Abstract
Chemoprevention by naturally occurring agents is a newer dimension in the management of neoplasia, including skin cancer. Solar ultraviolet (UV) radiation is the major cause of skin cancer. We recently demonstrated that resveratrol (3,5,4′-trihydroxystilbene), a polyphenolic antioxidant found in grapes and red wine, imparts protection from UVB-mediated cutaneous damages in SKH-1 hairless mice. The mechanism of action of resveratrol is not clearly understood. Here, we investigated the involvement of nuclear factor kappa B (NF-κB), which is known to play a critical role in skin biology and the development of skin cancer, as the mechanism of chemoprevention of UV damage by resveratrol. In the normal human epidermal keratinocytes, resveratrol blocked UVB-mediated (40 mJ/cm2) activation of NF-κB in a dose-dependent (5, 10, and 25 µM resveratrol for 24 hours) as well as time-dependent (5 µM resveratrol for 12, 24, and 48 hours) fashion. Resveratrol treatment of keratinocytes also inhibited UVB-mediated 1) phosphorylation and degradation of IκBα, and 2) activation of IKKα. We suggest that NF-κB pathway plays a critical role in the chemopreventive effects of resveratrol against the adverse effects of UV radiation including photocarcinogenesis.
Keywords: resveratrol, skin cancer, NF-κB, ultraviolet, keratinocyte
Introduction
In the US, nonmelanoma skin cancer, which includes basal and squamous cell carcinoma, is the most frequently diagnosed form of cancer accounting for nearly half of all cancer types [1]. According to an estimate, more than a million new cases of skin cancers are diagnosed annually in the US [1]. The epidemiological studies have shown that there is an increased risk of several other lethal cancer types in the individuals with a history of skin cancer [2]. Therefore, it is warranted to intensify our efforts for the development of novel approaches for prevention and therapy of this cancer type.
Chemoprevention by naturally occurring compounds (at nontoxic dose) appears to be a potential strategy for the management of neoplasia [3–5]. The expanded horizon of cancer chemoprevention also includes the chemotherapy of precancerous lesions [5]. Our recent work has demonstrated that a topical application of resveratrol (trans-3,4′,5-trihydroxystilbene) to SKH-1 hairless mice results in significant inhibitions of ultraviolet (UV) B-mediated 1) skin edema, 2) inflammation, 3) cyclooxygenase (COX) and ornithine decarboxylase (ODC) induction, and 4) generation of hydrogen peroxide (H2O2) and lipid peroxidation in the skin [6]. However, the mechanism of the action of resveratrol is not clearly understood.
The nuclear transcription factor nuclear factor kappa B (NF-κB; a redox-sensitive transcriptional factor) is known to play a critical role in the pathogenesis of cancer and many other pathological conditions [7–11], inasmuch as it is being extensively investigated as a potential target of anticancer drug design [11]. In fact, in the skin, NF-κB plays a particularly central role in epidermal biology (see Ref. [12]). It is believed that, perpetually subjected to the harmful UV rays of the sun, the proliferative cells of the epidermis may rely on NF-κB activation for protection and survival (see Ref. [12]). This study was designed to investigate our hypothesis that NF-κB plays a critical role in the chemoprevention of UV damages imparted by resveratrol.
Thus, the genesis and rationale for this work are based on three important facts: 1) solar UV radiation, which is known to cause the generation of many reactive oxygen species (ROS), is the major cause of skin cancer and many other skin-related hyperproliferative disorders such as actinic keratoses that are regarded to be the precursor of neoplastic condition [13,14]; 2) the nuclear transcription factor NF-κB is known to play key role in skin biology and development of cancer [12]; and 3) resveratrol (Figure 1) — a naturally occurring polyphenolic phytoalexin found in grapes, red wine, peanuts, mulberries, and fruits — is an exceptionally strong antioxidant that has shown promise for the management of certain cancer types including skin cancer [15–17]. Further, our recent studies have shown that resveratrol imparts prevention from the damages caused by UVB radiation in a mouse model [6].
Figure 1.
Chemical structure of trans-resveratrol.
