Abstract
DTI-015 (BCNU in 100% ethanol) utilizes solvent facilitated perfusion for the intratumoral treatment of gliomas. The ethanol solvent vehicle facilitates a rapid and thorough saturation of the tumor with the dissolved anticancer agent BCNU. We conducted a phase I/II dose escalation study of DTI-015 in 40 heavily pretreated patients with inoperable recurrent malignant glioma. The study goals were to establish a maximally tolerated dose (MTD) for DTI-015 and assess its safety and activity. Patients received stereotactic intratumoral injection of DTI-015 under magnetic resonance imaging guidance. Dose escalation was performed in two phases. First, DTI-015 volume was escalated at a set BCNU concentration of 12.5 mg/ml; second, BCNU mg dose was escalated by increasing BCNU concentration to 30, 45, 60, and 75 mg/ml. A MTD of 5 ml and 240 mg was established. Twenty-five of 28 DTI-015 treatments (89%) using ≤MTD were administered safely without producing high-grade drug-related adverse events. Median survival for GBM patients administered DTI-015 at ≤MTD was 55 weeks. Magnetic resonance imaging demonstrated stable disease in 72% of evaluable patients with a median of 10.5 weeks. The results suggest that DTI-015 administered at ≤MTD is well tolerated and active in patients with inoperable recurrent GBM.
Keywords: stereotactic injection, malignant glioma, GBM, recurrent tumor, clinical trial
Introduction
Approximately 14,000 new cases of malignant glioma are diagnosed each year in the US [16]. Despite a multimodality approach using surgery, radiotherapy and chemotherapy, the prognosis is poor, with a median survival of approximately 1 year following diagnosis for patients with glioblastoma multiforme. Effective drug treatments for malignant gliomas have proven elusive, due in part, to the presence of the blood brain barrier. A number of approaches have been used in an attempt to overcome this obstacle to drug delivery in the brain. High-dose intravenous delivery of chemotherapeutic agents has been attempted but is limited by systemic toxicity. BCNU has been delivered using intraarterial administration in an attempt to increase tumor dosing. This, however, results in extensive neurological toxicity, and provides no increase in survival compared to intravenous BCNU [4,8,13].
Using local delivery of antitumor agents, through the interstitial space, investigators have sought to bypass the blood brain barrier, thereby increasing amounts of anticancer drugs that can be delivered to the tumor. Gliadel® incorporates BCNU in a solid polymer wafer depot, and in clinical studies demonstrated a small but significant benefit in combination with surgery in patients with recurrent gliomas [3]. Gliadel® and other depot approaches, however, deliver the drug through the interstitial space by diffusion, which limits tissue penetration to a few millimeters.
To extend the penetration distance of anticancer drugs in brain tumors, Bobo et al. [1], Laske et al. [6], and Morrison et al. [9] developed convection enhanced delivery (CED), which uses pressurized interstitial injection of anticancer agents in aqueous fluids delivered at low flow rates. In preclinical testing, this approach has been demonstrated to produce large distribution volumes [1,5,7]. Our research with DTI-015 (a combination of BCNU in ethanol) suggests that drug delivery through the intratumoral route may be accomplished effectively by solvent facilitated perfusion, which utilizes water-miscible organic solvent delivery vehicles, rather than the aqueous vehicles used in convection enhanced delivery. We have hypothesized that the dual properties of the solvent, of water miscibility and membrane permeability, facilitate a rapid and thorough penetration of high concentrations of the anticancer agent solute throughout solid tumors following intratumoral injection [11,12,14].
Based upon the efficacy demonstrated in tumor models [10,11,14], a phase I/II trial was conducted to study the safety and activity of DTI-015 in patients with inoperable recurrent malignant glioma.
Patients and Methods
Clinical Study Protocol
Clinical study Protocol ID 95-115 was conducted under physician-sponsored IND #48,726 using DTI-015 in patients with inoperable recurrent malignant gliomas. The study protocol was approved by the Institutional Review Boards (IRBs) of the MD Anderson Cancer Center (MDACC) in Houston and the Cleveland Clinic and initiated in December 1995. Patients gave informed consent before entering the study. Described here are the results as of October 24, 2001.
