Abstract
Either ribavirin (RBV) or cyclophosphamide (CY) can shift an immune response from Th2 toward a Th1 cytokine profile. CY is used in this role in various current cancer immunotherapy attempts but with mixed success. More potent and reliable immunoadjuvants and Th1 response biasing methods are needed. RBV is used today mainly to augment interferon-alpha treatment of hepatitis C. RBV shifts an immune response from Th2 toward Th1 more effectively than CY and may be a safe and useful adjuvant for current cancer immunotherapeutic efforts. RBV is thought to act by inhibition of tetrahydrobiopterin synthesis. Tetrahydrobiopterin is an essential cofactor for all known isoforms of nitric oxide synthase. Lowered nitric oxide favors Th1 development as high levels favor Th2 weighting.
Keywords: adjuvant, cancer immunotherapy, hepatitis C, inflammation, interferon-alpha, lymphocyte, macrophage, nitric oxide
Introduction
This paper reviews some aspects of ribavirin (RBV) that may be of particular interest to cancer immunologists. It leads to several surprising conclusions. As a help to understanding this notion, a brief prelude reviewing the use of cyclophosphamide (CY) to increase tissue destructive immune responses will be presented. It will be shown that there are mechanistic similarities between RBV and CY in their shaping of immune responses.
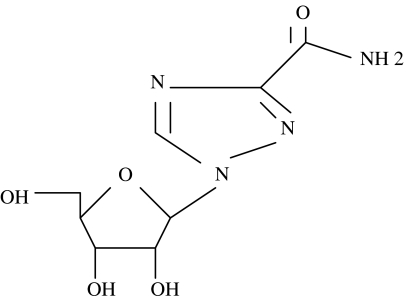
RBV is a 244Da guanosine analogue depicted in Figure 1 that is considered a broad-spectrum anti-viral agent with activity against both DNA and RNA viruses. RBV inhibits inosinic acid dehydrogenase [1], and consequently can reduce lymphocyte guanosine pools [1]. RBV's oral availability is good; it is relatively well tolerated with a half-life of 298 hours. Steady state is seen clinically after several weeks of use. The major side effect is hemolytic anemia, although not all of those so affected need stop treatment. Induction of mood lability and depression are occasionally a problem. RBV doubles sustained viral clearance rates to interferon-alpha (IFN-alpha) treatment of chronic hepatitis C infection (HCV) in humans.
Figure 1.
Chemical structure of ribavirin: 244 Da, terminal half-life in humans, 298 hours.
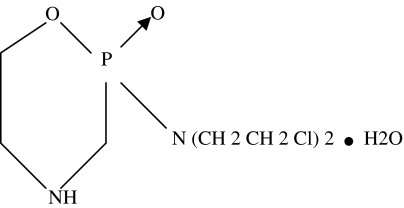
CY, depicted in Figure 2, is a 279Da pro-drug alkylating agent used widely in cytotoxic, cytoablative treatment attempts for several cancers [2]. It is thought to work by creating interstrand cross links in DNA resulting in cell death, particularly in rapidly dividing cells. Consequently, CY is profoundly immunosuppressive to both cell-mediated and antibody responses [2]. Given as a single much lower dose than that used in cytoablative cancer treatments, CY can show an immune response enhancing effect. CY is used to enhance the evoked immune response in several past and current experimental cancer immunotherapy attempts.
Figure 2.
Chemical structure of cyclophosphamide, 279 Da.
This paper reviews data indicating that as immunization adjuvant, both RBV and CY can under certain circumstances and by different mechanisms, similarly shape an immune response toward one more effective in killing malignant, infected, or otherwise antigenetically altered host cells. The data indicate that RBV may be safer and more potent and reliable than CY in this regard and should be tried in augmentation of current cancer immunotherapy attempts.
Lymphocytes, Th1 and Th2
CD4+ cells (T helper lymphocytes, Th) interact with dendritic cells (usually monocyte lineage cells) most commonly within the T lymphocyte-rich areas of the lymph node. It is within the lymph node that much of the qualitative direction or proportion determination of Th cells takes place, to become a Th1 or Th2 lymphocyte [3]. Th1 lymphocytes secrete IFN-gamma and Il-2 among other cytokines, and Th2 lymphocytes secrete Il-4 and Il-10 among others and principally augment the cell-mediated and humoral immune responses respectively.