We employed the normal human epidermal keratinocytes for our present work. Our choice of cells is based on the fact that cancer begins in normal cells and, therefore, the chemoprevention studies should be conducted in normal cells. Our data demonstrated that resveratrol inhibits UVB-mediated activation of NF-κB pathway. Therefore, we suggest that resveratrol imparts chemopreventive effects for UV responses that include skin carcinogenesis, at least in part, through the inhibition of NF-κB activation by blocking IκB kinase (IKK) degradation of IκBα. Our study provides a molecular basis for the chemopreventive effects of resveratrol against skin carcinogenesis, in particular, and the process of oncogenesis, in general.
Materials and Methods
Reagents
Resveratrol (>99% pure) was purchased from Sigma (St. Louis, MO). The antibodies against IκBα and phospho-IκBα were purchased New England Biolaboratories, (Beverly, MA). NF-κB/p65 antibody was obtained from Geneka Biotechnology (Montreal, Canada) and IKKα antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The DC Bio-Rad protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA). Electrophoretic mobility shift assay (EMSA) kit was purchased from Geneka Biotechnology. Enzyme-linked immunosorbent assay (ELISA) kit for NF-κB/p65 was purchased from Pierce (Rockford, IL). The precast Tris-glycine gels and chemiluminescence detection kits were obtained from Amersham Life Science (Arlington Heights, IL).
Cell Culture
The normal human epidermal keratinocytes were isolated from neonatal foreskin specimens as described earlier [18], and primary cultures were initiated and maintained in a replicative state with complete, serum-free MCDB 153 keratinocyte medium. Complete medium was supplemented with 0.1 mM calcium, 0.2% (vol/vol) bovine pituitary extract, 10 ng/ml EGF, 5 mg/ml insulin, 5x10-7 M hydrocortisone, 1x10-4 M ethanolamine, and 1x10-4 M phosphoethanolamine, and supplemented with amino acids [19]. Cells were grown until they were 60% to 80% confluent, at which time they were subjected to different treatment protocols.
UVB Source
The UVB sources employed in this study were FS40 lamps (Westinghouse, Pittsburgh, PA), which emit an energy spectrum with high fluency in the UVB region (with a peak at 313 nm). UV radiation that is not normally present in natural solar light was filtered out using Kodacel cellulose film. After filtration with Kodacel film, the majority of the resulting wavelengths of UV radiation were in the range of 290 to 320 nm. The emitted UVB dose was regularly quantitated with an IL-443 phototherapy radiometer (International Light, Newburyport, MA) equipped with an IL SED 240 detector fitted with a W side-angle quartz diffuser and an SC5 280 filter.
Treatment of Cells
Resveratrol (dissolved in DMSO) was used for the treatment of cells. For dose-dependent studies, the cells (70–80% confluent) were treated with resveratrol (0, 5, 10, and 25 µM) for 24 hours. Twenty-four hours following treatment with resveratrol, the cells were washed with phosphate-buffered saline (PBS; pH 7.4) and exposed to UVB (40 mJ/cm2). Six hours following UVB exposure, cells were harvested, lysed, and nuclear and cytosolic (post-nuclear) fractions were prepared. For time-dependent studies, the cells (50–60% confluent) were treated with 5 µM resveratrol for 12, 24, and 48 hours and then washed with PBS and exposed to UVB (40 mJ/cm2). Six hours following UVB exposure, the cells were harvested, and nuclear and cytosolic cellular lysates were prepared.
Preparation of Nuclear and Cytosolic Lysates
For the preparation of nuclear and postnuclear fractions, the cells were washed with cold PBS (pH 7.4) and suspended in 0.4 ml of lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 2.0 µg/ml leupeptin, 2.0 µg/ml aprotinin, 0.5 mg/ml benzamidine) in a microfuge tube. The cells were incubated on ice for 15 minutes, after which 12.5 µl of 10% Nonidet P-40 was added and the contents were mixed on a vortex and then centrifuged for 1 min at 4°C at 14,000g. The supernatant was saved as “postnuclear fraction” and stored at -80°C. The nuclear pellet was resuspended in 25 µl of ice -cold nuclear extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2.0 µg/ml leupeptin, 2.0 µg/ml aprotinin, 0.5 mg/ml benzamidine) and incubated on ice for 30 minutes with intermittent mixing. The tube was centrifuged for 5 minutes at 14,000g at 4°C and the supernatant (nuclear extract) was stored at -80°C. The protein concentration was determined by the DC Bio-Rad assay (Bio-Rad Laboratories) as per the manufacturer's protocol.
