Abstract
We have isolated a selectively deglycosylated form of vitamin D binding protein (DBP-maf) generated from systemically available DBP by a human pancreatic cancer cell line. DBP-maf is antiproliferative for endothelial cells and antiangiogenic in the chorioallantoic membrane assay. DBP-maf administered daily was able to potently inhibit the growth of human pancreatic cancer in immune compromised mice (T/C=0.09). At higher doses, DBP-maf caused tumor regression. Histological examination revealed that treated tumors had a higher number of infiltrating macrophages as well as reduced microvessel density, and increased levels of apoptosis relative to untreated tumors. Taken together, these data suggest that DBP-maf is an antiangiogenic molecule that can act directly on endothelium as well as stimulate macrophages to attack both the endothelial and tumor cell compartment of a growing malignancy.
Keywords: angiogenesis, carcinoma, pancreas, vitamin D binding protein, macrophages
Introduction
There is considerable evidence that tumor growth is an angiogenesis-dependent event [1]. Certain tumor-generated molecules can inhibit tumor growth and induce dormancy of metastases by inhibiting tumor angiogenesis, (the growth of new blood vessels into the tumor mass). These molecules function by inhibiting the migration and proliferation of endothelial cells. Recently discovered examples of such angiogenesis inhibitors include angiostatin [2], endostatin [3], and antiangiogenic ATIII [4]. Antiangiogenic therapies hold great potential to prevent the growth of primary and metastatic disease, as these therapies target the genomically stable endothelial compartment of a tumor. Many conventional anticancer therapies lose efficacy over time, because the genomically unstable population of tumor cells that they target can acquire drug resistance.
At least three antiangiogenic molecules have been previously isolated from the conditioned media of tumors where, clinically, metastases grow rapidly after removal of the primary disease. Accordingly, we screened several human pancreatic tumors for the ability to inhibit the growth of a secondary implant of tumor cells, using a well-established in vivo model [5]. One such tumor, BxPC-3, was able to inhibit the growth of secondary tumor implants by as much as 80%. Using conventional chromatography techniques, we isolated an antiangiogenic molecule from the conditioned media of BxPC-3 cells. Sequence analysis determined that this protein was the bovine form of vitamin D binding protein (DBP).
DBP is a multidomain protein that binds vitamin D metabolite at its amino-terminal domain and actin at its carboxy-terminal domain [6]. Furthermore, the carboxy-terminal domain contains an O-linked glycosylation site on a threonine residue in human DBP [7]. This site is occupied with a mucin-type trisaccharide, consisting of N-acetylgalactosamine with a dibranched galactose and sialic acid. Sequential hydrolysis of the terminal sialic acid and galactose results in a molecule with core N-acetylgalactosamine attached to the threonine residue [7]. Such selective deglycosylation is shown to occur naturally as part of the inflammatory response. Membrane-bound β-galactosidase and sialidase of activated B- and T-lymphocytes can hydrolyse the terminal galactose and sialic acid [7]. The resultant molecule is a potent activator of macrophages, and is termed DBP-maf (macrophages activating factor).
Previous data have shown that DBP-maf, generated specifically by selective in vitro deglycosylation, has a role to play in the treatment of an Ehrlich ascites tumor in a mouse model [8,9]. Further administration of DBP-maf as an adjuvant immunotherapy to photodynamic therapy of cancer [10] had a synergistic effect on tumor cures using a squamous cell carcinoma model in mice. In both tumor models, it was hypothesized that DBP-maf elicited its effect by activating macrophages, which then directly attacked the tumor cells. We present evidence that BxPC3 pancreatic cancer cells can generate DBP-maf from DBP indicating that DBP-maf may be generated in vivo. We further present evidence suggesting that DBP-maf is anti-tumorigenic in part through an antiangiogenic mechanism. Further, DBP-maf, as well as being directly antiangiogenic activates macrophages which can then infiltrate solid tumors. This proposed amplification of the antiangiogenic effect of DBP-maf may explain the currently observed potency of this molecule as an anti-tumorigenic therapy.
Materials and Methods
Cell Culture and Conditioned Media Collection
Human pancreatic cancer cell lines BxPC-3, SU88.86, and ASPC-1 (American Type Culture Collection, Rockville, MD) were incubated in RPMI 1640 (Gibco, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS) (Gibco), 100 µg/ml penicillin G, and 100 µg/ml streptomycin (Pharmacia, Sweden). Cells were maintained in T-75 tissue culture flasks (Falcon, Bedford, MA) and grown in 5% CO2/95% at 37°C in an humidified incubator. Conditioned media were produced by adding RPMI 1640/5% FCS (100 ml) to near confluent BxPC-3 cells in 900 cm2 roller bottles (Corning, Acton, MA), and incubating for 72 hours. Conditioned media were collected, filtered (0.45 µm), and stored at 4°C.