Patient Selection
The protocol required patients between 18 and 75 years of age with histologic proof of recurrent, previously irradiated supratentorial glioblastoma multiforme, anaplastic astrocytoma, anaplastic oligodendroglioma, or anaplastic ependymoma. It must have been judged that gross total resection of the patient's tumor was not possible or the patient had refused to have a resection of the tumor. Patients must have had a Karnofsky performance score (KPS) rating ≥60, with an inoperable recurrent tumor volume of ≥0.5 and ≤13 cm3 and undergoing a stereotactic biopsy or open craniotomy for clinical reasons other than injection of DTI-015. Patients must have been fully recovered from the acute effects of any prior chemotherapy or radiotherapy. For females of child-bearing potential, the patient must not have been pregnant as evidenced by a menstruation in the last 8 weeks or by a negative urine human chorionic gonadotropin (HCG) pregnancy test.
The exclusion criteria excluded patients receiving any radiotherapy or chemotherapy during 4 weeks before entering the study or any treatment with nitrosoureas or mitomycin during 6 weeks before entering the study, patients with active uncontrolled infection, serious liver or bone marrow disorder, evidence of renal failure, bleeding diathesis, and patients using anticoagulant medication. Ovoid or spherical tumors were allowed as were tumors with central necrosis or cystic areas as long as an enhancing rim of thickness ≥5 mm was present.
Patient Evaluation and Management
Each patient underwent a thorough medical examination before intratumoral injection of DTI-015. These included medical history, physical examination, KPS determination, neurological examination, mini-mental state examination, tumor imaging by magnetic resonance imaging (MRI) scans, and laboratory examinations. Starting the day before injection, the patients' steroid dose was set to 10 mg intravenous or oral every 6 hours or the dose of the previous day whichever was larger and also loaded and maintained on anticonvulsants preferably dilantin. Toxicity was assessed by the use of NCI common toxicity grading criteria.
DTI-015 Injection and Tumor Volume Calculations
An MRI was performed before the injection of DTI-015. The tumor volume was calculated using the three MRI slices (one each in the coronal, sagittal, and axial view) having the largest tumor area in that view. From the coronal slice, the largest superior-inferior tumor diameter and the largest lateral tumor diameter were determined. In the same way, the largest superior-inferior tumor diameter and largest anterior-posterior tumor diameter were measured from the sagittal view, as were the largest anterior-posterior and lateral diameters from the axial view. The two diameters for each dimension were averaged to yield a lateral (dL), anterior-posterior (dAP), and superior-inferior diameter (dSI). Tumor volume was calculated as (4/3) πx(dL/2)x (dAP/2)x(dSI/2).
A twist drill was used to make a puncture in the scalp and the skull, and the stereotactic needle was passed through the dura to the center of the tumor. The malignant nature of the tumor was confirmed by histological evaluation of frozen sections from stereotactic biopsy material before injection of DTI-015. The volume of DTI-015 injected was determined as a percentage of tumor volume. DTI-015 (BCNU in ethanol) was supplied by Direct Therapeutics (Redwood City, CA).
Multiple 1 ml syringes connected by means of a 10-cm IV tubing to a Nashold reusable 5-mm window biopsy needle were used to inject DTI-015 at the flow rates of 0.3 to 1.5 ml/minute. Once the infusion was completed, the tubing was clamped and the needle was left in place for 5 minutes. The needle was removed and the scalp wound closed with a single suture.
Dose Escalation and Maximally Tolerated Dose (MTD)
Preclinical studies of DTI-015 injection into normal rat and feline brain demonstrated that both volume and milligram dose (concentration) could contribute to the toxicity of DTI-015 (unpublished results). Therefore, dose escalation was executed in two phases to distinguish the effects of these two independent variables. In the first phase (dose levels I to III), volume was escalated using a set BCNU concentration of 12.5 mg/ml. Because the goal of intratumoral therapy is to treat the tumor without causing damage to surrounding normal brain, the absolute volume administered has to take into account the tumor volume being injected. Consequently, DTI-015 is administered as a percentage of the treated tumor volume and volume escalation was accomplished by increasing the volume of DTI-015 as a percentage of tumor volume (25%, 50%, 75%). In the second phase (dose levels IV to VIII), BCNU milligram dose was escalated by increasing BCNU concentration (30, 45, 60, and 75 mg/ml). Escalation to the next higher dose was performed provided at least three patients at each prior level showed no or minimal toxicity (NCI toxicity grade 1 or 2). The MTD of DTI-015 was determined by analyzing the frequency with which high-grade drug-related adverse events (HGR-AEs) were produced as a function of injection volume (ml), BCNU dose (mg), BCNU concentration (mg/ml), injection volume per tumor volume (ml/cm3 tumor), and BCNU dose per tumor volume (mg/cm3 tumor).