Immunoblot Analysis
For Immunoblot analysis, 30 to 50 µg of protein was resolved over 8% to 12% Tris-glycine gels and transferred onto a nitrocellulose membrane. The nonspecific sites were blocked by incubating the blot with 5% nonfat dry milk in buffer (10 mM Tris, 100 mM NaCl, 0.1% Tween-20) for 1 hour at room temperature or overnight at 4°C. The blot was washed with wash buffer (10 mM Tris, 100 mM NaCl, 0.1% Tween-20) for 2x10 minutes and then incubated overnight with appropriate primary antibody specific for the protein to be assessed. The antibodies were used at dilutions specified by the manufacturer. The blot was washed for 2x10 minutes and then incubated with the corresponding secondary antibody HRP conjugate (Amersham Life Science) at 1:2000 dilution for 1 hour at room temperature. The blot was washed for 2x10 and 4x5 minutes, and the protein was detected by chemiluminescence using ECL kit (Amersham Life Science) and autoradiography with XAR-5 film (Amersham Life Science). For every immunoblot, equal loading of protein was confirmed by stripping the blot and reprobing with β-actin antibody.
Electromobility Shift Assay
EMSA for NF-κB/p65 was performed by employing Lightshift™ Chemiluminescent EMSA kit (Pierce) as per the manufacturer's protocol. Briefly, to start with, DNA was biotin-labeled using the Biotin 3′ end labeling kit (Pierce). In a 50-µl reaction buffer in a microfuge tube, 5 pmol of double-stranded NF-κB oligonucleotide 5′-AGTTGAGGG GACTTTCCCAGGC-3′; 3′-TCAACTCCCCTGAAAGGGTCCG-5′ was incubated in 10 µl of 5x TdT (terminal deoxynucleotidyl transferase) buffer, 5 µl of 5 µM biotin-N4-CTP, 10 U of diluted TdT, and 25 µl of ultrapure water at 37°C for 30 minutes. The reaction was stopped with 2.5 µl of 0.2 M EDTA. To extract labeled DNA, 50 µl of chloroform:isoamyl alcohol (24:1) was added to each tube followed by centrifugation briefly at 13,000g. The top aqueous phase containing the labeled DNA was removed and saved for binding reactions. Each binding reaction contained 1x binding buffer (100 mM Tris, 500 mM KCl, 10 mM dithiothreitol, pH 7.5), 2.5% glycerol, 5 mM MgCl2, 50 ng/µl poly (dI-dC), 0.05% NP-40, 2 to 3 µl of nuclear extract, and 20 to 50 fmol of biotin end-labeled target DNA. Binding reactions were incubated at room temperature for 20 minutes. To this reaction mixture was added 5 µl of 5x loading buffer, and the content was subjected to gel electrophoresis on a native polyacrylamide gel and transferred to a nylon membrane. When the transfer was complete, DNA was cross-linked to the membrane at 120 mJ/cm2 using a UV cross-linker equipped with 254-nm bulbs. The biotin end-labeled DNA was detected using streptavidin-horseradish peroxidase conjugate and a chemiluminescent substrate. The membrane was exposed to X-ray film (XAR-5; Amersham Life Science) and developed with a Kodak film processor.
