) and alter homotypic cell-to-cell adherence. Additionally, these tumor cells become increasingly motile and have enhanced invasive properties. Subsequently, tumor cells detach from the primary tumor site and are released into the local microvascular environment (intravasation).
Currently, it is unclear how circulating tumor cells survive in this third microenvironment, avoiding innate immune response, shear forces, and anchorage independence. Normally, cells that require anchorage to ECM for survival undergo spontaneous apoptosis when their adherence to the ECM is lost. However, tumor cells develop the ability to survive in an anchorage-independent environment through acquired phenotypic and genotypic alterations (e.g., expression of galectin-3 and edg-2) [15]. Several investigators have demonstrated protection against anoikis—the loss of anchorage-dependent cell death—in circulating tumor cells and have presented this as a possible means for tumor cell survival. Similarly, heterotypic and homotypic aggregation of neoplastic cells has been implicated as a possible mechanism for tumor cell survival. Studies have demonstrated that the ability of tumor cells to adhere in a homotypic fashion correlates with the metastatic potential and aggressiveness of a given tumor [16].
There is growing support for the release of tumor cells into the circulation as an early stage of metastasis that may precede the clinical identification of the primary tumor, and a significant correlation between the level of circulating tumor cells (either by reverse transcription-polymerase chain reaction [RT-PCR], immunomagnetic separation, or fluorescent-activated cell sorting [FACS]) and disease progression [19] has been demonstrated. Despite the fact that the impact of circulating tumor cells on the metastatic potential, disease progression, or potential tumor burden of a given cancer is not clearly understood, the presence of tumor cells in the peripheral blood and bone marrow is generally accepted and provides the opportunity to study specific tumor cell populations thought to be responsible for metastasis and increased tumor burden.
Methods for Detecting Circulating Tumor Cells in Urologic Cancers
Several methods for detecting circulating cancer cells in peripheral blood from patients with various urologic cancers have been developed over the past few decades. These methods are outline in Table 1 and discussed in greater detail below.
Table 1.
Identification of Circulating Tumor Cells in Peripheral Blood.
| Cancer | Method of Detection | Markers | Conclusions | Key References |
| Bladder | RT-PCR | UPII, CK20 CK19, EGFR, PSMA | EGFR and UPII expression may be useful tumor markers | 22,29,32,61 |
| IHC | CK8, CK18, CK19 | CTCs were detected only in patients with metastatic disease | 34 | |
| MACS | MACS improved sensitivity and specificity of CTC detection | 62,63 | ||
| FACS | E-cadherin, CD103 | Flow cytometry allowed isolation of bladder cancer cells by differential expression of E-cadherin | 64 | |
| Prostate | RT-PCR | PSA, PSMA, PSCA | PSA as promising marker for molecular staging in PCa | 49,65 |
| IHC | Cytokeratin, PSMA, DAPI, E-cadherin, p53, PSA | IHC and in situ hybridization allow characterization of isolated cancer cells | 66,67 | |
| MACS | Enrichment of circulating tumor cells for enhanced genetic analysis | 24,68 | ||
| FACS | 5E10, Muc1, CK | Flow cytometric isolation is efficient for CTC detection | 17,69 | |
| Renal | RT-PCR | PSMA, MN/CA9 | PSMA and MN/CA9 may be useful as biomarkers for renal cancer | 21,39,55 |
| IHC | N/A | |||
| MACS | Increased sensitivity of detection of cell number and tumor grade | 58 | ||
| FACS | N/A | |||
| Testicular | RT-PCR | α-Fetoprotein | Inconsistent findings using PCR to detect circulating tumor cells in patients with testicular cancer | 59,60 |
| IHC | N/A | |||
| MACS | N/A | |||
| FACS | N/A | |||
RT-PCR
Both qualitative and quantitative RT-PCR have been used as methods to calculate the levels of circulating tumor cells from whole blood in several types of urologic cancer. RT-PCR is a highly sensitive amplification method of specific cDNA sequences based on the design of oligonucleotide primer probes that recognize the target gene of interest. Studies have demonstrated the ability of PCR to detect one circulating tumor cell in 1 to 10 million normal cells using primer probes to several different target genes [18]. The advent of PCR technology has provided a specific and sensitive way to distinguish cells based on differential gene expression and genetic profiling. The primer probes used to detect circulating tumor cells from peripheral blood and bone marrow are designed based on two strategies: 1) amplification of tissue-specific markers (i.e., prostate-specific antigen, or PSA), and 2) general tumor cell characteristics (i.e., epithelial cell markers).
The limitations of using PCR to detect circulating tumor cells include: 1) amplification of nonspecific products, and 2) lack of consistent protocol and primer design between investigators necessary for interlaboratory comparisons [14,21]. This inherently increases the possibility of nonspecific amplification products due to protocol and primer design. Nonspecific products include amplification of products from an alternative cell type (e.g., PSA from a nonprostate cell), detection of pseudogenes due to inadequate primer design, and detection of products from nonmalignant cells present in the circulation. The lack of adequate tissue-specific or tumor-specific markers may result in PCR amplification of false-positives and may be a result of physical contamination from venipuncture as well as tumor cell heterogeneity (i.e., epithelial, albumin, and estrogen receptors).