Postinjection Follow-Up
Patients were followed for at least 2 days postoperatively with particular attention to any adverse reaction to the procedure or treatment. Vital signs, neurological examination, and all adverse events were closely followed and recorded. Patients had monthly clinical follow-ups including brain MRI scans. Disease progression was based on changes in tumor volume using T1-weighted gadolinium contrast enhancement according to classic NCI response criteria.
Results
Patient Summary
Forty patients [26 male, 14 female between the ages of 23 and 73 years (median, 50)] were enrolled in the study, 38 at MDACC, and two at the Cleveland Clinic. Three patients were Hispanic, one was East Indian, and the rest were Caucasian. The histologic diagnosis was AA for 9 patients, anaplastic oligodendroglioma for 2, GBM for 26, gliosarcoma for 1, infiltrating astrocytoma for 1, and mixed oligoastrocytoma for 1. The median KPS was 90 (range 50 to 100), median age 50 (range 23 to 73), and median tumor volume 6.1 cm3 (range 0.9 to 23.4 cm3) (Table 1).
Table 1.
Patient Characteristics.
| Total patients | 40 |
| Male:Female | 26:14 |
| Caucasian | 36 |
| Hispanic | 3 |
| East Indian | 1 |
| Age median (range) | 50 (23–73) years |
| Karnofsky median (range) | 90 (50–100) |
| Histology | |
| Glioblastoma multiforme | 26 |
| Gliosarcoma | 1 |
| Anaplastic astrocytoma | 9 |
| Oligodendroglioma | 2 |
| Infiltrating astrocytoma | 1 |
| Mixed oligoastrocytoma | 1 |
| Tumor volume median (range) | 6.1 (0.9–23.4) cm3 |
The study patients were extensively pretreated. Tumor resections before the study were documented for all except five patients and 14 of 40 (35%) patients had three or more prior surgical procedures. All had previously been treated with radiation, and 27 of 40 patients (68%) had received at least two courses of chemotherapy before entering the study. Treatment with nitrosoureas (lomustine [CCNU] and BCNU) before entry into the study was documented for 23 of 40 (58%) of the patients.
DTI-015 Treatment
The 40 patients received a total of 46 treatments. Thirty-five patients received one treatment, four received two treatments, and one received three treatments. Twenty-seven of the treatments consisted of one injection, 18 of the treatments consisted of two injections, and 1 treatment consisted of three injections.
A summary of number of patients, treatments, volume, and milligram dose of DTI-015 administered at each dose level is shown in Table 2. The total volumes of DTI-015 administered ranged from 0.44 to 10.5 ml, and the total doses of BCNU ranged from 7.5 to 369 mg per treatment.
Table 2.
DTI-015 Treatment Regimens.
| No. of patients/No. of treatments | Dose level | BCNU concentration (mg/ml) | DTI-015 volume (as percentage Tv) | Total DTI-015 volume (ml) | Total BCNU dose (mg) |
| 4/5 | I | 12.5 | 25 | 0.6–2.0 | 7.5–25.0 |
| 5/5 | II | 12.5 | 50 | 2.1–8.3 | 26.25–103.0 |
| 10/13 | III | 12.5 | 75 | 0.7–10.5 | 8.75–131.25 |
| 5/5 | IV | 30 | 50 | 0.4–5.0 | 13.2–150 |
| 1/1 | V | 60 | 50 | 2.6 | 153 |
| 5/5 | VI | 45 | 75 | 1.4–7.6 | 63–342 |
| 8/8 | VII | 60 | 75 | 1.2–5.8 | 74–350 |
| 4/4 | VIII | 75 | 75 | 2.7–4.9 | 203–369 |
Forty patients received 46 treatments. Two patients received treatment at two dose levels, one patient received treatment at dose levels II and III, and one patient received treatment at dose levels VII and VIII. This accounts for the number of patients in column 1 totaling 42.
Adverse Events and MTD Determination
Protocol 95–115 called for identification of adverse events that were both high grade [3, 4, or 5 (severe, life-threatening, or fatal, respectively)] and related to investigational treatment (HGR-AEs). Fifteen of the 46 treatments (33%) administered to 14 patients resulted in high-grade events judged related (possibly, probably, or definitely) to DTI-015 treatment.
The frequency of HGR-AEs increased at both the higher injection volumes and the higher BCNU milligram doses administered. The distribution of HGR-AEs as a function of dose level cohort is shown in Table 3.
Table 3.