Bovine Capillary Endothelial (BCE) Assays
The anti-endothelial activity of protein fractions obtained during purification procedures was determined using a proliferation assay. BCE cells were isolated from the cortices of freshly harvested bovine calf adrenal glands, cultured, and used from passage 9–14. BCE cells were maintained in DMEM with 10% heat-inactivated bovine calf serum (BCS), antibiotics, and 3 ng/ml recombinant human basic fibroblast growth factor (bFGF) (Scios Nova, CA). Cells were washed with PBS and dispersed in a 0.05% solution of trypsin. A cell suspension was made with DMEM/10% BCS/1% antibiotics and the concentration adjusted to 32,000 cells/ml. Cells were plated onto gelatinized 24-well culture plates (0.5 ml/well) and were incubated at 37°C in 10% CO2 for 24 hours. The media were replaced with 0.5 ml of DMEM/5% BCS/1% antibiotics, and the test sample applied. After 20 min of incubation, bFGF was added to each well (1 ng//ml). After 72 hours, cells were dispersed in trypsin, resuspended in Isoton II (Fisher Scientific, PA) and counted by Coulter counter.
Chick Chorioallantoic Membrane (CAM) Assay
Chick embryos were prepared as reported [11]. The sample was dissolved in 0.45% methylcellulose (10 µl) and micropipetted onto the outer third of the chorioallantoic membrane of day 6 embryos. This procedure was accomplished by using a 3x–6x stereoscope. Antiangiogenic activity was measured by observing the avascular zone around the sample between 48 and 96 hours.
Purification of Human and Bovine DBP
Human and bovine DBP were purified from respective sera using 25-hydroxyvitamin D3 affinity chromatography as described [12] with suitable modifications. Human or bovine sera was diluted with equal volume of column buffer (50 mM Tris HCl, pH 8.3, 150 mM NaCl, 1.5 mM EDTA) and applied to the 25-hydroxyvitamin D3 affinity column at 4°C. The column was washed with the column buffer (20 times the bed volume) and bound proteins eluted with 1 M acetate buffer, pH 5.0. The salts were dialyzed away and proteins were further fractionated on a hydroxyapatite column equilibrated with 10 mM potassium phosphate buffer, pH 7.0. The column was washed with excess equlibration buffer and bound DBP eluted with 75 mM potassium phosphate buffer, pH 7.0.
Purification of DBP-maf from BxPC-3 Conditioned Media
BxPC-3 conditioned media were applied to a 25-hydroxyvitamin D3 affinity column (equilibrated with 50 mM Tris HCl, pH 8.3 buffer at 4°C). The column was washed with RPMI 1640 followed by 50 mM Tris HCl, pH 8.3 buffer. Bound DBP/DBP-maf was eluted with 1 M acetate buffer, pH 5.0. The proteins were further purified using hydroxyapatite chromatography as described above.
Generation of DBP-maf from DBP
Sialidase and β-galactosidase (2 U each) were coupled to 0.6 g of CNBr activated Sepharose (Pharmacia) according to manufacturer's instructions. The immobilized enzymes were stored at 4°C after a final wash with coupling buffer until needed.
DBP (100 µg) was incubated with a mixture of immobilized sialidase and β-galactosidase (0.2 U of activity each) in PBS-Mg (10 mM sodium phosphate buffer, pH 5.5, 0.9% sodium chloride and 1 mM MgSO4) at 37°C on an end-to-end shaker for 2 hours. The gel was sedimented by centrifugation at 600xg at 4°C for 5 minutes. The supernatant was collected and sterilized by filtering through a 0.22-µm filter. Possible endotoxin contamination of the DBP-maf preparations was eliminated by removing endotoxin using Detoxi Gel (Pierce, Rockford, IL).
Macrophage Activation Assay
RAW 264.7 cells were plated at a density of 50,000 cells per well in 24-well plates and grown overnight in RPMI 1640 containing 5% FBS at 37°C. The cells were treated with various preparations of DBP or DBP-maf (1 ng/ml per well) in buffer containing 1 mg/ml of ovalbumin for 3 hours (n=6) at 37°C. Reduced fluoresceine (dihydrofluoresceine diacetate) was added to each well to a final concentration of 2 mM and incubated for a further 1 hour at 37°C. The fluorescence of the cell free supernatant was determined using a Hitachi F2000 Fluorescence Spectrophotometer (excitation at 485 nm and emission at 530 nm).
Detection of Glycosidases in BxPC-3 Conditioned Media
Cells (50,000) were washed three times with PBS and suspended in 1 ml of PBS containing 0.4 mM 2-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (sialidase assay), 2-(4-methylumbelliferyl)-β-d-galactoside (galactosidase assay) for 4 hours at 25°C with mixing. At the end of 4 hours, 0.2 ml of the reaction mixture was withdrawn into a clean tube and the reaction stopped by adding 1 ml of ethanol. The tubes were centrifuged and the supernatant collected. 1 M NaOH (25 µl) was added to the supernatant. The fluorescence emission was measured at 450 nm. The excitation was at 365 nm. Values were expressed as arbitrary fluorescence units.
Animal Studies
All animal work was performed in the animal facility at Children's Hospital, Boston in accordance with federal, local, and institutional guidelines. Male 6 to 8 weeks old immune-compromised mice (SCID, Mass General Hospital, Boston, MA) were acclimated, caged in groups of four or less in a barrier care facility and their backs were shaved. All mice were fed a diet of animal chow and water ad libitum. Animals were anesthetized with methoxyflurane (Pittman-Moore, Mundelein, IL) before all procedures and observed until fully recovered. Animals were euthanized by CO2 asphyxiation.