The most characterized molecular marker for PCR-based detection of circulating tumor cells in urologic cancers as well as other solid tumors is the cytokeratin family. Cytokeratins are intermediate filament proteins found in epithelial cells and are commonly used to distinguish epithelial cells from a heterogenous cell population. However, cytokeratins are neither specific for tissue type, nor do they distinguish the origin of the epithelial cells. For example, cytokeratin 20 (CK20) is a cytokeratin originally thought to be specific to the gastrointestinal epithelium, although its expression has since been identified in granulocytes, bone marrow, and whole blood samples from healthy individuals [22,23]. However, in an alternative experiment, CK20 expression was not identified in venous peripheral blood isolated from healthy individuals implicating CK20 as a possible tumor detection marker [23]. These studies emphasize the conflicting results obtained from RT-PCR-based detection methods and highlight the need for standardized laboratory techniques and preparation protocols to insure proper and consistent interpretation of results. For this reason, gene-specific amplification by RT-PCR is now being performed in conjunction with alternative methods.
Immunohistochemistry
The use of immunohistochemistry for identification and detection of circulating tumor cells relies on antibody recognition of a specific tissue-type marker or cancer-specific marker. For example, studies in bladder cancer have used immunohistochemistry techniques with antibodies for several of the cytokeratins, CD45 (a tyrosine phosphatase), carcinoembryonic antigen (CEA), urokinase plasminogen activator receptor (uPA-R/CD87), and plasminogen activator inhibitor type-2 (PAI2). Similarly, de la Taille et al. [23] have used antibodies that recognize PSA and prostatic acid phosphatase (PAP) to detect circulating prostate cancer cells.
However, opposing data obtained from immunohistochemical techniques have resulted in conflicting opinions on the reliability and specificity of immunohistochemistry. The basis for these assays is their ability to recognize celltype -specific markers and is dependent on the specificity of manufactured antibodies and interpretational biases. The antibodies used for immunohistochemical-based detection methods must be both specific as well as sensitive to be able to distinguish between circulating tumor cells and normal cells. Immunohistochemical analysis of bladder cancer circulating tumor cell tests for molecular markers such as cytokeratin, nucleic acid staining, CEA, or uPA-R. Although immunohistochemistry can produce significant results, its value is contingent on the efficacy of specific antibodies and discernible expression of proteins. Due to the shortcomings of immunohistochemistry, including sensitivity, reproducibility, and limited quantitation, it often serves as merely a confirmatory experiment to alternative assays.
Magnetic Cell Sorting (MACS)
A relatively new technique has been developed using magnetic nanoparticles coupled to antibodies to specifically separate circulating tumor cells from whole blood. Immunomagnetic cell selection is an attractive method for studying the biology of circulating tumor cells. This method produces an enriched sample of circulating epithelial cells that can be subsequently used for DNA separation, mRNA purification, cell isolation and detection, development of immunoassays, capture of biomolecules, and protein purification [21,26].
The process of immunomagnetic cell selection is based on the recognition of epithelial cell-specific antibodies coupled to magnetic beads. The magnetic beads allow for isolation and separation of epithelial cells by serial magnetic incubations. Several companies have designed enrichment protocols based on immunomagnetic separation (e.g., www.immunicon.com, www.miltenyibiotec.com, www.stemcell.com). Briefly, an example of a separation and enrichment protocol from whole blood is as follows: epithelial cells are labeled using ferromagnetic nanoparticles coupled to an epithelial cell adhesion molecule antibody and placed in a magnetic chamber. Epithelial cell markers are useful in detecting cancer cells due to the extremely low levels of circulating epithelial cells in normal individuals. A leukocyte marker is used to distinguish leukocytes that sometimes separate with the epithelial cells. Cells are labeled with an anti-cytokeratin monoclonal antibody (mAb) and placed into a magnetic field chamber and visualized using a fluorescent microscope [27]. The cells labeled with the ferromagnetic nanoparticles are drawn to, and align themselves along, magnetic lines within the chamber [28]. A microscope scans the ferromagnetic lines and captures images of the cells using four different wavelengths, and the images are stored in a computer. The investigator reviews the cells and counts the number of cancer cells in the blood sample based on predefined criteria (Figure 2). To ensure unbiased identification of circulating tumor cells, multiple investigators may review these images.
Figure 2.
Illustration of cells isolated from peripheral blood by immunomagnetic cell selection and analyzed with a fluorescent microscope. Cells that stain positive for 4′,6-diamidino-2-phenylindole (DAPI; nuclear stain), positive for CK19 (epithelial cell), and negative for CD45 (lymphocyte) are identified as a circulating tumor cells. Cells that stain positive for CD45 are identified as lymphocytes.