ELISA for NF-κB/p65
For the detection of NF-κB/p65 by ELISA, we employed commercially available Trans-AM kit (Active Motif, Carlsbad, CA) using the vendor's protocol. The assay system uses an oligonucleotide containing NF-κB consensus site (5′-GGGACTTTCC-3′) that binds to the nuclear extract and can detect NF-κB, which can recognize an epitope on activated p65 bound to its target DNA. In the absence of the competitive binding with the wild-type or mutated consensus oligonucleotide, 30 µl of binding buffer was added to each well in duplicate. Alternatively, 30 µl of binding buffer containing 20 pmol (2 µl) of appropriate oligonucleotide, in duplicate, was added to corresponding well. Ten-micrograms of protein from the nuclear lysate of each sample was diluted in 20 µl of lysis buffer and added to each well. For positive control, 20 µl of lysis buffer containing 1 µl of control cell extract was added per well. For blank, 20 µl of lysis buffer per well was used. The plate was covered with a sealer and incubated for 1 hour at room temperature with mild agitation (100 rpm on a rocking platform). Each well was washed three times with 200 µl of 1x wash buffer. One hundred microliters of diluted primary antibody (1:1000 dilution in 1x antibody-binding buffer) was added to each well and incubated at room temperature for 1 hour without agitation. The wells were washed again three times with 1x wash buffer. Then, 100 µl of diluted HRP conjugate antibody (1:1000 dilution in 1 x antibody binding buffer) was added to each well and incubated for 1 hour. The wells were again washed four times with 1x wash buffer followed by an addition of 100 µl of developing solution. The content was incubated for 5 minutes at room temperature, which developed a blue color in the samples and positive control wells. One hundred microliters of stop solution was added to each well and the absorbance was read at 450 nm.
Results and Discussion
In the recent past, chemoprevention by naturally occurring nontoxic agents found in the diets and beverages has been appreciated as a potential strategy for the management of neoplasia including skin cancer [3–5]. Resveratrol (Figure 1) is a polyphenolic compound found in a variety of fruits and vegetables and is abundant in grapes and red wine. Some epidemiological studies have suggested the cardioprotective and chemopreventive properties of resveratrol (see Ref. [1]). The lower risk of heart diseases and cancer in certain populations, e.g., the French and the Greek, has been linked with a regular consumption of red wine (rich in resveratrol; concentration 10–20 µM), a phenomenon dubbed as French paradox [20–25]. Resveratrol has been shown to possess strong antiproliferative and anti-inflammatory properties in some assay systems [17]. Studies have shown that resveratrol imparts skin cancer chemopreventive action by inhibiting cellular events at all the three stages (initiation, promotion, and progression) of the process of carcinogenesis [16]. Jang et al. [16], in the year 1997, have shown that topical application of resveratrol affords substantial protection against chemically induced skin carcinogenesis in CD-1 mice. Our recent studies have shown that resveratrol imparts prevention of the UVB radiation-mediated damages, which are regarded to be critical in the development of skin cancer, in the SKH-1 hairless mouse skin [6]. How resveratrol exerts its chemopreventive effects is only partially understood.
Because the inflammatory, growth-modulatory, and oncogenic effects of many chemicals are mediated through NF-κB [7–11], we hypothesized that the inhibition of NF-κB pathway is responsible for the chemopreventive effects of resveratrol against UVB-mediated damages in skin including skin carcinogenesis. Many lines of evidence suggest in favor of this possibility. For example, a variety of agents, which promote tumorigenesis (such as UV radiation, phorbol ester, okadaic acid, and TNF-α), are known to activate NF-κB (see Refs. [22,26]). It is also important to mention here that resveratrol has been shown to block the activation of NF-κB in certain cell types [22]. Further, several genes that are involved in oncogenesis, tumor metastasis, and inflammation are regulated by NF-κB [22,26]. Studies have also demonstrated a critical role for NF-κB in cellular transformation [27].