Cell-mediated and antibody responses can be promoted by either Th1 or Th2 cells but cell-mediated prominent responses are Th1 associated, Th2 are antibody prominent. Il-2 and IFN-gamma promote strong cell-mediated, cytotoxic responses, and strong macrophage activation with hundred-fold increased NO production and increased microbiocidal qualities particularly suited to killing of Mycobacteria and intracellular organisms like Listeria monocytogenes (Th1/Th2 reviewed in Ref. [3]). Destruction of virus-infected cells, or malignant cells as aimed for in cancer immunotherapies, has been shown to often be Th1 mediated, although not universally so [3]. In addition, elimination of hepatitis C infection and destruction of pancreatic islet beta cells in type I or insulin-dependent diabetes mellitus (IDDM) are predominantly Th1 driven. Th2-driven antigen responses tend to be IgG1, IgE, and mast cell and eosinophil prominent responses more suited to elimination of extracellular organisms, soluble circulating foreign proteins, larger antigen loads such as helminths, and other parasites. Th2 development has elements of a default response; though it is actively promoted by cytokines like Il-4, lower antigen-receptor avidity, larger antigen load, and other influences [3]. Immune responses usually involve both Th1 and Th2 responses — the reviewed data and our discussion of CY and RBV will be focused on response weighting to one or the other.
Cyclophosphamide
Non-obese diabetic mice (NOD) are a strain that spontaneously develop IDDM as early as 15 to 20 weeks of age [4,5]. They manifest an inflammatory lymphocytic infiltrate in the pancreas islets, insulitis, long before overt IDDM. This prediabetic insulitis is mediated predominantly by Th2 lymphocytes [4,5]. At this stage, there is little or no beta cell loss. The chronic Th2 prominent insulitis lasts months to a year. As NOD mice age, the islet infiltrating lymphocytes become more balanced between Th1 and Th2, and beta cell loss rate increases [6]. At the point of massive loss of beta cells and insulin dependence, a predominantly Th1 infiltrate is seen [7]. Beta cells are exquisitely sensitive to destruction by Th1 cytokines such as Il-1 beta, TNF-alpha, and IFN-gamma [8].
A single pulse of low-dose CY will accelerate the above transition, ending typically after 2 weeks post injection with most mice exhibiting full IDDM [8,9]. After the CY pulse, lymphocyte Il-12 gene activation is seen [10] and Il-12 antagonists given post-CY dampen the induced insulitis acceleration [11]. Enhancement of cytotoxic T cell functions after low-dose CY is partially due to CY-mediated impairment of suppressor T cells [12]. CY-accelerated insulitis, like the slower naturally occurring one, is associated with loss of islet Th2 response, islet beta cell destruction being Th1 mediated [7,8]. Th2 polarizing agents prevent or delay CY acceleration of IDDM in NOD mice, and Th2 cytokines Il-10 and Il-4 are protective to the islets by retarding Th2 to Th1 transition [8].
The mechanism by which CY accelerates Th2 to Th1 weighting shift in the insulitis of NOD mice is unclear but IFN-gamma producers (Th1 lymphocytes) seem to be relatively resistant to CY cytotoxic effects [13], conferring selective advantage on that lymphocyte subclass. Il-4 is important to Th2 lymphocyte development. It is produced mainly by lymphocytes that have undergone several divisions (reviewed in Refs. [2,3]). By selective killing of actively dividing lymphocytes, the negative selection creates a relative positive selection of Th1 cells.
Th1 weighting shift induced by CY is being used in attempts to increase effectiveness of cancer immunotherapies meeting with varying success in human malignancy and animal models. Single low-dose CY has given a weighting shift from Il-10 (Th2) to increased Il-2 and IFN-gamma (Th1) responses in a murine lymphoma model with resultant decreased metastasis [14]. Such preimmunization low-dose CY pulse has also shown benefit in experimental murine models of melanoma [15], plasmacytoma [16], fibrosarcoma [17], and sarcoma [18]. Th1-mediated, macrophage-effected elimination of experimental murine tumors can be seen post-CY plus Il-12 but with neither used alone [19]. Four-fold peritumoral TNF-alpha and a Th2 to Th1 shift was documented in the fibrosarcoma model when lipopolysaccharide was added to CY pulse [17].