Treatments Resulting in HGR-AEs and DTI-015 Dose Level Cohorts.
| Dose level | Dosing | HGR-AEs/Total treatments | Percentage HGR-AEs |
| I | 12.5/25 | 1/5 | 20% |
| II | 12.5/50 | 0/5 | 0% |
| III | 12.5/75 | 4/13 | 31% |
| IV | 30/50 | 2/5 | 40% |
| V | 60/50 | 0/1 | 0% |
| VI | 45/75 | 2/5 | 40% |
| VII | 60/75 | 4/8 | 50% |
| VIII | 75/75 | 2/4 | 50% |
The HGR-AEs encountered at 12.5 mg/ml (during the volume escalation phase of the study) were predominantly from treatments using volumes above 5 ml (4 of 5), whereas those encountered during the concentration escalation phase of the trial are predominantly from treatments using BCNU dose ≥240 mg (8 of 10).
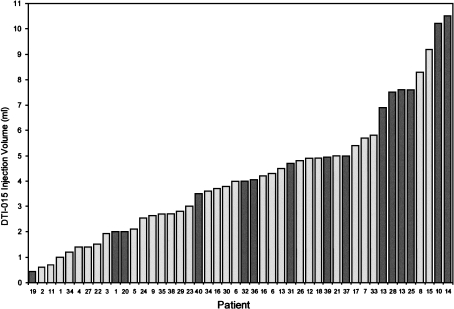
The frequency of HGR-AEs as a function of injection volume for all 40 patients (46 treatments) is shown in Figure 1. Overall, 9 of 35 treatments (26%) using a DTI-015 injection volume of ≤5 ml resulted in HGR-AEs, compared to 6/11 treatments (55%) using >5 ml injection volume yielding a maximally tolerated DTI-015 volume of 5 ml.
Figure 1.
Bar chart showing DTI-015 injection volume (ml) and patient number ordered by increasing injection volume. HGR-AEs are indicated by dark bars.
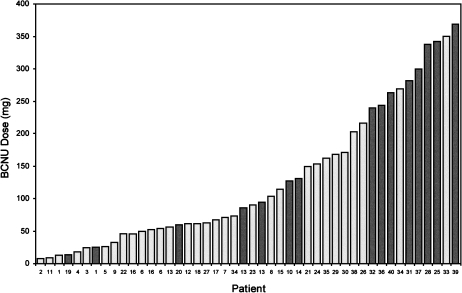
Regarding BCNU milligram dose, 7 of 36 treatments (19%) using <240 mg BCNU resulted in HGR-AEs, compared to 8 of 10 treatments (80%) using ≥240 mg BCNU (Figure 2) yielding a maximally tolerated BCNU dose of <240 mg.
Figure 2.
Bar chart showing BCNU dose (mg) and patient number ordered by increasing milligrams. HGR-AEs are indicated by dark bars.
Taken together, 3 of 28 (11%) DTI-015 treatments using DTI-015 at ≤5 ml and <240 mg BCNU resulted in HGR-AEs compared to 12 of 18 treatments (67%) using DTI-015 at >5 ml or ≥240 mg BCNU (Table 4). Based upon these results, the MTD is defined by both volume injected and total dose administered as 5 ml and 240 mg, respectively.
Table 4.
HGR-AEs and DTI-015 Dose.
| BCNU dose (mg) | DTI-015 injection volume | |
| ≤5 ml | >5 ml | |
| <240 | 3/28 (11%) | 4/8 (50%) |
| ≥240 | 6/7 (86%) | 2/3 (67%) |
The ≥240 mg BCNU treatments resulting in HGR-AEs were all delivered at 75% tumor volume. In addition to high absolute milligram dose, they therefore also utilized high milligram/tumor proportionate dosing of 45 to 56 mg BCNU/cm3 tumor.
Adverse Events Produced at DTI-015 Doses ≤MTD
The three treatments administered at ≤MTD which resulted in HGR-AEs produced a total of three events. Patient 1 exhibited a grade 3 cerebral edema following DTI-015 injection plus surgical resection and lived 31 weeks following treatment during which time the edema did not resolve. Patient 19 presented with severe leg and mild arm weakness before DTI-015 that progressed to a grade 3 hemiparesis following injection that then resolved and the patient lived 79 weeks following treatment. Patient 20 presented with a severe and worsening weakness before DTI-015 that progressed to grade 3 hemiplegia following injection. This did not resolve before the patient's expiration 3 weeks following treatment due to unrelated pneumonia. Twenty-five of 28 (89%) DTI-015 treatments at ≤MTD were administered safely without producing any HGR-AEs.