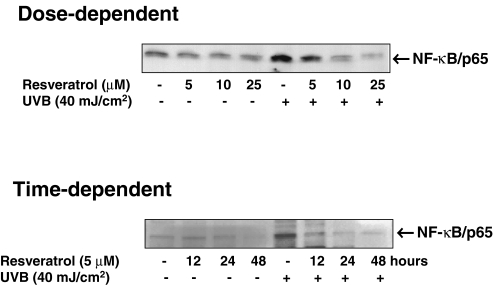
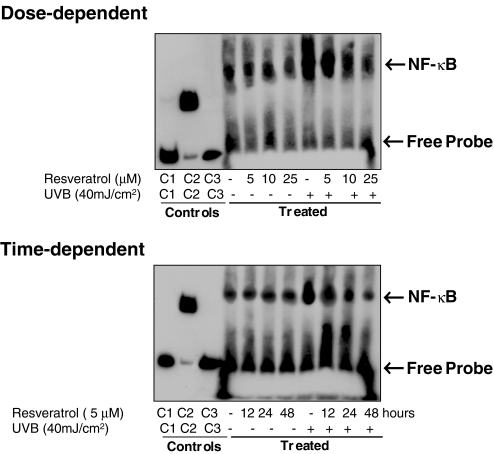
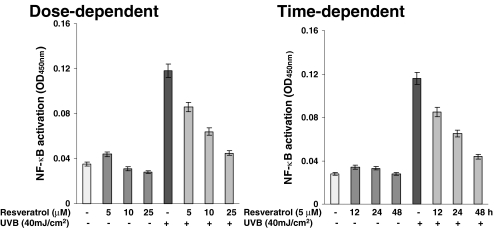
To start with our experimental strategy, in dose- as well as time-dependent protocols, we first evaluated the effect of resveratrol on UVB-mediated modulation in NF-κB. We used multiple methods to achieve our goal. As shown by the data in Figure 2, the immunoblot analysis of the nuclear cell lysates (obtained from the treated and control cells) clearly demonstrated that a 40-mJ/cm2 (a near physiological dose) dose of UVB radiation results is a significant activation of NF-κB/p65. A prior treatment of the keratinocytes with resveratrol resulted in a significant dose-dependent (5, 10, and 25 µM for 24 hours) as well as time-dependent (12, 24, and 48 hours of treatment with 5 µM resveratrol) inhibition of NF-κB activation. Similar results were obtained when we measured the effect of resveratrol on UVB-mediated modulations in keratinocyte employing EMSA (Figure 3) and ELISA (Figure 4). This is an important information because NF-κB is a redox-sensitive transcriptional factor [28,29], which is activated in response to a change in the redox status of the cells (in favor of oxidants) as a result of an oxidative stress condition. UVB exposure to the skin is known to produce oxidative stress in the cells [30]. Our recent studies have shown that in mouse skin, UVB exposure results in the generation of H2O2 and lipid peroxides (LPOs); and the pretreatment of skin with resveratrol significantly prevents damages to the skin [6]. This study, together with our present observations, suggests that resveratrol imparts chemoprevention of UVB-mediated damages to the skin through inhibition of NF-κB activation and its subsequent nuclear translocation.
Figure 2.
Effect of resveratrol on UVB-mediated activation of NF-κB/p65: immunoblot analysis. The normal human keratinocytes were treated with resveratrol (5, 10, and 25 µM for 24 hours for dose-dependent studies or 5 µM for 12, 24, and 48 hours for time-dependent studies) after which the cells were washed and exposed to UVB radiation in PBS. Six hours following UVB exposure, the nuclear lysates were prepared. The immunoblot analysis was performed for NF-κB/p65 using appropriate antibodies as described under Materials and Methods. For every immunoblot, equal loading of protein was confirmed by stripping the blot and reprobing with β-actin antibody (data not shown). The data shown here are from representative experiment repeated four times with similar results.
Figure 3.
Effect of resveratrol on UVB-induced activation of NF-κB: EMSA. The normal human keratinocytes were treated with resveratrol (5, 10, and 25 µM for 24 hours for dose-dependent studies or 5 µM for 12, 24, and 48 hours for time-dependent studies) after which the cells were washed and exposed to UVB radiation in PBS. Six hours following UVB exposure, the nuclear lysates were prepared. The EMSA was performed as detailed under Materials and Methods. C1, C2 and C3 represent controls; biotin-EBNA control DNA, biotin-EBNA control DNA+EBNA extract, biotin-EBNA control DNA+EBNA extract +200-fold molar excess of unlabeled EBNA DNA, respectively. The data shown here are from representative experiment repeated three times with similar results.
Figure 4.