Once the circulating tumor cells have been isolated from blood, analysis by flow cytometry using antibodies specific for subpopulations of cells (i.e., stem cells) or specific cellular morphologic and functional properties can provide further understanding of the biology of tumor cells. Similarly, these same techniques can be employed under sterile conditions, allowing the purification and enrichment of a tumor cell population that can be placed as xenografts into immunedeficient mice, or grown in culture for further molecular and biologic investigations.
There are distinct advantages to using the immunomagnetic cell selection method for enriching circulating tumor cell populations. One advantage is the visualization and quantification of circulating tumor cells compared with other methods including PCR and immunohistochemistry. A second advantage is that the immunomagnetic selection detects only intact cells, requiring a nucleus and a stained membrane. PCR detects living cells, dead cells, and free DNA, resulting in potential false-positives. Similarly, the limitations of the immunomagnetic enrichment system include cost, time-consuming process, variability due to nonstandardized methods and reagents, and inherent circulating tumor cell variability between patients.
Several studies have started combining the previously mentioned methods of detection (i.e., RT-PCR, immunohistochemistry, and flow cytometry) in an effort to more efficiently and specifically identify circulating tumor cells. Hu et al. [29] combined magnetic separation with immunocytochemistry and flow cytometry to enrich and detect circulating tumor cells from breast cancer patients. They reported an increased ability to isolate, detect, and identify circulating tumor cells from whole blood by combining magnetic separation and immunocytochemistry. They also reported a significant correlation between circulating breast cancer cells and clinical disease state. Perhaps the best method of detection employs a combination of enrichment techniques with specific tissue-type or cell-type marker detection.
Detection of Circulating Tumor Cells in Various Types of Urologic Cancer
Studies have reported attempts to isolate, identify, and classify circulating tumor cells from patients diagnosed with various urologic cancers. The detection method of circulating tumor cells has mainly been based on gene expression and phenotypic characteristics common to neoplastic epithelial cells.
Bladder Cancer
In the United States, approximately 38,000 men and 15,000 women are diagnosed with bladder cancer each year [30]. Bladder tumors are grouped into several types based on morphologic criteria and biopsy pathology. The three main types of cancers that affect the bladder are: urothelial carcinoma (also known as transitional cell carcinoma, or TCC), squamous cell carcinoma, and adenocarcinoma. TCC is responsible for approximately 90% of bladder cancers, whereas squamous cell carcinoma and adenocarcinoma account for the remaining 10%. The cancer origin is predominantly derived from the lining of the bladder and is often referred to as a superficial tumor. However, the cancer may turn invasive and invade the muscle wall, resulting in the progression of metastatic disease to nearby organs.
A number of investigators have used RT-PCR techniques to detect and identify micrometastases of bladder cancer from peripheral blood samples [31–33]. Gazzaniga et al. [34] reported the use of endothelial growth factor receptor (EGFR) expression compared with cytokeratin 19 (CK19) and CK20 by RT-PCR and Southern blot analysis from blood collected from bladder cancer patients. This study demonstrated that identification of circulating tumor cells from peripheral blood by cytokeratin expression is complicated by false-positives from normal healthy patients. Additionally, they demonstrated the importance of alternative, supportive biomarkers if molecular staging were to be pursued as a prognostic indicator of disease. This group was able to show no evidence of uroplakin II (UPII) or EGFR in samples from healthy patients, yet 74% of patients with confirmed metastatic bladder cancer were positive for EGFR expression by RT-PCR.
Further studies have used immunohistochemical techniques to detect circulating tumor cells in peripheral blood from bladder cancer patients [35]. The use of antibodies directed toward CK8, CK18, and CK19 was aimed to identify epithelial cells after isolation of mononuclear cells by Ficoll gradients and fixation. Desgrandchamps et al. [35] were able to identify epithelial cells in blood samples from 32 patients with TCC of the bladder; however, they could not distinguish between stage or grade of cancer using immunohistochemistry of circulating epithelial cells. These data suggest that although the use of molecular targets initially thought to be specific for circulating tumor cells (i.e., cytokeratins) is useful in identifying metastasis, further work is needed to develop gene expression as a means of prognosis and diagnosis in patients.
Prostate Cancer
Prostate carcinoma is the predominant cancer diagnosed in American men and is the second leading cause of cancer-related deaths in men. The American Cancer Society estimates that approximately 220,900 men will be diagnosed with clinically defined prostatic carcinoma and roughly 29,000 men will die from advanced metastatic prostate cancer in 2003 [30]. Biochemical relapse (a rise in PSA levels) after definitive treatment occurs in approximately 40% of prostate cancer patients and is indicative of metastatic disease.