Adverse Events Produced at DTI-015 Doses >MTD
The 12 treatments administered at >MTD resulting in HGR-AEs produced a total of 37 events: patient 10 exhibited hemiplegia and hematoma; patient 13 exhibited seizure following a first treatment and then headache, nausea, and vomiting following a third treatment; patient 25 exhibited headache, fatigue, and lethargy; patient 28 exhibited hydrocephalus, speech impairment, and depressed level of consciousness; patient 31 exhibited confusion, speech impairment, and right-sided weakness; patient 32 exhibited right-sided weakness; patient 36 exhibited left hand incoordination and weakness; patient 37 exhibited muscle weakness, speech impairment, cognitive changes, and left hemiparesis. The milliliter and milligram dose each of these patients received is shown in Figures 1 and 2.
Three deaths were considered possibly related to DTI-015 administered at the highest milliliter volume, milligram per milliliter concentration, and milligrams studied. Patient 14 did not meet protocol eligibility criteria but was treated on a compassionate basis. He had brain herniation and a KPS of 50 at the time of enrollment. He received the highest injection volume administered in the study (10.5 ml), experienced respiratory distress, respiratory failure, hypertension, unresponsiveness, dilated pupils, raised intracranial pressure, and eventually succumbed because of respiratory failure on day 3 posttreatment. Patient 39 exhibited ataxia, decreased level of consciousness, neuropathy, speech impairment, urinary incontinence, and dehydration following DTI-015 administration (75 mg/ml, 369 mg) and although his condition improved upon discharge died 7 weeks following the administration of DTI-015. Patient 40 experienced two focal seizures immediately following DTI-015 (75 mg/ml, 264 mg), was administered phenobarbital, then experienced a decreased level of consciousness, decreased respiratory rate, and hypoxia requiring intubation and ICU care. In addition, the patient experienced left lower lobe pneumonia, urinary tract infection, and a candida superinfection and expired 4 weeks following treatment.
All HGR-AEs were CNS associated and occurred in the immediate postoperative period. There were none of the systemic adverse reactions that might be expected from administration of BCNU, including no episodes of delayed myelosuppression following treatment with DTI-015 up to the maximum dose of BCNU administered (369 mg). There were no reports of adverse changes in any laboratory parameters.
Survival
As of October 24, 2001 three of the 40 patients are still alive. The median survival time for the entire population (n=40) was 31 weeks and 13 weeks for non-GBM patients (n=14). The median survival of patients with GBM (n=26) was 32 weeks. The median survival of GBM patients treated with DTI-015 doses ≤MTD (n=16) was 55 weeks, whereas the median survival of GBM patients treated with doses >MTD (n=10) was 19 weeks.
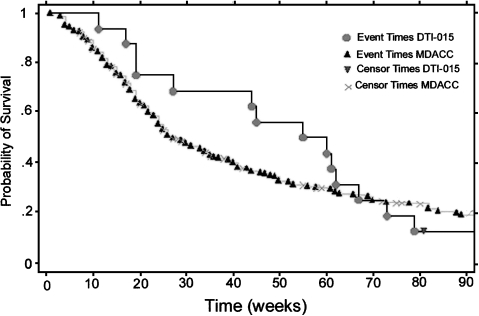
Figure 3 shows the Kaplan Meier survival curves for GBM patients receiving DTI-015 doses ≤MTD compared to the MDACC historical data for recurrent operable and inoperable GBM patients (median survival time 25 weeks, Ref. [15]).
Figure 3.
Kaplan Meier survival curve for recurrent inoperable GBM patients following DTI-015 [median survival time of 55±15 (SE) weeks], compared to MDACC recurrent GBM historical data [median survival time of 25±5 (SE) weeks] which includes operable and inoperable patients.
Magnetic Resonance Imaging
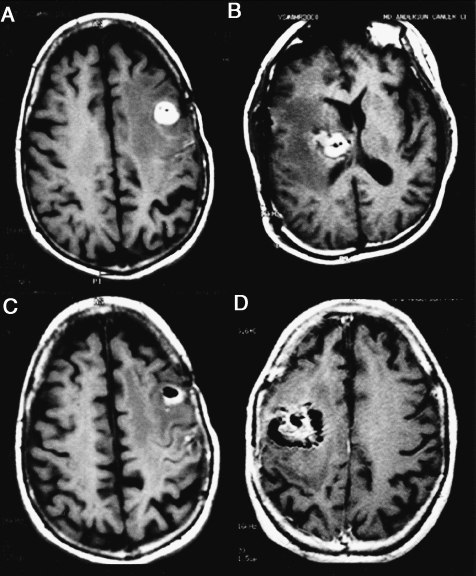
Following intratumoral DTI-015 injection, the signal detected on T1-weighted MR images acquired in the absence of contrast medium (Figure 4, A–D) was typically hyperintense with hypodense foci, some of which were confluent spots. This signal was present in all evaluable patients (n=25) and often lasted for several months.