Effect of resveratrol on UVB-induced activation of NF-κB/p65: ELISA. The normal human keratinocytes were treated with resveratrol (5, 10, and 25 µM for 24 hours for dose-dependent studies or 5 µM for 12, 24, and 48 hours for time-dependent studies) after which the cells were washed and exposed to UVB radiation in PBS. Six hours following UVB exposure, the nuclear lysates were prepared for ELISA. The effect on NF-κB/p65 was evaluated using ELISA as detailed in Materials and Methods. The data are expressed as mean±SEM from four experiments, conducted at least in triplicate.
Currently, NF-κB is regarded as a target for anticancer drug design [11]. It is important to mention here that NF-κB plays a critical role in epidermal biology [12]. It is believed that, perpetually subjected to the harmful UVB exposure, the proliferative cells of the epidermis may rely on NF-κB activation for protection and survival (Ref. [12] and references therein). Recently, gain or loss of function studies in mice suggest an equally important role in balancing growth and differentiation in the epidermis (see Ref. [12]). Mice that are null for each of the Rel family members have been generated. The p65 single and double null mice die by E16 at a time when epidermal stratification and differentiation are still developing, suggesting a connection of this targeting event with epidermal differentiation (see Ref. [12]).
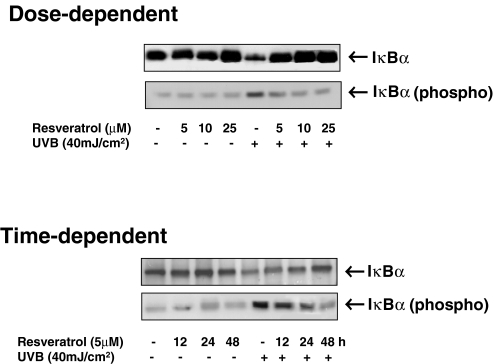
It is well known that in a living cell, NF-κB is present in the cytoplasm in the form of a complex with its inhibitory protein IκBα [7–11]. This complex has been shown to primarily exist in an inactive stage, and upon activation, IκBα undergoes a phosphorylative degradation through an ubiquitin-dependent pathway, which results in a translocation of NF-κB to nucleus [7–11]. In the nucleus, NF-κB activates a variety of genes responsible for different physiological functions [7–11]. In the next series of experiments, we evaluated the effect of resveratrol on UVB exposure-mediated modulation in IκBα in normal human keratinocytes. As shown by the immunoblot analysis (Figure 5), UVB exposure (40 mJ/cm2) to the keratinocytes was found to result in a significant decrease in the level of IκBα in the cytosol (postnuclear cell lysate), and pretreatment of keratinocytes with resveratrol resulted in a dose- as well as time-dependent restoration of IκBα degradation. IκBα (a 35- to 37-kDa protein) is the most widely studied member of the IκB family of proteins that is known to bind to the p65 subunit of p50–p65 heterocomplex through ankyrin repeats [31,32]. The phosphorylative degradation, through the phosphorylation at serine-32 of IκBα, is a major mechanism of NF-κB activation [33]. Therefore, employing a phospho-specific antibody that was raised against a peptide corresponding to a short amino acid sequence containing phosphorylated serine-32 of IκBα of human origin, we studied the effect of resveratrol UVB-mediated modulations on serine-32 phorphorylation of IκBα. Our results (Figure 5) clearly showed that UVB exposure of the keratinocytes results in increased phosphorylation of IκBα at serine-32, whereas resveratrol pretreatment results in a significant dose- and time-dependent inhibition of the phosphorylation.
Figure 5.
Effect of resveratrol on UVB-induced degradation and phosphorylation of IκBα. The normal human keratinocytes were treated with resveratrol (5, 10, and 25 µM for 24 hours for dose-dependent studies or 5 µM for 12, 24, and 48 hours for time-dependent studies) after which the cells were washed and exposed to UVB radiation in PBS. Six hours following UVB exposure, the postnuclear lysates (cytosol) were prepared. The immunoblot analysis was performed for IκBα or phospho-IκBα using appropriate antibodies as described under Materials and Methods. For every immunoblot, equal loading of protein was confirmed by stripping the blot and reprobing with β-actin antibody (data not shown). The data shown here are from representative experiment repeated four times with similar results.