In human breast and other cancers, low-dose CY has shown some immunological and clinical benefit with immunization with a proprietary vaccine (Theratope, an STn-keyhole limpet hemocyanin conjugate given with a proprietary adjuvant Detox) [20,21]. Theratope with CY augmentation is currently in two large phase 3 trials for breast and colon cancer. However, there have been failures to show benefit of low-dose CY: Human trials in breast and colon cancer of MUC-1 conjugated to mannan have shown no clinical or immunological effects of low-dose CY [22], nor was CY of clinical or immunological benefit in a human melanoma vaccine trial [23]. In addition, of concern are observations of pulse CY with methylpresnisolone being used successfully used to induce Th1 to Th2 shift with increased Il-4 in humans [24].
Conclusions about current clinical use of single low-dose CY before cancer immunotherapies: CY can in some situations shift an immune response from what would have been Th2 weighted to a Th1-weighted response but there have been clinical failures to achieve this. Th1 biasing attribute of prevaccination CY can be used with therapeutic cancer immunization attempts to increase tissue destructive immune responses but constructive responses are unreliably achieved. Under some circumstances, depending on dose, timing with respect to antigen and others, CY can be tolerogenic, or Th2 shifting, or generally immunosuppressive, particularly if given after antigen. People with cancer have presumably already been presented with the relevant antigen at the time of our immunization efforts so these threats are real. There is no immunological reason preventing concurrent Th1 biasing by CY and tolerogenizing or immunosuppressive effects all occurring within the same host, the net effect being whichever predominates. Even should Th1 predominate, it would be expected to be weakened by the tolerogenic or Th2 biasing component. Better Th1 biasing treatments are needed.
Ribavirin
It is variously estimated that 1% to 3% of humanity is currently chronically infected with hepatitis C virus, HCV, a single-stranded positive sense RNA virus of the Flaviviridae family [25]. The replication time is 24 hours and an average total body burden is 1012 copies. About a third of those infected eventually die of cirrhosis or hepatocellular carcinoma because of their infection. Less than 20% of those acutely infected clear the virus, the rest becoming chronically infected for decades, a significant fraction of whom die of HCV-related illnesses [25]. HCV infection is also a major cause worldwide of disability due to chronic mild encephalopathy, chronic pain, fatigue, and malaise [26].
There are four immunological curiosities about HCV infection, currently unexplained:
HCV is not itself hepatocellular toxic. Florid replication can occur for years in livers of near-normal histology.
The observed steady-state HCV numbers over years with a replication time of 24 hours implies viral clearance time of 24 hours. HCV is therefore constantly being cleared and produced. Several HCV antigens are recognized as foreign and both antibody and cell-mediated immune responses can be demonstrated to these antigens yet established infection is rarely cleared without treatment.
HCV does not generally replicate in hepatocyte cultures in vitro.
RBV as monotherapy has no measurable effect on viral numbers in infected patients [27–31]. Liver transaminases are lowered during RBV monotherapy of HCV but usually rise to pretreatment values when RBV is stopped [29,31]. IFN-alpha monotherapy over a 1-year period results in about 20% of patients showing sustained clearance of HCV, though more will have temporary reductions in viral numbers during treatment [25,32]. Addition of RBV (1000 to 1200 mg/day p.o.) to IFN-alpha raises sustained clearance to 40% [32].
These four curiosities would be explained if the theory that there is some aspect of the inflammatory, immune, or other host response to HCV or HCV-infected hepatocytes that is required for replication were correct.
The current theory on how HCV avoids clearance by the immune system is that its high genetic variability, with consequent antigenic drifting results in immune system clearance of one antigenic variant as the next comes along [25]. That there is high genetic variability and antigenic drift during an HCV infection seems to be proven but there is no evidence that the immune system completely clears any specific HCV subpopulation during a chronic infection, and there is good evidence that the drift does not encompass T-cell recognized epitopes [28]. No significant effect of RBV on HCV amino acid sequence evolution was seen [28].