Figure 4.
Post-DTI-015 MR images (T1-weighted axial images without contrast) demonstrating a combined hyperintense and hypointense signal 24 hours following injection. Patients 27 (A and C), 16 (B), and 26 (D).
The size of the signal abnormality and the fact that it was superimposed upon the tumor images made routine assessment of tumor shrinkage (complete or partial response) impractical in most patients. MRI, however, was routinely useful in assessing stable disease (an unchanging T1-weighted contrast enhanced signal) and progressive disease (an increasing T1-weighted contrast enhanced signal), as demonstrated for patient 6 (Figure 5). Eighteen of 25 (72%) evaluable patients exhibited stable disease with a median of 10.5 weeks (range 4 to 40 weeks) and 13 of these patients were stable at last follow-up (median 8 weeks, range 4 to 16 weeks). Seven of 25 evaluable patients (28%) progressed at first follow-up with a median of 4 weeks (range 4 to 8 weeks).
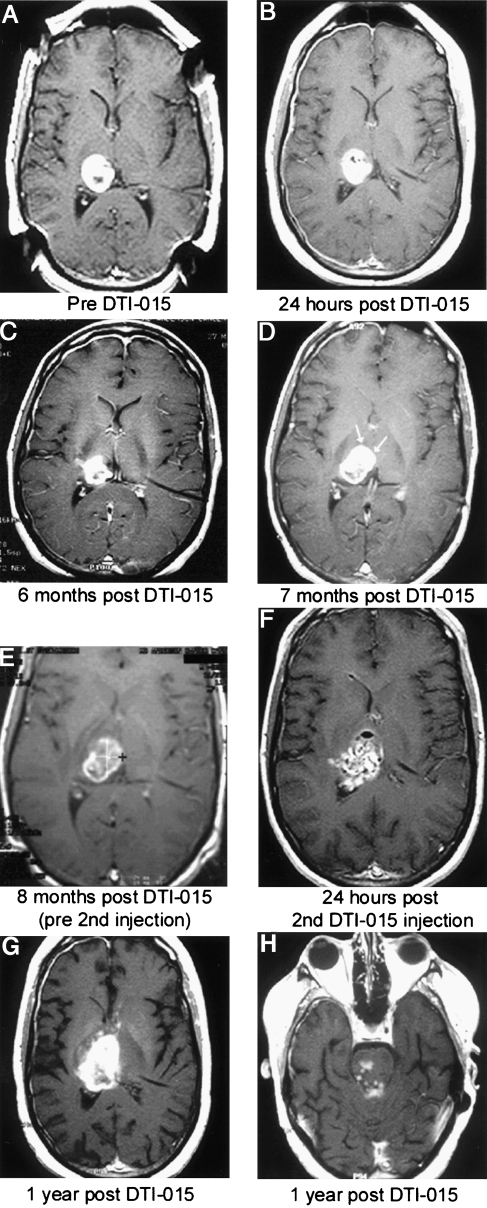
Figure 5.
Patient 6 presented 7 months following diagnosis with a recurrent right thalamic glioblastoma multiforme, which had been increasing in size every 3 weeks following radiation and chemotherapy. All MR images are axial T1-weighted images with contrast. Preinjection MRI tumor volume was 8 ml (A). Twenty-four hours post-DTI-015 (B). Tumor volume decreased to 5.4 ml at 6 months (C). At 7 months, a contrast enhancing area of new tumor growth occurred (D, arrows). A second DTI-015 injection was performed at 8 months (E). Twenty-four hours post-second DTI-015 injection, a mixed hyperintense and hypointense signal is seen (F). One year post-initial DTI-015 treatment, areas of contrast enhancement appeared anterior and superior to the tumor (G) and inferior in the brainstem (H) indicating tumor regrowth. The patient survived 59 weeks from the first DTI-015 injection.
Although atypical, tumor volume reduction was seen in some patients with minimal DTI-015-induced MRI signal (Figure 6).