PSA monitoring and radiographic imaging analysis have been the standards for detecting prostate cancer recurrence and progression. However, the identification of recurrence at earlier time points would be extremely advantageous for immediate therapeutic intervention [36]. Prostate cancer has been an active area of research for circulating tumor cells due to several known prostate-specific genes (i.e., PSA and prostate-specific membrane antigen [PSMA]). There have been several reports describing methods and strategies used to identify and characterize circulating prostate cancer cells and to correlate these findings with disease progression [14,37–39]. Many of these studies have relied on the sensitivity of PCR-targeted amplification of PSA and PSMA [40–45].
A variety of studies have reported increased dissemination and hematogenous spread of prostate cancer cells following prostatectomy by RT-PCR analysis [46,47]. Similarly, the ability to detect changes in circulating tumor cells between patients with localized disease and metastatic disease has been demonstrated, as well as a correlation between PSA levels and circulating tumor cells [14,48–51]. Gelmini et al. [51] reported the detection of circulating prostate cells in peripheral blood from patients with metastatic prostate cancer. This study amplified the PSA gene using quantitative RT-PCR from blood samples taken from prostate cancer patients. They reported a reliable detection of PSA in prostate cancer patients as well as a reduction in PSA detection after definitive treatment. Additionally, no detection of PSA was reported in healthy controls.
Alternative methods have been used to detect circulating prostate cancer cells including flow cytometry, immunohistochemistry, and immunomagnetic separation [52–54]. Flow cytometry was used to measure circulating PSA-positive cells obtained from 40 diagnosed, untreated prostate cancer patients and demonstrated a significant correlation with metastatic disease [52].
Renal Cancer
The incidence of renal cell carcinoma is estimated to be 31,900 new cases, resulting in approximately 11,900 renal cancer-related deaths in 2003, placing renal carcinoma in the top 10 leading cancers [30]. Renal carcinoma typically has a poor prognosis due to the fact that early detection is difficult. Currently, there are no specific renal tumor markers (e.g., PSA for prostate cancer) that are beneficial for diagnosis or monitoring. Few studies have focused on detecting and isolating circulating tumor cells from renal carcinoma patients [55–58]. Ashida et al. [59] reported the detection of mutations in the von Hindel-Lindau tumor-suppressor gene in patients with renal cell carcinoma by nested RT-PCR. Additionally, the expression of MN/CA9 (a carbonic anhydrase isoenzyme) has been the target of RT-PCR amplification in peripheral blood samples taken from patients with renal cell carcinoma [58]. However, although PCR offers a highly sensitive method to detect genes, the specificity of the amplified target genes is a limiting factor for its diagnostic or prognostic value. De la Taille et al. [41] described the use of nested RT-PCR to evaluate PSMA expression in peripheral blood from renal cancer patients. PSMA has been thought to be a prostate cell-specific gene and, therefore, amplification of this gene in blood from renal cancer patients brings into question the specificity and reliability of this assay.
Due to the relatively limited number of studies aimed at detecting and characterizing circulating renal carcinoma cells from peripheral blood samples, implementing the detection of circulating tumor cells as a prognostic indicator remains premature. Several techniques and combinations of techniques are currently being explored. Bilkenroth et al. [60] have demonstrated an increased sensitivity of renal carcinoma detection from peripheral blood by using MACS followed by immunohistochemical labeling of cytokeratins to identify tumor cells. The major advantage of this study was the ability 2to isolate a highly enriched population of circulating epithelial (tumor) cells from a small volume of peripheral blood drawn from patients (i.e., 8 ml of blood). Further studies are necessary to specifically identify the origin of the circulating tumor cells and to, perhaps, isolate and identify the circulating cells necessary for metastasis.
Testicular Cancer
Testicular cancer is one of the leading cancers among young men. The incidence of testicular cancer is estimated to be 7600 new cases, resulting in approximately 400 renal cancer-related deaths in 2003 [30]. To date, there has been limited efforts aimed at detecting and isolating circulating tumor cells from patients with testicular cancer. The most recent studies used nested PCR techniques to detect circulating malignant cells from peripheral blood isolated from patients with germ cell testicular tumors of various stages and treatment regimens [65,66]. These studies demonstrated the ability to detect increased levels of α-fetoprotein (AFP) and β-human chorionic gonadotropin (β-hCG) in peripheral blood from patients with advanced testicular cancer. Although these studies have demonstrated the ability to detect the presence of circulating tumor cells in patients with testicular cancer by PCR, further studies are required to fully investigate this line of inquiry for possible prognostic value. Additionally, the alternative methodologies mentioned above should be explored and used for detecting and isolating circulating tumor cells from patients with testicular cancer.