Double Side Tumor Model
A primary tumor of pancreatic cancer cells was generated by injecting 2.5x106 cells (0.2 ml) in the left flank. Tumors were measured with a dial-caliper, and volumes were determined using the formula width2xlengthx0.52. When the tumor volume was at least 400 to 500 mm3 (2% to 2.5% of body weight), which occurred within 14 to 21 days, the secondary tumor was transplanted into the contralateral flank (2.5x106 cells in 0.2 ml). Control mice received an identical injection of secondary tumor cells at the same time. Tumors were measured every third day. The extent of suppression of the secondary tumor by the primary tumor was calculated as the difference in tumor volume.
Tumor Cell Implantation and Treatment
A tumor cell suspension of 2.5x106 cells in 0.2 ml RPMI 1640 was injected into the subcutaneous dorsa of mice in the proximal midline. The mice were weighed and tumors were measured as described above, blinded, every 3 to 5 days in two diameters with a dial-caliper. When the tumor volumes were approximately 100 mm3, mice were randomized into different groups. Experimental groups received DBP-maf at the stated dose in 0.2 ml PBS, administered intraperitoneally. The control group received comparable injections of saline or human DBP, expressed in Escherichia coli. Tumors were measured every third to fifth day and the ratio of treated to control tumor volume (T/C) was determined for the last time point.
Histology
Mice were killed at the end of the experiments. Representative tumor tissues (three of each group) were harvested and fixed in 10% neutral buffered formalin at 4°C for 12 hours. All tissues were paraffin embedded. Sections (5 µm) were first stained with hematoxylin-eosin to evaluate tissue viability and quality. The microvessel density was determined by immunocytochemical staining using a Vectastain avidin-biotin detection system (Vector Labs, Burlingame, CA) with anti-CD 31 (monoclonal, dilution: 1:250, Pharmingen, San Diego, CA) according to the manufacturers' instructions. At low magnification (x40 to x100), regions of highest vessel density (“hot spot” regions) were scanned and counted at x200 magnification (0.738 mm2 field) by an observer who did not know the treatment schedule. At least six fields were counted in a representative tumor section and the highest count was taken. Tumor sections were stained for proliferation with anti-PCNA (monoclonal, dilution: 1:50, Signet, Dedham, MA) or for apoptosis with terminal deoxynucleotide transferase (TUNEL) (Apotag; Intergen, Purchase, NY). They were evaluated at low magnification for viable regions. PCNA-positive (red stain) and apoptotic tumor cells (brown stain) were counted at x400 magnification and expressed as a percentage of the total cells in the field. At least 1000 cells in six random fields were counted. Macrophages were immunostained with either rat anti-mouse Mac-3 antiserum (1:200, Pharmingen) or with rat anti-mouse cd45 antiserum (leukocyte common antigen, Ly-5′1:100, Pharmingen). After the same secondary antibody as above, avidin-biotin conjugated to alkaline phosphatase (Vector) was used with a New Fuschin chromagen (BioGenex, San Ramon, CA).
Statistics
The rate of tumor growth was plotted against treatment day. Analysis of covariance on the rate of change was used to compare the slopes for the treated and the control groups. Student's t test analysis was used to compare the mean microvessel density, TUNEL staining, and PCNA staining between control and treated groups.
Results
Purification of Anti-Endothelial DBP-maf from Pancreatic Cancer Cell Conditioned Media
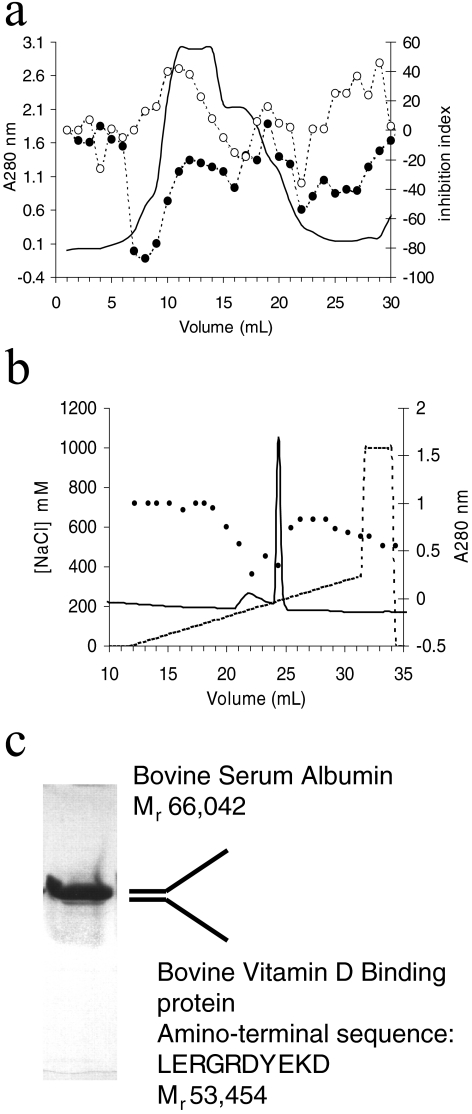