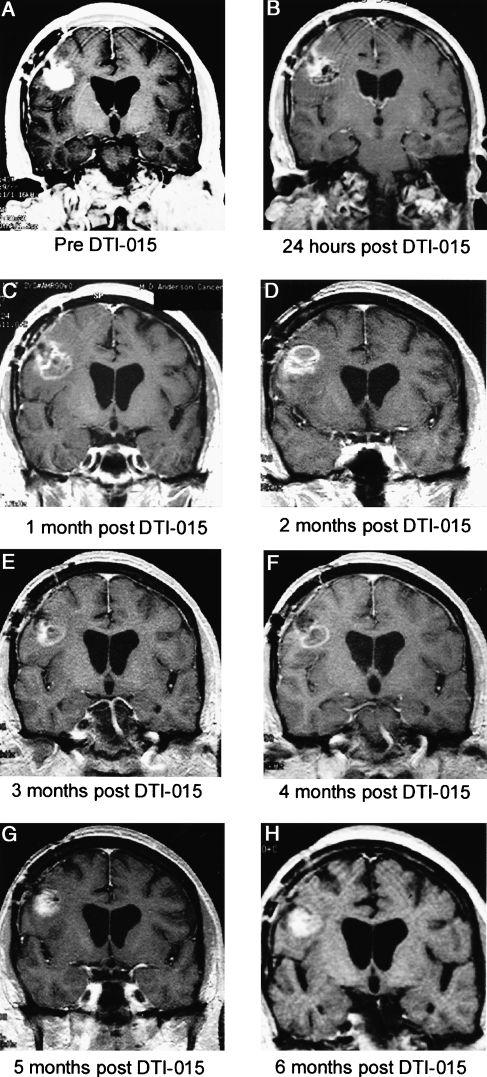
Figure 6.
Patient 29 presented 7 months following diagnosis with a recurrent posterior frontal lobe glioblastoma multiforme following two resections, chemotherapy, and radiation. All MR images are coronal T1 weighted with contrast. Preinjection tumor volume was 3.7 ml (A). Twenty-four hours post-DTI-015 demonstrates a predominantly hypointense signal intensity and decreased contrast enhancement (B). The tumor demonstrated a small ring lesion with central hypointensity at 1–4 months (C–F) and then a slight increase in enhancement at 5 months (G). Six months postinjection (H), the area inferior to the injection progressed as demonstrated by increasing contrast enhancement. At 8 months post-DTI-015 injection, this region and the injected area were resected. The resected region injected with DTI-015 had a “hard rock” consistency. Histological examination of this region demonstrated necrosis, with microscopic foci of tumor at the edges (although no tumor growth). A biopsy of the wall of a previous resection cavity demonstrated tumor mass and diffuse infiltrating tumor.
Discussion
This phase I/II trial was undertaken to identify a MTD of DTI-015 and investigate its safety and activity when administered by stereotactic injection in patients with inoperable malignant glioma. A dual phase dose escalation was employed and identified the MTD of DTI-015 in the study population to be 5 ml and 240 mg. When administered at ≤MTD, DTI-015 was well tolerated, and produced a median survival time of 55 weeks in heavily pretreated patients with inoperable recurrent GBM.
Our goal was to establish the MTD by systematically increasing dosage until unacceptable toxicity was produced. Not surprisingly, we found that DTI-015 toxicity in humans is related to both injection volume and milligram dose as predicted by preclinical studies in normal feline and rat brain (unpublished results). When DTI-015 was administered at >MTD, a high frequency of HGR-AEs resulted (67%, 12 of 18 treatments) with a severity that peaked at the highest milliliter (10.5), milligram (369), and milligram per milliliter (75) doses studied.
In sharp contrast, when administered at ≤MTD, DTI-015 was well tolerated resulting in only three HGR-AEs from 28 treatments with 25 of these treatments (89%) being administered safely. The safety of DTI-015 exhibited in this trial would seem especially encouraging given the inoperable nature of the tumors treated.
It is not clear if 240 mg is an absolute dose that should not be exceeded or if this dose can in fact be exceeded if administered at less than 75% tumor volume, that is, administered into tumors larger than those included in this study. An ongoing multicenter trial evaluating DTI-015 in inoperable recurrent GBM sized up to 33.4 cm3 should help answer this question.
The median survival for patients with recurrent inoperable GBM of 55 weeks following treatment with DTI-015 at ≤MTD compares favorably with survival results from other studies. The historical cumulative experience of 225 patients with recurrent GBM enrolled in eight consecutive phase II chemotherapy studies at MDACC (including operable and inoperable patients) was 25 weeks [15]. Recent studies of temozolomide and procarbazine at first recurrence in patients with GBM reported median progression-free survivals of 9 to 12 weeks and overall survival times of 23 to 29 weeks after chemotherapy was initiated [2,17]. Although proof of efficacy can only be demonstrated in a randomized prospective clinical trial, the current findings of a two-fold increase in survival with DTI-015 compared to these studies of systemic chemotherapy agents are quite encouraging.