IκB is clearly an important signaling molecule in skin biology. Mice null for IκB are seemingly normal at birth, but they eventually runt and develop dermatitis (see Ref. [12]). Marked alterations in epidermal morphology include excessive proliferation of basal keratinocytes and a paucity of keratohyalin granules that typify the granular layer, a late-stage feature of terminal differentiation. Additionally, keratin 14, a marker of the proliferative compartment of the epidermis, and keratin 16 (typically induced in a variety of hyperproliferative skin disorders) are expressed in the suprabasal compartment of IκBα-/- epidermis, normally reserved for terminally differentiating cells (see Ref. [12]). Thus, our studies showing that resveratrol pretreatment to the keratinocytes causes the restoration of UVB-mediated phosphorylative degradation of IκBα is an important information, which implies that this naturally occurring agent may provide protection from cutaneous damages including skin cancer.
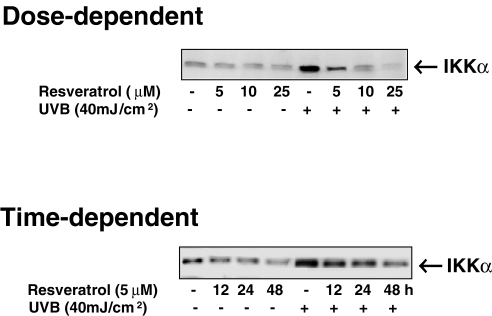
In fact, numerous extracellular stimuli can activate NF-κB through signaling pathways, which activate an IKK complex [34] that phosphorylates IκB on serine-32 and serine-36. The phosphorylation of IκB leads to its ubiquitination and ultimate degradation by the proteasome [7–12], allowing NF-κB to translocate to the nucleus where it activates the expression of genes. Activation of the NF-κB/Rel family of transcription factors regulates the expression of genes that participate in pathways involving inflammation, cell proliferation, and apoptosis [7–12], including the inflammatory mediators such as COX-2 [35]. Because our studies have shown that resveratrol blocks UVB-mediated activation of NF-κB in keratinocytes and that this effect is mediated through a phosphorylative degradation of IκBα, we asked whether or not IKK plays a role in this process. As shown in Figure 6, our data demonstrated that resveratrol indeed inhibits the UVB-mediated upregulation in the levels of IKK, in a dose- as well as time-dependent fashion. IKK is the key regulatory complex required for NF-κB activation of gene transcription [34].
Figure 6.
Effect of resveratrol on UVB-induced activation of IKKα. The normal human keratinocytes were treated with resveratrol (5, 10, and 25 µM for 24 hours for dose-dependent studies or 5 µM for 12, 24, and 48 hours for time-dependent studies) after which the cells were washed and exposed to UVB radiation in PBS. Six hours following UVB exposure, the postnuclear lysates (cytosol) were prepared. The immunoblot analysis was performed using appropriate antibodies as described under Materials and Methods. For every immunoblot, equal loading of protein was confirmed by stripping the blot and reprobing with β-actin antibody (data not shown). The data shown here are from representative experiments repeated four times with similar results.
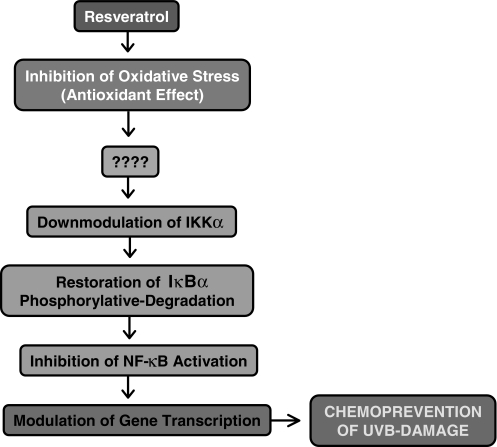
Taken together, our study suggests that NF-κB pathway plays a critical role in the chemopreventive effects of resveratrol against the adverse effects of UV radiation including photocarcinogenesis. A simplified explanation of the mechanism of resveratrol action is given in Figure 7. Thus, our results, for the first time, provide a molecular basis to explain the photochemopreventive properties of resveratrol. The upstream molecular target of resveratrol action is not known at this time. However, the involvement of NF-κB-inducing kinase or MEKK1 is an intriguing possibility in this direction.