The pancreatic cancer cell line, BxPC-3, was able to inhibit the growth of a secondary tumor implant by up to 80% (Table 1) as determined in an in vivo model in extensive use in our laboratory. A different pancreatic cancer cell line (ASPC-1) was unable to inhibit the growth of secondary tumors, suggesting that BxPC-3 was producing a circulating tumor-suppressive activity. However, the conditioned media obtained from BxPC-3 cells were unable to inhibit the proliferation of endothelial cells (data not shown). This was possibly due to the relatively high concentrations of growth factors such as bFGF (6 to 8 pg/ml) and VEGF (3000 to 6000 pg/ml) present in the conditioned media after 24 to 48 hours. These proangiogenic cytokines bind heparin. We therefore fractionated the conditioned media on heparin-Sepharose. Material that did not bind to heparin-Sepharose exhibited anti-endothelial activity as determined using an endothelial cell proliferation assay. This activity was further purified using a combination of Q-Sepharose and monoQ anion exchange chromatography. Importantly, media containing 5% FCS did not contain any anti-endothelial activity when fractionated using the same protocol (Figure 1a). Anti-endothelial fractions eluting from the monoQ column (Figure 1b) exhibited two bands by SDS-PAGE (Figure 1c). These two proteins were resolved using C4 RP-HPLC. Mass spectrometry and sequence analysis indicated that one of the proteins (Mr 66,042) was BSA, whereas the other protein (Mr 53,454) had the amino-terminal sequence LERGR-DYEKD. This sequence is 90% similar to the amino-terminal sequence of human DBP. We assigned an identity of bovine DBP to our anti-endothelial protein.
Table 1.
Inhibition of Secondary Tumor Growth by a Primary Pancreatic Cancer Tumor in an In Vivo Mouse Model.
| Primary Tumor | Secondary tumor | ||
| Lewis lung | HT1080 | BxPC3 | |
| BxPC3 | 84% | 73% | 81% |
| ASPC-1 | 10% | 7% | Not determined |
Tumor cells were prepared and implanted into SCID mice. At the end of the experiment, tumor volume was measured. The volume of an inhibited tumor was compared to the volume of an uninhibited control tumor, and the ratio expressed as percentage inhibition. Thus, a secondary tumor having a volume that is 16% of the control tumor volume exhibits 84% inhibition in growth.
Figure 1.
Purification of anti-endothelial factor from human pancreatic cancer cell, BxPC-3. BxPC-3 conditioned media (1 L conditioned for 48 hours at 37°C in the presence of 5% FCS) were applied to a HiTrap (5 ml) heparin-Sepharose column (Pharmacia) equilibrated in 50 mM Tris, pH 7.4. The unbound material contained anti-endothelial activity, as assayed by an endothelial cell proliferation assay. This activity (●) was further purified using Q-Sepharose (a) and monoQ anion exchange chromatography (b). Note that control media containing 5% FCS exhibited no anti-endothelial activity (◯) when subjected to the fractionation protocol. The fraction exhibiting anti-endothelial activity (●) eluting from monoQ at ∼0.2 M NaCl (from a continuous gradient of NaCl) contained two bands (c). These bands were very similar in migration and pI. They were resolved from each other using C4 RP-HPLC, and sequence analysis, coupled with mass spectrometry analysis, determined that they were BSA and a protein with 90% homology to human vitamin D binding protein within the amino terminal 10 residues. We therefore assigned an identity of bovine vitamin D binding protein to this band.
Selective Deglycosylation Converts DBP to an Antiangiogenic Factor
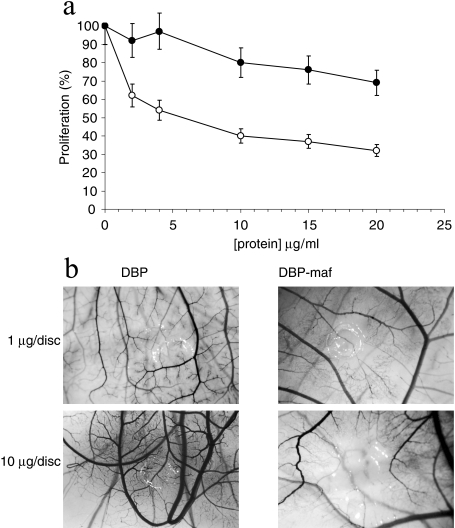
Bovine DBP purified from BxPC-3 conditioned media was able to inhibit the proliferation of endothelial cells with an apparent IC50 value of 5 to 10 µg/ml (Figure 2a). However, bovine DBP purified from BCS was unable to inhibit the proliferation of endothelial cells (Figure 2a).
Figure 2.