Although the MRI signal changes following injection of DTI-015 prohibited an assessment of initial tumor shrinkage in most patients, MRI was valuable in determining stable disease (an unchanging T1-weighted contrast enhanced signal) and progressive disease (an increasing T1-weighted contrast enhanced signal). The incidence of stable disease achieved in 72% of evaluable patients for a median of 10.5 weeks suggests DTI-015-produced anti-tumor activity. The explanation of the DTI-015-induced MRI signal appearance is unknown. Nonetheless, over long time periods of 6 to 12 months, the majority of these MRI signal changes regressed. Although the apparent distribution of DTI-015 shadowed the T1-weighted tumor image on the MRI scans, there is insufficient data at this time to correlate this treatment-induced signal with clinical activity. Diffusion MRI is currently being evaluated for its potential to map the spatial distribution of DTI-015 anti-tumor activity in gliomas.
In conclusion, the results of this trial have identified a MTD of DTI-015. When administered at ≤MTD, DTI-015 is well tolerated and results in a median survival of 55 weeks in heavily pretreated patients with inoperable recurrent GBM. Based on these findings, a randomized prospective clinical trial of DTI-015 is planned to definitively evaluate its efficacy in patients with GBM.
Acknowledgements
The authors thank Raymond Sawaya, MD (MD Anderson Cancer Center) for his support in the initiation and conduct of this study, Gene Barnett, MD (Cleveland Clinic) for contributing two patients, Ken Hess, PhD (MD Anderson Cancer Center) for providing the historical survival data for recurrent GBM patients at MD Anderson Cancer Center and Julie Carter, PhD (Direct Therapeutics) for help in preparing this manuscript.
References
- 1.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group [see comments] Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 4.Kleinschmidt-Demasters BK. Intracarotid BCNU leukoencephalopathy. Cancer. 1986;57:1276–1280. doi: 10.1002/1097-0142(19860401)57:7<1276::aid-cncr2820570703>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Laske DW, Morrison PF, Lieberman DM, Corthesy ME, Reynolds JC, Stewart-Henney PA, Koong SS, Cummins A, Paik CH, Oldfield EH. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87:586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- 6.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 8.Miller DF, Bay JW, Lederman RJ, Purvis JD, Rogers LR, Tomsak RT. Ocular and orbital toxicity following intracarotid injection of BCNU (carmustine) and cisplatinum for malignant gliomas. Ophthalmology. 1985;92:402–406. doi: 10.1016/s0161-6420(85)34036-8. [DOI] [PubMed] [Google Scholar]
- 9.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266:R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 10.Pietronigro D, Drnovsky F, Cravioto H, Ransohoff J. DTI-015 produces cures in T9 gliosarcoma. Neoplasia. 2003;5:17–22. doi: 10.1016/s1476-5586(03)80013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietronigro D, Drnovsky F, Cravioto H, Ransohoff J. Stereotactic intratumoral injection of DTI-015 in a rat intracranial T9 gliosarcoma model. Proc Am Assoc Cancer Res. 1999;40:583. [Google Scholar]
- 12.Pietronigro D, Frey K, Desmond T, Carter J, Ross BD. Rapid facilitated distribution of high 14C-BCNU concentrations following intratumoral injection of DTI-015 in rat 9L brain tumors. Proc Am Assoc Cancer Res. 2000;41:523. [Google Scholar]
- 13.Shapiro WR, Green SB, Burger PC, Selker RG, VanGilder JC, Robertson JT, Mahaley MS. A randomized comparison of intra-arterial versus intravenous BCNU, with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg. 1992;76:772–781. doi: 10.3171/jns.1992.76.5.0772. [DOI] [PubMed] [Google Scholar]
- 14.Simpson-Herren L, Pietronigro D. Intratumoral injection of DTI-015 in the Walker 256 subcutaneous model. Proc Am Assoc Cancer Res. 1999;40:583. [Google Scholar]
- 15.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 16.Wrensch MR, Minn Y, Bondy M. Epidemiology. In: Bernstein M, Berger M, editors. Neuro-oncology, The Essentials. New York: Thieme Medical Publishers; 2000. pp. 2–17. [Google Scholar]
- 17.Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]