Figure 7.
Schematic representation of the mechanism of the chemopreventive effects of resveratrol against UVB-mediated damages.
Footnotes
Part of this work was conducted at the Department of Dermatology, Case Western Reserve University and the Research Institute of University Hospitals of Cleveland, 11100 Euclid Avenue, Cleveland, OH 44106, USA. This work, in part, was supported by US Public Health Services grants RO3 CA 98368 and RO3 CA 89723.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Kahn HS, Tatham LM, Patel AV, Thun MJ, Heath CW. Increased cancer mortality following a history of non melanoma skin cancer. JAMA. 1998;280:910–912. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad N, Mukhtar H. Cutaneous photochemoprevention by green tea: a brief review. Skin Pharmacol Appl Skin Physiol. 2001;14:69–76. doi: 10.1159/000056336. [DOI] [PubMed] [Google Scholar]
- 4.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci. 2002;7:d784–d792. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999;57:78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x. [DOI] [PubMed] [Google Scholar]
- 6.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2002 doi: 10.1016/s0041-008x(02)00014-5. (In Press) [DOI] [PubMed] [Google Scholar]
- 7.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 9.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 10.Neumann M, Marienfeld R, Serfling E. Rel/NF-kB transcription factors and cancer: oncogenesis by dysregulated transcription. Int J Oncol. 1997;11:1335–1347. doi: 10.3892/ijo.11.6.1335. [DOI] [PubMed] [Google Scholar]
- 11.Waddick KG, Uckun FM. Innovative treatment programs against cancer: II. Nuclear factor-kappaB (NF-kappaB) as a molecular target. Biochem Pharmacol. 1999;57:9–17. doi: 10.1016/s0006-2952(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman CK, Fuchs E. It's got you covered. NF-kappaB in the epidermis. J Cell Biol. 2000;149:999–1004. doi: 10.1083/jcb.149.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dore JF, Pedeux R, Boniol M, Chignol MC, Autier P. Intermediate-effect biomarkers in prevention of skin cancer. IARC Sci Publ. 2001;154:81–91. [PubMed] [Google Scholar]
- 14.Black HS, deGruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, deFabo EC, Ullrich SE, Tyrrell RM. Photocarcinogenesis: and overview. J Photochem Photobiol B Biol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 15.Cadenas S, Barja G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med. 1999;26:1531–1537. doi: 10.1016/s0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 16.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 17.Savouret JF, Quesne M. Resveratrol and cancer: a review. Biomed Pharmacother. 2002;56:84–87. doi: 10.1016/s0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 18.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 19.Wille JJ, Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984;121:31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- 20.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent reservation, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 21.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 22.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 23.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 24.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the “French paradox”? Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg DM, Hahn SE, Parkes JG. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chim Acta. 1995;237:155–187. doi: 10.1016/0009-8981(95)06069-p. [DOI] [PubMed] [Google Scholar]
- 26.Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- 27.Foo SY, Nolan GP. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 28.Ginn-Pease ME, Whisler RL. Redox signals and NF-kappaB activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 29.Hutter D, Greene JJ. Influence of the cellular redox state on NF-kappaB-regulated gene expression. J Cell Physiol. 2000;183:45–52. doi: 10.1002/(SICI)1097-4652(200004)183:1<45::AID-JCP6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Black HS, deGruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, deFabo EC, Ullrich SE, Tyrrell RM. Photocarcinogenesis: and overview. J Photochem Photobiol B Biol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa] B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 32.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 33.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 34.DiDonato JA. IKK alpha on center stage. Sci STKE. 2001;97(PE1):1–4. doi: 10.1126/stke.2001.97.pe1. [DOI] [PubMed] [Google Scholar]
- 35.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001:480–481. 243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]