Inhibition of angiogenesis in a BCE proliferation assay and the CAM assay by DBP-maf but not DBP. Bovine DBP (◯) purified from BxPC-3 conditioned media was able to inhibit proliferation of BCE cells in a dose-dependent manner, whereas DBP (●) purified from bovine serum, diluted in control media, was unable to inhibit proliferation as effectively. Note that there is a slight inhibition at higher concentrations of DBP (a). Purified human DBP and selectively deglycosylated human DBP-maf were tested in the CAM assay at 1 and 10 µg. Note that there are substantial zones of angiogenesis inhibition in the CAM treated with DBP-maf (b). These images are representative of at least five assays performed at each dose.
Similarly, human DBP, expressed in an E. coli system, was unable to inhibit angiogenesis in the CAM (data not shown). Thus, we hypothesized that the BxPC-3 cells were modifying bovine DBP in some way to generate an anti-endothelial protein. A known biochemical modification of DBP is deglycosylation to produce DBP-maf. Furthermore, it is well known that certain cancer cells can produce glycosidases. BxPC-3 cells produce sialidase (40750±353 fluorescent units, see Materials and Methods section) and β-galactosidase (8570±86 fluorescent units, see Materials and Methods section). To test this hypothesis, we purified human DBP, deglycosylated it using immobilized β-galactosidase and sialidase as described (see Materials and Methods section), and tested this DBP-maf in the CAM assay. DBP-maf was able to inhibit angiogenesis in the CAM assay (Figure 2b).
DBP Purified from BxPC-3 Conditioned Media can Activate Macrophages in a Similar Manner to Human and Bovine DBP-maf
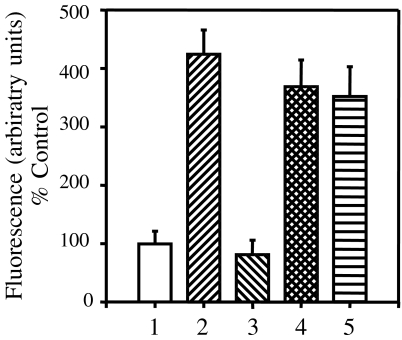
To confirm that BxPC-3 cells were able to convert DBP to the deglycosylated macrophage activating factor form, we tested the ability of DBP purified from BxPC-3 conditioned media to activate macrophages. Human and bovine DBP were purified as described, and enzymatically converted to DBP-maf. These DBP-maf proteins were able to activate macrophages (Figure 3) as determined by a superoxide generation assay (see Materials and Methods section). The increase in superoxide generation by macrophages exposed to DBP-maf was three- to four-fold. DBP purified from BxPC-3 conditioned media activated macrophages, with a similar increase in superoxide production of three- to four-fold over bovine DBP (Figure 3). These data indicate that the DBP purified from BxPC-3 conditioned media has been converted to the DBP-maf form.
Figure 3.
Macrophage activation by DBP-maf variants. Various DBP preparations were generated and tested for their ability to activate macrophages, as measured by a superoxide production assay human DBP [1] and bovine DBP [3] were unable to activate macrophages. Human DBP-maf [2] and bovine DBP-maf [4] were able to activate macrophages. Bovine DBP purified from BxPC-3 conditioned media [5] was also able to activate macrophages. Each value is the mean of quadruplicates, and these data are representative of at least three separate experiments.
Systemically Administered DBP-maf is Able to Inhibit the Growth of Solid Tumors
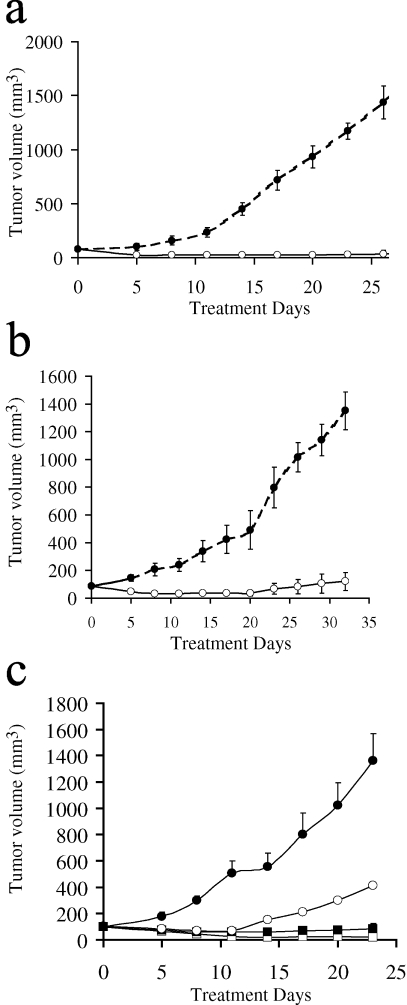
Tumors were implanted on the flank of a mouse, and when the tumor had attained a volume of 100 mm3, therapy was started. Control animals received saline or E. coli expressed human DBP and treated animals received intraperitoneal injection of DBP-maf (4 ng/kg per day) for 28 to 30 days. The growth of human pancreatic tumors (BxPC-3 and SU88.86) in immune compromised mice was inhibited significantly (Figure 4, a and b), with essentially no growth of the treated tumor observed over the course of the experiment [P<.001 as determined by analysis of covariance (see Materials and Methods section)]. The growth of Lewis Lung carcinoma in a normal C57BL6/J mouse was also markedly inhibited by DBP-maf at this dose although there was a measurable increase in the volume of the treated tumor over the course of the experiment (data not shown). We observed a dose response to the therapy when we treated BxPC-3 tumors (Figure 4c). Animals receiving human DBP expressed in E. coli exhibited tumor growth, whereas animals receiving the maximum dose in this experiment (4 µg/kg per day) showed no growth of established tumors with evidence of regression of established tumors [P<.001 as determined by analysis of covariance (see Materials and Methods section)]. Animals receiving 4 ng/kg per day also exhibited little or no tumor growth even after 24 days on therapy, whereas animals receiving 4 ng/kg every 4 days displayed some growth in established tumors (Figure 4c).