Conclusions
The initial detection methods of circulating tumor cells have been based on histopathologic techniques and have proven to be time-consuming and subject to reviewer interpretation. The development of PCR led to increased sensitivity and specificity of detection, and removed the subjective influence inherent in earlier methods of detecting circulating tumor cells. However, several concerns regarding the specificity of PCR in detecting circulating tumor cells have been raised due to the inconsistency of results and amplification of false products. Recently, a technique has been developed using magnetic nanoparticles coupled to epithelial cell-specific antibodies that recognize and isolate circulating tumor cells from whole blood, allowing for enrichment of tumor cell sample by a noninvasive clinical procedure. Further investigation is required to fully develop a series of methodologies and protocols aimed at detecting and isolating circulating tumor cells from peripheral blood. The combination of the methods discussed above may offer an effective, powerful means to understanding the mechanism of metastasis and may lead to new ways of monitoring disease progression and clinical outcome.
The importance of understanding the mechanisms inherent to circulating metastatic tumor cells that allow the cells to survive the microenvironment of the circulation is an exciting area of ongoing research. Identifying these mechanisms is the next wave of cancer research with great potential for novel therapeutic targeting of tumor cell survival in the circulation.
Abbreviations
- RT-PCR
reverse transcription-polymerase chain reaction
- FACS
fluorescent-activated cell sorting
- cDNA
complimentary deoxyribonucleic acid
- PSA
prostatespecific antigen
- CD45
a tyrosine phosphatase
- CK8
cytokeratin 8
- CK18
cytokeratin 18
- CK19
cytokeratin 19
- CK20
cytokeratin 20
- uPA-R/CD87
urokinase plasminogen activator receptor
- PAI2
plasminogen activator inhibitor type-2
- PAP
prostatic acid phosphatase
- CEA
carcinoembryonic antigen
- mRNA
messenger ribonucleic acid
- TCC
transitional cell carcinoma
- EGFR
endothelial growth factor receptor
- UPII
uroplakin II
- PSMA
prostatespecific membrane antigen
- MN/CA9
a carbonic anhydrase isoenzyme
- MACS
magnetic cell sorting
- IHC
immunohistochemistry
- OBp
osteoblastic progenitor cells
- OCp
osteoclastic progenitor cells
- OB
osteoblast
- OC
osteoclast
- S
stromal cell
- TFAg
Thomas Friedrich antigen
- gal 3
galectin-3 receptor
- PTHrp
parathyroid hormone-related protein
- SDF
stromal-derived factor
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146. [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571. [PubMed] [Google Scholar]
- 3.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 4.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–281. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 5.McKiernan JM, Buttyan R, Bander NH, de la Taille A, Stifelman MD, Emanuel ER, Bagiella E, Rubin MA, Katz AE, Olsson CA. The detection of renal carcinoma cells in the peripheral blood with an enhanced reverse transcriptase-polymerase chain reaction assay for MN/CA9. Cancer. 1999;86:492–497. doi: 10.1002/(sici)1097-0142(19990801)86:3<492::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol. 2003;169:1975–1982. doi: 10.1097/01.ju.0000067461.30468.6d. [DOI] [PubMed] [Google Scholar]
- 7.Butler T, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–516. [PubMed] [Google Scholar]
- 8.Glaves D, Mayhew E. Selective therapy of metastasis: I. Quantitation of tumorigenic circulating and covert cancer cells disseminated from metastatic and nonmetastatic tumors. Cancer Drug Deliv. 1984;1:293–302. doi: 10.1089/cdd.1984.1.293. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 10.Chung LW. Prostate carcinoma bone-stroma interaction and its biologic and therapeutic implications. Cancer. 2003;97:772–778. doi: 10.1002/cncr.11140. [DOI] [PubMed] [Google Scholar]
- 11.Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, McCauley RS, Keller ET, Pienta KJ. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97:739–747. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- 12.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998;17:331–336. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 13.Onn A, Fidler IJ. Metastatic potential of human neoplasms. In Vivo. 2002;16:423–429. [PubMed] [Google Scholar]