RBV mediates immune response weighting shift, Th2 toward Th1. How it does so is unclear. Low-dose (1 µg per Petri dish) RBV increases in vitro murine antibody response to sheep erythrocytes [33] by suppression of T suppressor subpopulations. In experimental immunization of mice to hepatitis B antigens, 25 times the amounts of Th1 cytokines Il-2 and IFN-gamma are seen in mice also receiving RBV [27]. Also noted in that murine study was decreased IgG1 (which is enhanced by Th2 cells) and increased IgG2 (enhanced by Th1 cells) after RBV. Th2 to Th1 skewing was observed in a murine hepatitis model both in vivo and in vitro after RBV or its L enantiomer [34]. RBV and its analogues are being explored to bias the immune system from Th2 toward Th1 in human disease treatment [34,35]. Because nondividing lymphocytes can meet their guanosine requirements largely by the salvage pathway but stimulated lymphocytes must meet these requirements by de novo synthesis [36], Th2 biased lymphocytes would be selected against during RBV treatment because they must pass through several cell divisions and have consequent exogenous guanosine requirements before taking on Th2 qualities [3].
The Nitric Oxide Connection
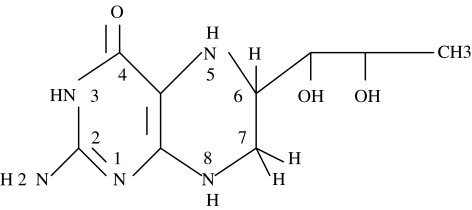
NO (.N == O) is a highly reactive free radical synthesized in vessel endothelium, immune cells, brain, and other tissues. NO is a killer. Among the many paths the immune system uses to kill cells, NO mediated cell death figures prominently. Although essential to host defense, at some point of increasing NO, toxicity to host lymphocytes and the NO synthesizing macrophages themselves becomes evident [37]. NO is synthesized by enzymatic cleavage of the guanido nitrogen of arginine by nitric oxide synthase, NOS, that exists in three different isoforms; constitutive (cNOS or NOS 3), inducible (iNOS or NOS 2), and neural (nNOS or NOS 1) [37]. TNF-alpha, Il-1 beta, and immune cell activation generally give rise to increasing amounts of iNOS mRNA followed by several hundred times the NO output of the given cell. All three isoforms of NOS have an absolute requirement for tetrahydrobiopterin, BH4 (Figure 3) to function catalytically in NO synthesis [37]. BH4 is synthesized in mammalian cells from guanosine 5′-triphosphate, GTP. Since RBV lowers intracellular guanosine pools, BH4 levels drop, and with it the ability to generate NO [37,38].
Figure 3.
5,6,7,8-Tetrahydrobiopterin, BH4. The pteridine nucleus is numbered in standard fashion.
iNOS is considerably upregulated in liver mononuclear cells during chronic infection with HCV [39–43]. This iNOS upregulation is diffuse, seen evenly throughout the liver even when fibrosis scores are low and antibody to HCV antigen immunostains few isolated and scattered islands of hepatocytes [43]. This is prima facie evidence that HCV is doing something to upregulate NO production beyond its immediate replicative microenvironment. Current IFN-alpha treatments of HCV actually further upregulate this already elevated intrahepatic NOS activity [44,45], thereby potentially increasing, as outlined below, an NO-mediated negative selection pressure on cytotoxic Th1 lymphocytes that are HCV specific.
Further evidence for NO-mediated negative selection pressure on HCV-reactive lymphocytes comes from studies on circulating levels of lipopolysaccharide, LPS, a major component of the cell wall of Gram-negative bacteria (endotoxin is the historical term for LPS). LPS is one of the most potent inducers of NO synthesis known. HCV patients who go on to durably clear their viremia under IFN-alpha plus RBV treatment have lower pretreatment LPS levels than those who fail to clear [46]. In addition, 100% of those to clear have end of treatment undetectable levels of LPS, whereas half of those failing to clear still show moderate LPS levels [46].
The additional 20% of complete sustained responders to RBV-IFN over IFN monotherapy may be attributable to RBV's prevention of IFN-alpha-mediated increase of NO in the milieu of cytotoxic lymphocytes. In the 20% of IFN-alpha monotherapy patients who would have cleared if RBV had been added, high levels of NO have caused death of activated anti-HCV T cells. Such a mechanism was documented by Saio et al. [47] where tumor-infiltrating macrophages synthesized and released NO that was highly proapoptotic to activated antitumor T lymphocytes.