Figure 4.
Treatment of tumors with DBP-maf. BxPC-3 cells (a) and SU88.86 cells (b) were implanted subcutaneously onto the flank of a SCID mouse. Once the tumors had attained a volume of 100 mm3, treatment was started. Animals were given 4 ng/kg per day of DBP-maf (◯). Therapy was administered intraperitoneally. Control animals (●) received saline injections only. n=3. BxPC-3 cells were implanted subcutaneously onto the flank of a SCID mouse (c). Once the tumor had attained a volume of 100 mm3, treatment was started. Animals received intraperitoneal injections of DBP-maf at 4 ng/kg every 4 days (◯), 4 ng/kg per day (■), or 4 µg/kg per day (□). Control animals received 4 µg/kg per day of E. coli expressed human DBP (●). n=5.
Histology Results
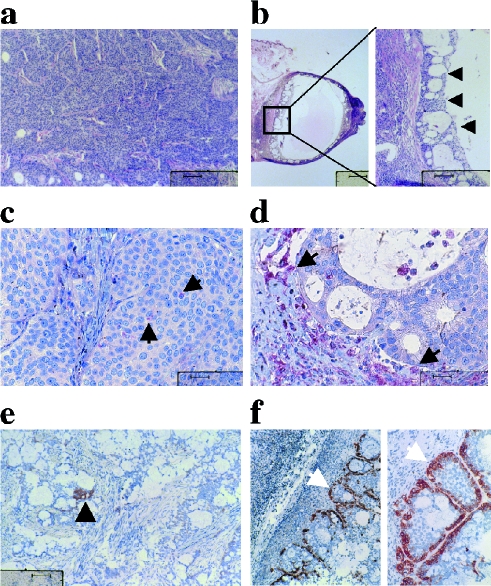
Histological analysis showed that treated tumors were comprised mainly of a small necrotic cyst with a layer of viable tumor cells at the outer perimeter, adjacent to a layer of healthy connective tissue (Figure 5b, arrowed). Untreated tumors (Figure 5a) showed no such morphology, instead comprising a mass of viable tumor cells. Furthermore, immunostaining directed against the macrophage specific marker Mac-3 revealed that treated tumors had been infiltrated by macrophages (Figure 5d, arrowed), whereas control tumors had much reduced staining for this antigen (Figure 5c). Staining for another macrophage marker, leukocyte common antigen, which will also stain NK-cells, also showed infiltration of the treated tumor by cells of leukocyte origin (Figure 5, f and e).
Figure 5.
Histological examination of control and treated tumors. Control untreated BxPC-3 tumor (a) and DBP-maf-treated tumors (b) were stained with H&E. Staining for the antigen Mac-3 revealed few macrophages in control tumors (c, arrow), whereas heavy infiltration of macrophage cells into the treated tumor (d) was apparent (arrow). Staining for leukocyte common antigen also showed heavy infiltration of DBP-maf-treated tumors by cells of leukocyte origin (f). Control tumors stained poorly for this antigen (e). In all panels, the bar is 40 µm except the first panel in b (400 µm).
Histological sections of treated and control tumors were analyzed for endothelial cells (CD 31), proliferation (PCNA), and apoptosis (TUNEL). Our data demonstrated a significant difference in the microvessel density between the treatment and the control groups as assessed by t test analysis. In control BcPC-3 tumors treated with PBS, the microvessel density per high power field was 133.39±9.3, whereas the DBP-maf (4 µg/ml) treated group had a value of 34±5.56 (P<.001). There was no significant difference in the proliferation rate between control tumors (59.27±2.96) and tumors treated with DBP-maf (60.72±2.46) (P<.5). In contrast, the apoptotic index of treated tumors was significantly higher (3.21±0.38) than in the control group (0.86±0.13: P<.001). These data show that in the tumors in which angiogenesis is inhibited, the high level of tumor cell proliferation in all groups is balanced by an increased apoptotic index and a simultaneously decreased microvessel density in the therapy group.