- 14.Wood DP, Banerjee M., Jr Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol. 1997;15:3451–3457. doi: 10.1200/JCO.1997.15.12.3451. [DOI] [PubMed] [Google Scholar]
- 15.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 16.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–2511. [PubMed] [Google Scholar]
- 17.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghossein RA, Osman I, Bhattacharya S, Ferrara J, Fazzari M, Cordon-Cardo C, Scher HI. Detection of prostatic specific membrane antigen messenger RNA using immunobead reverse transcriptase polymerase chain reaction. Diagn Mol Pathol. 1999;8:59–65. doi: 10.1097/00019606-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ellis WJ, Vessella RL, Corey E, Arfman EW, Oswin MM, Melchior S, Lange PH. The value of a reverse transcriptase polymerase chain reaction assay in preoperative staging and follow-up of patients with prostate cancer. J Urol. 1998;159:1134–1138. [PubMed] [Google Scholar]
- 20.Ghossein RA, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res. 1999;5:1950–1960. [PubMed] [Google Scholar]
- 21.Bustin SA, Gyselman VG, Siddiqi S, Dorudi S. Cytokeratin 20 is not a tissue-specific marker for the detection of malignant epithelial cells in the blood of colorectal cancer patients. Int J Surg Invest. 2000;2:49–57. [PubMed] [Google Scholar]
- 22.Retz M, Lehmann J, Roder C, Weichert-Jacobsen K, Loch T, Romahn E, Luhl C, Kalthoff H, Stockle M. Cytokeratin-20 reverse-transcriptase polymerase chain reaction as a new tool for the detection of circulating tumor cells in peripheral blood and bone marrow of bladder cancer patients. Eur Urol. 2001;39:507–515. doi: 10.1159/000052496. (discussion, 516–507). [DOI] [PubMed] [Google Scholar]
- 23.de la Taille A, Colombel M, Amsellem S, Muscatelli B, Radvanyi F, Mazeman E, Abbou CC, Chopin D. The PSA gene: value in detection of circulating prostate cancer cells. Prog Urol. 1997;7:930–936. [PubMed] [Google Scholar]
- 24.Moreno JG, O'Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, Terstappen LW. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology. 2001;58:386–392. doi: 10.1016/s0090-4295(01)01191-8. [DOI] [PubMed] [Google Scholar]
- 25.Martin VM, Siewert C, Scharl A, Harms T, Heinze R, Ohl S, Radbruch A, Miltenyi S, Schmitz J. Immunomagnetic enrichment of disseminated epithelial tumor cells from peripheral blood by MACS. Exp Hematol. 1998;26:252–264. [PubMed] [Google Scholar]
- 26.Tibbe AG, de Grooth BG, Greve J, Dolan GJ, Rao C, Terstappen LW. Magnetic field design for selecting and aligning immunomagnetic labeled cells. Cytometry. 2002;47:163–172. doi: 10.1002/cyto.10060. [DOI] [PubMed] [Google Scholar]
- 27.Hu XC, Wang Y, Shi DR, Loo TY, Chow LW. Immunomagnetic tumor cell enrichment is promising in detecting circulating breast cancer cells. Oncology. 2003;64:160–165. doi: 10.1159/000067776. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Lu JJ, Kakehi Y, Takahashi T, Wu XX, Yuasa T, Yoshiki T, Okada Y, Terachi T, Ogawa O. Detection of circulating cancer cells by reverse transcription-polymerase chain reaction for uroplakin II in peripheral blood of patients with urothelial cancer. Clin Cancer Res. 2000;6:3166–3171. [PubMed] [Google Scholar]
- 30.Qhiu L, Cong X, Ja L. The expression and clinical significance of keratin 19, 20 mRNA in different tumor cell lines and tumor tissues. Zhonghua Zhong Liu Za Zhi. 2000;22:32–35. [PubMed] [Google Scholar]
- 31.Soria JC, Morat L, Durdux C, Housset M, Cortez A, Blaise R, Sabatier L. The molecular detection of circulating tumor cells in bladder cancer using telomerase activity. J Urol. 2002;167:352–356. [PubMed] [Google Scholar]
- 32.Gazzaniga P, Gradilone A, Frati L, Agliano AM. Epidermal growth factor receptor mRNA expression in peripheral blood of bladder cancer patients: a potential marker to detect treatment failure. Clin Cancer Res. 2001;7:4288–4289. [PubMed] [Google Scholar]
- 33.Desgrandchamps F, Teren M, Dal Cortivo L, Marolleau JP, Bertheau P, Villette JM, Cortesse A, Teillac P, Le Duc A, Hamdy FC. The effects of transurethral resection and cystoprostatectomy on dissemination of epithelial cells in the circulation of patients with bladder cancer. Br J Cancer. 1999;81:832–834. doi: 10.1038/sj.bjc.6690771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tombal B, Van Cangh PJ, Loric S, Gala JL. Prognostic value of circulating prostate cells in patients with a rising PSA after radical prostatectomy. Prostate. 2003 Aug 1;56(3):163–170. doi: 10.1002/pros.10237. [DOI] [PubMed] [Google Scholar]