A similar situation has been commonly recognized in parasitology and mycology. High levels of NO can be required for parasite killing but high levels of NO also can kill responding host T lymphocytes. At some point of increasing NO, the latter force predominates and the parasite wins the battle. At somewhat lower doses, the parasite is killed, the host wins. At lower yet levels of NO, the parasite wins again with the failure of NO-mediated parasite killing (Ref. [48] for paracoccidioidomycosis and Ref. [49] for echinococcosis are examples).
Analogous to these examples from parasitology, according to the hypothesis of this paper, HCV upregulates NO synthesis, high NO levels help HCV survive, RBV lowers NO, permitting anti-HCV lymphocyte function to clear HCV-infected cells.
The huge increases of NO seen during overwhelming sepsis contribute to the observed immunosuppression of that state [50]. The excessive NO produced by activated macrophages are seen to inhibit antigen-driven T lymphocyte proliferation in diverse other situations as well [50–53]. IFN-gamma produced by activated Th1 lymphocytes (or in response to therapeutic use of IFN-alpha) induces several hundred-fold increase in NO production by macrophages [53]. High NO levels are preferentially cytotoxic to antigen-responding Th1 lymphocytes compared to Th2 cells [52]. Thus NO, produced by macrophage lineage cells, limits expansion of the antigen-responding T lymphocyte population. In cancer immunotherapies and in HCV, too soon it seems.
The above discussion of RBV and NO goes a long way toward explaining the immunological curiosities of HCV mentioned earlier.
Discussion
Th1-biased immune responses, often sought in current experimental cancer immunotherapy, are thought to be more effective in malignant cell killing than Th2-biased responses. Low-dose CY is used for such biasing in current trials of cancer vaccines in humans but RBV may be more effective in this role. NO contributes to the development and evolution of all three phases of an immune-response initiation, maintenance, and ending. Excessive or inadequate NO levels can impair host defenses. In pathologically high NO states, RBV, by depriving NOS of BH4, causes NO synthetic ability to fall. The brake on evolution of effective anti-HCV or anti-malignant cell immune responses is thereby lessened. The evidence from RBV use in IFN-alpha-based treatment of HCV indicate that RBV may be more effective in this role in cancer immunotherapies than is the currently used CY. Experimental proof of this notion would be checking RBV's ability to accelerate IDDM in NOD mice compared to the well-characterized actions of CY.
RBV could prove useful in other diseases where NO overproduction is pathophysiologically important. For example, all multiple myeloma (MM) cells investigated overexpressed NOS [54]. MM is a bone-eroding malignant expansion of a post-germinal center B lymphocyte clone. Current treatments are rarely curative but IFN-alpha can prolong temporary remission after cytotoxic chemotherapy. If overproduction of NO is part of MM cell's survival strategy as conjectured for HCV, then RBV addition should prolong yet further IFN-alpha remissions in MM.
References
- 1.Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide to proliferating T lymphocytes from healthy humans. J Biol Chem. 1995;270:29682–29689. [PubMed] [Google Scholar]
- 2.Brodsky RA. High dose cyclophosphamide for a plastic anemia and autoimmunity. Curr Opin Oncol. 2002;14:143–146. doi: 10.1097/00001622-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic D, Liu Z, Gause WC. Th1 and Th2 cell commitment during infectious disease. Trends Immunol. 2001;22:450–473. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 4.Martin S, Hibino T, Faust A, Kleemann R, Kolb H. Differential expression of ICAM-1 and LFA-1 versus L-selectin and VCAM-1 in insulitis of NOD mice and association with both Th-1 and Th2 infiltrates. J Autoimmun. 1996;9:637–643. doi: 10.1006/jaut.1996.0083. [DOI] [PubMed] [Google Scholar]