Discussion
DBP purified from the conditioned media of human pancreatic cancer cells BxPC-3 is able to activate macrophages. Our data indicate that glycosidases produced by BxPC-3 pancreatic carcinoma cells generate active DBP-maf from its fully glycosylated precursor, present in plasma and in tissue culture serum. DBP-maf is antiangiogenic in the CAM assay. Interestingly, nonglycosylated recombinant DBP expressed in E. coli has no antiangiogenic activity suggesting that some residual carbohydrate is necessary for antiangiogenic activity. DBP-maf, generated from purified human DBP, was effective as an anti-tumorigenic drug. Administration of low daily doses (4 ng/kg) of DBP-maf caused marked inhibition of solid tumor growth in murine models. Furthermore, DBP-maf was able to regress established pancreatic tumors in immune compromised mice when administered at higher doses. Histological examinations of treated and control tumor specimens revealed that tumors treated with DBP-maf had a low microvessel density, and substantial macrophage infiltration into the tumor mass. Conversely, untreated tumors had little observable macrophage infiltration into a highly vascularized tumor mass. Treated tumors also demonstrated an increase in apoptotic index as compared to control tumors.
DBP-maf has been used successfully to treat Ehrlich ascites tumor in a mouse model [ 8,9]. Although DBP-maf showed no efficacy against a squamous cell carcinoma in a murine model when administered alone [10], it proved to be curative when administered as an adjuvant to photo-dynamic therapy. We now show that tumor-dependent DBP-maf inhibits pancreatic cancer at doses as low as 4 ng/kg per day.
This selectively deglycosylated form of the protein also activates macrophages [13]. We have also shown that DBP-maf, either generated enzymatically or purified from BxPC-3 conditioned media, can activate macrophages. Macrophages are terminally differentiated cells that produce a number of potent angiogenic cytokines and growth factors as well as ECM degrading enzymes. Thus, macrophages can influence various stages of angiogenesis either positively or negatively [14]. There are also differences in the angiogenic potential of classically versus alternatively activated macrophages [15]. Alternatively activated macrophage-rich lesions tend to be highly vascularized, whereas classically activated macrophage-rich lesions tend not to be [14]. Our histology data do show a poorly vascularized, macrophage-rich tumor mass in the case of DBP-maf treated tumors. This obvious targeting of the tumor by activated macrophages may help explain the potency of this therapy against tumors. The additional macrophage activating activity may also explain why the in vivo anti-tumor activity is observed at substantially lower doses than in vitro anti-endothelial activity.
The activated macrophages may secrete an antiangiogenic factor that contributes to the potency of this molecule. The identity of this factor is unknown at present. We assayed the DBP-maf stimulated macrophage conditioned media for cytokines known to have a role in angiogenesis. DBP-maf did not induce the secretion of IFNγ or IL-12, both of which were undetectable by ELISA in the macrophage conditioned media (data not shown). The absence of IFNγ in the macrophage conditioned media argues against the direct involvement of this cytokine and also any involvement of IL-18. IL-18 is reported to be antiangiogenic and antitumorigenic [16]. However, it is a potent IFNγ-inducing cytokine, thus, one would expect to find IFNγ if IL-18 was involved. IL-12 has also been shown to be a potent antiangiogenic cytokine [17] which again mediates its effect by inducing IFNγ. We have directly demonstrated the absence of IL-12 in the conditioned media of macrophages exposed to DBP-maf.
It has been hypothesized that DBP-maf activates macrophages as the first step in an immune developmental process involving macrophages acting as antigen presentation cells and lymphocytes generating antibodies against tumor antigens [10,18]. It has been suggested that the effectiveness of multiple administrations of DBP-maf against Ehrlich ascites tumor was due to a developed immunity against the tumor [8]. In support of this concept, Ehrlich ascites tumor cells cannot grow in mice previously immunized with killed Ehrlich ascites [19]. It is also known that the tumoricidal capacity of macrophages is observed preferentially through the IgG (Fc-receptor)-mediated pathway [20–22] and DBP-maf is a potent adjuvant in immunization for antibody production. However, we have observed anti-tumorigenic activity with DBP-maf therapy in immune compromised mice, deficient in B and T lymphocytes. Such an observation argues against a wholly immune-mediated mechanism. Furthermore, we saw no anti-proliferative effect against pancreatic cancer cells by DBP-maf itself (data not shown). Based on the observed anti-endothelial activity and antiangiogenic activity of DBP-maf presented here, we argue for another mechanism, which we hypothesize to be antiangiogenic.
While we were preparing this manuscript, another laboratory published data demonstrating that DBP-maf is indeed antiangiogenic [23]. They demonstrated that DBP-maf can inhibit not only endothelial cell proliferation, but also tube formation, and chemotaxis stimulated by bFGF, angiopoietin-2, and VEGF. Furthermore, DBP-maf was able to inhibit angiogenesis in the corneal pocket assay. By using specific monoclonal antibodies, they were able to implicate CD36 as a potential receptor of DBP-maf and mediator of this antiangiogenic effect.
It has been noted that cancers can secrete endo and exoglycosidases [24–26]. Evidence has been presented showing that cancerous cells secrete an α-N-acetylgalactosaminidase which cleaves the entire O-linked oligosaccharide from DBP. Such a deglycosylation renders the molecule incapable of being converted to DBP-maf. It has been speculated that this may be a mechanism whereby a cancer can evade an inflammatory response [18]. In fact, the presence of α-N-acetylgalactosaminidase activity in the bloodstream of a cancer patient can serve as a prognostic index for the disease [18]. It is not surprising then that a cancer cell may also be able to secrete the enzymes responsible for efficient conversion of DBP to DBP-maf. Thus, BxPC-3 in secreting glycosidases capable of generating DBP-maf from DBP may be enhancing a regulatory mechanism for the fine control of angiogenesis at inflammatory sites.