- 35.Ts'o PO, Pannek J, Wang ZP, Lesko SA, Bova GS, Partin AW. Detection of intact prostate cancer cells in the blood of men with prostate cancer. Urology. 1997;49:881–885. doi: 10.1016/s0090-4295(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 36.Berteau P, Dumas F, Gala JL, Eschwege P, Lacour B, Philippe M, Loric S. Molecular detection of circulating prostate cells in cancer: II. Comparison of prostate epithelial cells isolation procedures. Clin Chem. 1998;44:1750–1753. [PubMed] [Google Scholar]
- 37.Grasso YZ, Gupta MK, Levin HS, Zippe CD, Klein EA. Combined nested RT-PCR assay for prostate-specific antigen and prostate-specific membrane antigen in prostate cancer patients: correlation with pathological stage. Cancer Res. 1998;58:1456–1459. [PubMed] [Google Scholar]
- 38.Corey E, Arfman EW, Oswin MM, Melchior SW, Tindall DJ, Young CY, Ellis WJ, Vessella RL. Detection of circulating prostate cells by reverse transcriptase-polymerase chain reaction of human glandular kallikrein (hK2) and prostate-specific antigen (PSA) messages. Urology. 1997;50:184–188. doi: 10.1016/S0090-4295(97)00262-8. [DOI] [PubMed] [Google Scholar]
- 39.de la Taille A, Cao Y, Sawczuk IS, Nozemu T, d'Agati V, McKiernan JM, Bagiella E, Buttyan R, Burchardt M, Olsson CA. Detection of prostate-specific membrane antigen expressing cells in blood obtained from renal cancer patients: a potential biomarker of vascular invasion. Cancer Detect Prev. 2000;24:579–588. [PubMed] [Google Scholar]
- 40.Deguchi T, Ehara H, Takahashi Y. Detection of PSA mRNA and PSMA mRNA by RT-PCR. Nippon Rinsho. 2002;60(Suppl 11):151–155. [PubMed] [Google Scholar]
- 41.Halabi S, Small EJ, Hayes DF, Vogelzang NJ, Kantoff PW. Prognostic significance of reverse transcriptase polymerase chain reaction for prostate-specific antigen in metastatic prostate cancer: a nested study within CALGB 9583. J Clin Oncol. 2003;21:490–495. doi: 10.1200/JCO.2003.04.104. [DOI] [PubMed] [Google Scholar]
- 42.Hara N, Kasahara T, Kawasaki T, Bilim V, Tomita Y, Obara K, Takahashi K. Frequency of PSA-mRNA-bearing cells in the peripheral blood of patients after prostate biopsy. Br J Cancer. 2001;85:557–562. doi: 10.1054/bjoc.2001.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millon R, Jacqmin D, Muller D, Guillot J, Eber M, Abecassis J. Detection of prostate-specific antigen- or prostate-specific membrane antigen-positive circulating cells in prostatic cancer patients: clinical implications. Eur Urol. 1999;36:278–285. doi: 10.1159/000020005. [DOI] [PubMed] [Google Scholar]
- 44.Badwe RA, Vaidya JS. Haematogenous dissemination of prostate epithelial cells during surgery. Lancet. 1996;347:325–326. [PubMed] [Google Scholar]
- 45.Hamdy FC, Neal DE. Haematogenous dissemination of prostate epithelial cells during surgery. Lancet. 1996;347:325. [PubMed] [Google Scholar]
- 46.Ghossein RA, Scher HI, Gerald WL, Kelly WK, Curley T, Amsterdam A, Zhang ZF, Rosai J. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J Clin Oncol. 1995;13:1195–1200. doi: 10.1200/JCO.1995.13.5.1195. [DOI] [PubMed] [Google Scholar]
- 47.Jaakkola S, Vornanen T, Leinonen J, Rannikko S, Stenman UH. Detection of prostatic cells in peripheral blood: correlation with serum concentrations of prostate-specific antigen. Clin Chem. 1995;41:182–186. [PubMed] [Google Scholar]
- 48.Seiden MV, Kantoff PW, Krithivas K, Propert K, Bryant M, Haltom E, Gaynes L, Kaplan I, Bubley G, DeWolf W. Detection of circulating tumor cells in men with localized prostate cancer. J Clin Oncol. 1994;12:2634–2639. doi: 10.1200/JCO.1994.12.12.2634. [DOI] [PubMed] [Google Scholar]
- 49.Gelmini S, Tricarico C, Vona G, Livi L, Melina AD, Serni S, Cellai E, Magrini S, Villari D, Carini M. Real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) for the measurement of prostate-specific antigen mRNA in the peripheral blood of patients with prostate carcinoma using the Taqman detection system. Clin Chem Lab Med. 2001;39:385–391. doi: 10.1515/CCLM.2001.061. [DOI] [PubMed] [Google Scholar]
- 50.Ablin RJ. Re: Circulating prostate-specific antigen-positive cells correlate with metastatic prostate cancer. Br J Urol. 1993;71:761–762. [PubMed] [Google Scholar]
- 51.Kiessling A, Schmitz M, Stevanovic S, Weigle B, Holig K, Fussel M, Fussel S, Meye A, Wirth MP, Rieber EP. Prostate stem cell antigen: identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int J Cancer. 2002;102:390–397. doi: 10.1002/ijc.10713. [DOI] [PubMed] [Google Scholar]