- 5.Rothe H, Faust A, Schade U, Kleemann R, Bosse G, Hibino T, Martin S, Kolb H. Cyclophosphamide treatment of female NOD mice causes enhanced iNOS and IFN-gamma, but not of Il-4. Diabetologia. 1994;37:1154–1158. doi: 10.1007/BF00418380. [DOI] [PubMed] [Google Scholar]
- 6.Faulkner-Jones BE, Dempsey-Collier M, Mandel TE, Harrison LC. Both Th1 and Th2 cytokine mRNA are expressed in NOD mouse pancreas in vivo. Autoimmunity. 1996;23:99–110. doi: 10.3109/08916939608995333. [DOI] [PubMed] [Google Scholar]
- 7.Nitta Y, Kawamoto S, Tashiro F, et al. Il-12 plays a pathologic role at the inflammatory loci in the development of diabetes in NOD mice. J Autoimmunity. 2001;16:97–104. doi: 10.1006/jaut.2000.0469. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Karanam M, Poole CA, Ross JM. Dual label immunohistochemical study of Il-4 and IFN-gamma cells within pancreas of NOD mouse during disease acceleration with cyclophosphamide. Autoimmunity. 2000;32:181–192. doi: 10.3109/08916930008994091. [DOI] [PubMed] [Google Scholar]
- 9.Martin S, van den Engel NK, Vinke A, et al. Dominant role of ICAM-1 in the pathogenesis of autoimmune diabetes in NOD mice. J Autoimmunity. 2001;17:109–117. doi: 10.1006/jaut.2001.0526. [DOI] [PubMed] [Google Scholar]
- 10.Rothe H, Burkart V, Faust A, Kolb H. Il-12 gene expression mediates the accelerating effect of cyclophosphamide in autoimmune disease. Ann N Y Acad Sci. 1996;795:397–399. doi: 10.1111/j.1749-6632.1996.tb52704.x. [DOI] [PubMed] [Google Scholar]
- 11.Trembleau S, Penna G, Gregori S, Gately MK, Adorini L. Deviation of pancreas infiltrating cells to Th2 by Il-12 antagonist administration inhibits autoimmune diabetes. Eur J Immunol. 1997;27:2330–2339. doi: 10.1002/eji.1830270930. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Efraim S. Immunomodulating anticancer alkylating drugs: targets and mechanisms of activity. Curr Drug Targets. 2001;2:197–212. doi: 10.2174/1389450013348597. [DOI] [PubMed] [Google Scholar]
- 13.Ablamunits V, Quintana F, Reshef T, Elias D, Cohen IR. Acceleration of diabetes by cyclophosphamide is associated with an enhanced IFN-gamma secretion pathway. J Autoimmun. 1999;13:383–392. doi: 10.1006/jaut.1999.0331. [DOI] [PubMed] [Google Scholar]
- 14.Matar P, Rozados VR, Gervasoni SLLI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588–596. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Zhang W, Wan T, Yu Y, Tao Q, Wang J. Enhanced antitumor immune responses of Il-2 gene modified tumor vaccine by combination with Il-1 and low dose cyclophosphamide. J Exp Clin Cancer Res. 1999;18:173–179. [PubMed] [Google Scholar]
- 16.Li L, Okino T, Kan N, et al. Analysis of effector cells in tumor bearing mice pre-treated with active specific immunization followed by cyclophosphamide. Biotherapy. 1998;11:223–228. doi: 10.1023/a:1008054611739. [DOI] [PubMed] [Google Scholar]
- 17.Inagawa H, Nishizawa T, Honda T, Nakamoto T, Takagi K, Soma G. Mechanisms by which chemotherapeutic agents augment the antitumor effects of TNF-alpha: involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions. Anticancer Res. 1998;18(5D):3957–3964. [PubMed] [Google Scholar]
- 18.Livingston PO, DeLeo AB, Jones M, Oettgen HF. Comparison of approaches for augmenting the serologic response to the individually specific methylcholanthrene induced sarcoma MethA: pretreatment with cyclophosphamide is most effective. J Immunol. 1983;131:2601–2605. [PubMed] [Google Scholar]
- 19.Tsung K, Meko JB, Tsung YL, Peplinski GR, Norton JA. Immune response against large tumors eradicated by treatment with cyclophosphamide and Il-12. J Immunol. 1998;160:1369–1377. [PubMed] [Google Scholar]