In conclusion, a human pancreatic cancer cell line is capable of generating DBP-maf from DBP. This molecule has inherent antiangiogenic activity and can stimulate macrophages. Systemic administration of DBP-maf can inhibit the rate of tumor growth of various solid tumors and can, in some cases, cause regression of established tumors. Such tumor inhibition and regression was observed in immunocompromised mice, arguing against a wholly immune response-mediated mechanism. We believe that DBP-maf is working, at least in part through an antiangiogenic mechanism and that this antiangiogenic mechanism may be amplified by DBP-maf activated macrophages. DBP-maf has efficacy as an anti-tumorigenic therapy at relatively low doses in mice compared to other antiangiogenic therapies [27,28]. Further characterization and study of this promising potential drug will hasten its progress to clinical applications for diseases with an angiogenic component, including but not limited to the treatment of cancer.
Acknowledgements
We thank Kristin Gullage for expert assistance with photography. We also thank Daniela Prox for technical assistance, particularly with the animal work.
Footnotes
This study was supported by National Institutes of Health grants P01 CA45548 to J.F. and DK44337 and DK47418 to R.R. and by a grant from the Deutsche Krebshilfe, Mildred Scheel Stiftung D\97\05974 to O.K. and a grant from Deutsche Forschungsgemeinschaft Be2199/1-1 to C.M.B.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly MS, Rosenthal R, Sage EH, Smith S, Holmgren L, Moses M, Shing Y, Folkman J. The suppression of tumor metastases by a primary tumor. Surg Forum. 1993;44:474–476. [Google Scholar]
- 6.Ray R. Molecular recognition in vitamin D-binding protein. Proc Soc Exp Biol Med. 1996;212:305–312. doi: 10.3181/00379727-212-44020. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto N, Kumashiro R. Conversion of vitamin D3 binding protein (group-specific component) to a macrophage activating factor by the stepwise action of beta-galactosidase of B cells and sialidase of T cells. J Immunol. 1993;151:2794–2802. [PubMed] [Google Scholar]
- 8.Yamamoto N, Naraparaju VR. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 1997;57:2187–2192. [PubMed] [Google Scholar]
- 9.Koga Y, Naraparaju VR, Yamamoto N. Antitumor effect of vitamin D-binding protein-derived macrophage activating factor on Ehrlich ascites tumor-bearing mice. Proc Soc Exp Biol Med. 1999;220:20–26. doi: 10.1046/j.1525-1373.1999.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 10.Korbelik M, Naraparaju VR, Yamamoto N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br J Cancer. 1997;75:202–207. doi: 10.1038/bjc.1997.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen M, Shing Y, Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994;47:31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- 12.Swamy N, Roy A, Chang R, Brisson M, Ray R. Affinity purification of human plasma vitamin D-binding protein. Protein Expr Purif. 1995;6:185–188. doi: 10.1006/prep.1995.1023. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43(Suppl):69–71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- 15.Kodelja V, Muller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–493. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 16.Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13:2195–2202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- 17.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N, Naraparaju VR, Asbell SO. Deglycosylation of serum vitamin D3-binding protein leads to immunosuppression in cancer patients. Cancer Res. 1996;56:2827–2831. [PubMed] [Google Scholar]
- 19.Morton D, Eilber FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68:158–163. [PubMed] [Google Scholar]
- 20.Yamamoto N, Hoober JK, Yamamoto N, Yamamoto S. Tumoricidal capacities of macrophages photodynamically activated with hematoporphoryin derivative. Photochem Photobiol. 1992;56:245–250. doi: 10.1111/j.1751-1097.1992.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiner LM, Moldofsky PJ, Gatenby RA, O'Dwyer J, O'Brien J, Litwin S, Comis RL. Antibody delivery and effector cell activation in a phase II trial of recombinant gamma-interferon and the murine monoclonal antibody CO17-1A in advanced colorectal carcinoma. Cancer Res. 1980;48:2568–2573. [PubMed] [Google Scholar]
- 22.Houghton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci USA. 1985;82:1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N. Effects of vitamin-D3-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. JNCI. 2002;94:1311–1319. doi: 10.1093/jnci/94.17.1311. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N, Naraparaju VR, Srinivasula SM. Structural modification of serum vitamin-D3 binding protein and immunosuppression in HIV-infected patients. AIDS Res Hum Retroviruses. 1995;11:1373–1378. doi: 10.1089/aid.1995.11.1373. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Bergeron JM, Linehan LA, Lalley PA, Sakaguchi AY, Bowman BH. Mapping and conservation of the group-specific component gene in mouse. Genomics. 1990;7:509–516. doi: 10.1016/0888-7543(90)90193-x. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Lindsay DD, Naraparaju VR, Ireland RA, Popoff SN. A defect in the inflammation-primed macrophage-activation cascade in osteopetrotic rats. J Immunol. 1994;152:5100–5107. [PubMed] [Google Scholar]
- 27.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 28.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]