- 52.Makarovskiy AN, Ackerley W, III, Wojcik L, Halpert GK, Stein BS, Carreiro MP, Hixson DC. Application of immunomagnetic beads in combination with RT-PCR for the detection of circulating prostate cancer cells. J Clin Lab Anal. 1997;11:346–350. doi: 10.1002/(SICI)1098-2825(1997)11:6<346::AID-JCLA7>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hioki T, Sugimura Y. Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 in patients with renal cell carcinoma. Hinyokika Kiyo. 1999;45:577–581. [PubMed] [Google Scholar]
- 54.Pontes JE, Pescatori E, Connelly R, Hashimura T, Tubbs R. Circulating cancer cells in renal-cell carcinoma. Prog Clin Biol Res. 1990;348:1–12. [PubMed] [Google Scholar]
- 55.Uemura H. Molecular detection of circulating cancer cells in patients with renal cell carcinoma. Hinyokika Kiyo. 1999;45:571–575. [PubMed] [Google Scholar]
- 56.Uemura H, Hirao Y. Clinical significance for detection of circulating cancer cells in renal cell carcinoma. Gan To Kagaku Ryoho. 2002;29:1712–1718. [PubMed] [Google Scholar]
- 57.Ashida S, Okuda H, Chikazawa M, Tanimura M, Sugita O, Yamamoto Y, Nakamura S, Moriyama M, Shuin T. Detection of circulating cancer cells with von Hippel-Lindau gene mutation in peripheral blood of patients with renal cell carcinoma. Clin Cancer Res. 2000;6:3817–3822. [PubMed] [Google Scholar]
- 58.Bilkenroth U, Taubert H, Riemann D, Rebmann U, Heynemann H, Meye A. Detection and enrichment of disseminated renal carcinoma cells from peripheral blood by immunomagnetic cell separation. Int J Cancer. 2001;92:577–582. doi: 10.1002/ijc.1217. [DOI] [PubMed] [Google Scholar]
- 59.Hautkappe AL, Lu M, Mueller H, Bex A, Harstrick A, Roggendorf M, Ruebben H. Detection of germ-cell tumor cells in the peripheral blood by nested reverse transcription-polymerase chain reaction for alpha-fetoprotein-messenger RNA and beta human chorionic gonadotropin-messenger. RNA Cancer Res. 2000;60:3170–3174. [PubMed] [Google Scholar]
- 60.Yuasa T, Yoshiki T, Tanaka T, Isono T, Okada Y. Detection of circulating testicular cancer cells in peripheral blood. Cancer Lett. 1999;143:57–62. doi: 10.1016/s0304-3835(99)00194-9. [DOI] [PubMed] [Google Scholar]
- 61.Li SM, Zhang ZT, Chan S, McLenan O, Dixon C, Taneja S, Lepor H, Sun TT, Wu XR. Detection of circulating uroplakin-positive cells in patients with transitional cell carcinoma of the bladder. J Urol. 1999;162:931–935. doi: 10.1097/00005392-199909010-00093. [DOI] [PubMed] [Google Scholar]
- 62.Meye A, Bilkenroth U, Schmidt U, Fussel S, Robel K, Melchior AM, Blumke K, Pinkert D, Bartel F, Linne C. Isolation and enrichment of urologic tumor cells in blood samples by a semi-automated CD45 depletion autoMACS protocol. Int J Oncol. 2002;21:521–530. [PubMed] [Google Scholar]
- 63.Zigeuner RE, Riesenberg R, Pohla H, Hofstetter A, Oberneder R. Immunomagnetic cell enrichment detects more disseminated cancer cells than immunocytochemistry. In vitro J Urol. 2000;164:1834–1837. [PubMed] [Google Scholar]
- 64.Cresswell J, Wong WK, Henry MJ, Robertson H, Neal DE, Kirby JA. Adhesion of lymphocytes to bladder cancer cells: the role of the alpha(E)beta(7) integrin. Cancer Immunol Immunother. 2002;51:483–491. doi: 10.1007/s00262-002-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hara N, Kasahara T, Kawasaki T, Bilim V, Obara K, Takahashi K, Tomita Y. Reverse transcription-polymerase chain reaction detection of prostate-specific antigen, prostate-specific membrane antigen, and prostate stem cell antigen in one milliliter of peripheral blood: value for the staging of prostate cancer. Clin Cancer Res. 2002;8:1794–1799. [PubMed] [Google Scholar]
- 66.Wang ZP, Eisenberger MA, Carducci MA, Partin AW, Scher HI, Ts'o PO. Identification and characterization of circulating prostate carcinoma cells. Cancer. 2000;88:2787–2795. doi: 10.1002/1097-0142(20000615)88:12<2787::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Mejean A, Vona G, Nalpas B, Damotte D, Brousse N, Chretien Y, Dufour B, Lacour B, Brechot C, Paterlini-Brechot P. Detection of circulating prostate derived cells in patients with prostate adenocarcinoma is an independent risk factor for tumor recurrence. J Urol. 2000;163:2022–2029. [PubMed] [Google Scholar]
- 68.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–281. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 69.Rokhlin OW, Weiner GJ, Cohen MB. 5E10: a prostate-specific surface-reactive monoclonal antibody. Cancer Lett. 1998;131:129–136. doi: 10.1016/s0304-3835(98)00150-5. [DOI] [PubMed] [Google Scholar]