- 20.MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emphasis Tumor Immunol. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of Theratope STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low dose i.v. cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Karanikas V, Thynne G, Mitchell P, et al. Mannan mucin-1 peptide immunization: influence of cyclophosphamide and the route of administration. J Immunol. 2001;24:172–183. [PubMed] [Google Scholar]
- 23.Euhus DM, Gupta RK, Morton DL. Induction of antibodies to a tumor associated antigen by immunization with a whole melanoma cell vaccine. Cancer Immunol Immunother. 1989;29:247–254. doi: 10.1007/BF00199212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DR, Balashov KE, Hafler DA, Khoury SJ, Weiner HL. Immune deviation following pulse cyclophosphamide methylprednisolone treatment of MS, increased Il-4 and associated eosinophilia. Ann Neurol. 1997;42:313–318. doi: 10.1002/ana.410420307. [DOI] [PubMed] [Google Scholar]
- 25.Boyer N, Marcellin P. Pathogenesis, diagnosis and management of hepatitis C. J Hepatol. 2000;32(1 Suppl):98–112. doi: 10.1016/s0168-8278(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 26.Kast RE. Chronic mild encephalopathy of hepatitis C virus: evidence for a common psychiatric illness. Psychiatry Online. 2002 (in press) [Google Scholar]
- 27.Hultgren C, Milich DR, Weiland O, Sallberg M. The antiviral compound ribavirin modulates T1/Th2 subset balance in hepatitis B and C virus specific immune responses. J Gen Virol. 1998;79(Pt 10):2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 28.Querenghi F, Yu Q, Billaud G, Maetens G, Trepo C, Zoulim F. Evolution of hepatitis C virus genome in chronically infected patients receiving ribavirin monotherapy. J Viral Hepatol. 2001;8:120–131. doi: 10.1046/j.1365-2893.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 29.Reichard O, Sonnerborg A, Weiland O. HCV RNA titers prior to, during, and after oral RBV treatment. J Med Virol. 1993;41:99–102. doi: 10.1002/jmv.1890410203. [DOI] [PubMed] [Google Scholar]
- 30.Di Bisceglie AM, Hoofnagle JH, Krawczynski K. Changes in HCV antigen in liver with antiviral therapy. Gastroenterology. 1993;105:858–862. doi: 10.1016/0016-5085(93)90905-r. [DOI] [PubMed] [Google Scholar]
- 31.Reichard O, Andersson J, Schvarcz R, Weiland O. RBV treatment for chronic HCV. Lancet. 1991;337:1058–1061. doi: 10.1016/0140-6736(91)91707-2. [DOI] [PubMed] [Google Scholar]
- 32.McHutchison JG, Shad JA, Gordon SC, et al. Predicting response to initial therapy with IFN plus ribavirin in chronic HCV using serum HCV RNA results during therapy. J Viral Hepat. 2001;8:414–420. doi: 10.1046/j.1365-2893.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 33.Powers CN, Peavy DL, Knight V. Selective inhibition of functional lymphocyte subpopulations by ribavirin. Antimicrob Agents Chemother. 1982;22:108–114. doi: 10.1128/aac.22.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam RC, Ramasamy K, Bard J, Pal B, Lim C, Averett DR. Ribavirin analogue ICN 17261 demonstrates reduced toxicity with retention of both immunomodulatory activity and reduction of hepatitis induced serum alanine transferase levels. Antimicrob Agents Chemother. 2000;44:1276–1283. doi: 10.1128/aac.44.5.1276-1283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy KS, Tam RC, Bard J, Averett DR. Monocyclic L nucleosides with type 1 cytokine inducing activity. J Med Chem. 2000;43:1019–1028. doi: 10.1021/jm9905514. [DOI] [PubMed] [Google Scholar]
- 36.Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide availability to proliferating T lymphocytes from healthy humans. J Biol Chem. 1995;270:29682–29689. [PubMed] [Google Scholar]
- 37.Mills CD. Macrophage arginine metabolism to orinthine/urea or NO/citrulline: a life or death issue. Crit Rev Immunol. 2001;21:399–426. [PubMed] [Google Scholar]
- 38.Hurshman AR, Marletta MA. Reactions catalysed by the heme domain of iNOS synthase: evidence for the involvement of BH4 in electron transfer. Biochemistry. 2002;41:3439–3456. doi: 10.1021/bi012002h. [DOI] [PubMed] [Google Scholar]
- 39.Hokari A, Zeniya M, Esumi H, et al. Detection of serum nitrite and nitrate in primary biliary cirrhosis: possible role of nitric oxide in bile duct injury. J Gastroenterol Hepatol. 2002;17:308–315. doi: 10.1046/j.1440-1746.2002.02689.x. [DOI] [PubMed] [Google Scholar]
- 40.Schweyer S, Mihm S, Radzun HJ, Hartmann H, Fayyazi A. Liver infiltrating T lymphocytes express IFN-gamma and iNOS in HCV infection. Gut. 2000;46:255–259. doi: 10.1136/gut.46.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mihm S, Fayyazi A, Ramadori G. Hepatic expression of iNOS transcripts in chronic HCV infection: relation to hepatic viral load and liver injury. Hepatology. 1997;26:451–458. doi: 10.1002/hep.510260228. [DOI] [PubMed] [Google Scholar]
- 42.Kane JM, III, Shears LL, II, Hierholzer C, Ambs S, Billiar TR, Posner MC. Chronic HCV infection: induction of hepatic NOS and proposed mechanisms for carcinogenesis. J Surg Res. 1997;69:321–324. doi: 10.1006/jsre.1997.5057. [DOI] [PubMed] [Google Scholar]
- 43.Lake-Bakaar G, Sorbi D, Mazzoccoli V. NO and chronic HCV and HIV infections. Dig Dis Sci. 2001;46:1072–1076. doi: 10.1023/a:1010770230422. [DOI] [PubMed] [Google Scholar]
- 44.Sharara AI, Perkins DJ, Misukonis MA, et al. IFN-alpha activation of human blood mononuclear cells in vitro and in vivo for NOS type 2 mRNA and protein expression: possible relationship of induced NOS 2 to the anti-HCV effects of IFN-alpha in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brassard DL, Grace MJ, Bordens RW. IFN-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- 46.Caradonna L, Mastronardi ML, Magrone T, et al. Biological and clinical significance of endotoxinemia in the course of HCV infection. Curr Pharm Des. 2002;8:995–1005. doi: 10.2174/1381612024606983. [DOI] [PubMed] [Google Scholar]
- 47.Saio M, Radoja S, Marino M, Frey AB. Tumor infiltrating macrophages induce apoptosis in activated CD8+ T cells by a mechanism requiring cell contact and mediated by both the cell associated form of TNF and nitric oxide. J Immunol. 2001;167:5583–5593. doi: 10.4049/jimmunol.167.10.5583. [DOI] [PubMed] [Google Scholar]
- 48.Nascimento FR, Calich VL, Rodriguez D, Russo M. Dual role for NO in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J Immunol. 2002;168:4593–4600. doi: 10.4049/jimmunol.168.9.4593. [DOI] [PubMed] [Google Scholar]
- 49.Li WJ, Gottstein B. NO mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology. 1999;97:107–116. doi: 10.1046/j.1365-2567.1999.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright KL, Ward SG. Interactions between phosphatidylinositol 3 kinase and NO: explaining the paradox. Mol Cell Biol Res Commun. 2000;4:137–143. doi: 10.1006/mcbr.2001.0273. [DOI] [PubMed] [Google Scholar]
- 51.Juedes AE, Ruddle NH. Resident and infiltrating CNS APC's regulate the emergence and resolution of EAE. J Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 52.Roozendaal R, Vellenga E, de Jong MA, et al. Resistance of activated Th2 cells to NO induced apoptosis is mediated by gamma glutamyltranspeptidase. Int Immunol. 2001;13:519–528. doi: 10.1093/intimm/13.4.519. [DOI] [PubMed] [Google Scholar]
- 53.van der Veen RC, Dietlin TA, Pen L, Gray JD, Hofman FM. Antigen presentation to Th1 but not Th2 cells by macrophages results in NO production and inhibition of T cell proliferation: IFN-gamma is essential but insufficient. Cell Immunol. 2000;206:125–135. doi: 10.1006/cimm.2000.1741. [DOI] [PubMed] [Google Scholar]
- 54.Mendes RV, Martins AR, de Nucci G, Murad F, Soares FA. Expression of NOS isoforms and nitrotyrosine immunoreactivity by B cell non-Hodgkin's lymphomas and multiple myeloma. Histopathology. 2001;39:172–178. doi: 10.1046/j.1365-2559.2001.01189.x. [DOI] [PubMed] [Google Scholar]