Abstract
WISP-2 mRNA and protein was overexpressed in preneoplastic and cancerous cells of human breast. Statistical analyses show a significant association between WISP-2 expression and estrogen receptor (ER) positivity. In normal breast, the expression was virtually undetected. The studies showed that WISP-2 is an estrogen-induced early response gene in MCF-7 cells and the expression was continuously increased to reach a maximum level at 24 h. The estrogen effect was inhibited by a pure antiestrogen (ICI 182,780). Human mammary epithelial cells, in which WISP-2 expression was undetected or minimally detected, responded to 17β-estradiol by upregulating the WISP-2 gene after transfection with ER-α, providing further evidences that WISP-2 expression is mediated through ER-α. Overexpression of WISP-2 mRNA by estrogen may be accomplished by both transcriptional activation and stabilization. MCF-7 cells exposed to progesterone had a rapid but transient increase in WISP-2 expression, and PR antagonist RU38486 blocked this mRNA induction. In combination with estradiol, progesterone acted as an antagonist inhibiting the expression of WISP-2 mRNA. Moreover, disruption of WISP-2 signaling in MCF-7 cells by use of antisense oligomers caused a significant reduction in tumor cell proliferation. The results are consistent with the conclusion that WISP-2 expression is a requirement for breast tumor cells proliferation.
Keywords: Wnt-1 induced signaling protein, estrogen, progesterone, antisense oligos
Introduction
Breast cancer is the most common cancer in women and the third leading cause of cancer mortality, following lung and stomach cancer [1–3]. The incidence rates of breast cancer in the U.S. have more than doubled in the past three decades [4]. The lifetime risk is 1 in 8. It is estimated that 1.2 million new diagnoses and 500,000 deaths from breast cancer will occur worldwide this year [5–8]. Breast cancer is the most commonly diagnosed cancer in women in America and worldwide. However, treatment of this disease at the advanced stages is usually ineffective. Therefore, identification of the genes involved in the development of breast cancer is a high priority for the medical management of the disease.
The Wnt signal transduction pathway plays a critical role in a variety human cancers, including colon and breast, by modulating multiple oncogenes and tumor suppressor genes [9–12]. Alterations in Wnt signaling pathways have also been consistently implicated in mammary gland tumorigenesis in rodents [9,13,14]. The mouse mammary tumor was potentiated by a Wnt-1 gene when it was activated by a retroviral insertion [13,15–17]. During the transformation, the activated Wnt-1 gene induces several down-stream target genes including Wnt-1 induced signaling/secreted protein-2 (WISP-2), a member of the connective tissue growth factor/cysteine-rich 61/neuroblastoma overexpressed (CCN) family of growth factors [18–20]. Although, a similar viral etiology or Wnt-1 expression has yet to be established for breast cancer in humans [13], our recent studies have demonstrated that the WISP-2 expression, undetected in non-transformed human mammary epithelial cells (HMEC), was upregulated in different human mammary tumor cell lines [21], and its expression was modulated by serum and correlated with the serum-induced tumor cell proliferation [22]. Collectively, these studies suggest that WISP-2 could be an important growth factor for development of breast cancer and more importantly a genetic marker for this disease [21].
To directly test the hypothesis that the WISP-2 is an important growth factor for the development of this disease and could be considered as genetic marker, we determined the WISP-2 expression at mRNA and protein levels in the biopsy samples of breast tumors with different variables. In addition, the impact of this gene on breast tumor cell proliferation was evaluated by blocking its activities and the effects of estrogen and progesterone on WISP-2 expression were determined.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was purchased from Sigma Chemical (St. Louis, MO), FBS was purchased from American Type Culture Collection (Manassas, VA), and reverse transcription-polymerase chain reaction (RT-PCR) kits were purchased from Perkin-Elmer (Foster City, CA). Biolase DNA polymerase was purchased from Bioline USA (Nevada, USA). Digoxigenin (DIG) high prime DNA labeling and detection kit (Cat nos. 1636090 and 1585614) was obtained from Roche Diagnostics GmbH (Indianapolis, IN). Estrogen receptor α (ER-α), progesterone receptor (PR), and P53 monoclonal and polyclonal antibodies were purchased from Lab Vision (Fremont, CA) and Santa Cruz Biotechnology (Santa Cruz, CA). A custom-made WISP-2 polyclonal antibody [22] was used for this study. Antiestrogen (ICI 182,780) and antiprogesterone (RU38486) were purchased from Tocris (Ellsville, MO). All other chemicals from commercial sources were of the highest purity available.
Tissue Samples
Frozen specimens including normal human breast tissues, non-neoplastic breast tissues and primary breast carcinomas were obtained from the Kansas Cancer Institute Tissue Repository Core facilities and Cooperative Human Tissue Network (CHTN, NCI), respectively. Twenty normal frozen tissue specimens were obtained from healthy women, undergoing surgery for breast reduction at the University of Kansas Medical Center. Twenty-four non-neoplastic specimens, confirmed by histopathological examination, were isolated from adjacent invasive carcinoma. Fifty-four primary breast carcinoma specimens included 10 pure invasive carcinoma and 44 invasive carcinomas mixed with preneoplastic lesions (i.e., simple hyperplasia, atypical hyperplasia, and intraductal carcinoma in situ (DCIS)] were analyzed. For H&E stain and immunohistochemistry, part of the frozen specimens were fixed in 4% formalin and embedded in paraffin. The categorization of simple hyperplasia, atypical hyperplasia, ductal carcinoma in situ, and ductal carcinoma was based on the primary pathological diagnosis.
Cell Lines and Culture Conditions
The MCF-7 human breast carcinoma cell line and non-transformed HMEC were used for these studies. The MCF-7 cells were obtained from American Type Tissue Culture Collection (Rockville, MD) and maintained in DMEM (Sigma) containing 10% FBS. ER-α- or vector only-transfected HMEC were a gift from Deborah Zajchowski (Berlex Biosciences, CA) and grown in DFCI-1 medium with 1% FBS as previously described [23,24].
Immunohistochemistry
Immunohistochemical analysis was performed according to our previous method [25]. Tissues sections were deparaffinized, blocked with tissue blocker (Zymed Laboratories, CA) for 10 min and immunostained by using Zymed broad range immunohistochemical kits with overnight incubation with the WISP-2 antibodies.
RNA Extraction, cDNA Synthesis, PCR Amplification, and Probe Preparation
Cytoplasmic RNA was extracted from breast tissue specimens and different cell lines using the Trizol (Life Technologies, Grand Island, NY) extraction procedure, as previously described [22].
For cDNA preparation, 1 µg of total RNA from each sample was subjected to reverse transcription using a Perkin-Elmer RT-PCR RNA amplification kit in a total reaction volume of 20 µl. The reverse transcription product was diluted to 50 µl, and 2 µl of this diluted product was PCR amplified in a 50-µl reaction mixture containing 0.5 µM of each appropriate oligonucleotide primer, 200 µM dNTP, and 1.5 U of Biolase DNA polymerase. The PCR profiles for WISP-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were the same as previously described. The PCR products were separated by electrophoresis through 3% NuSieve 3:1 agarose gel followed by ethidium bromide staining and visualized by UV fluorescence. For each analysis, a negative control was prepared using all reagents except MuLV and 1 µl of the matching RNA yielded no detectable products, indicating that all RNAs were free of DNA contamination. The efficiency of cDNA synthesis from each sample was estimated by coamplification of GAPDH gene with specific primers. The sequences of primers are as follow: WISP-2 (GenBank accession number: XM 0095188): 5′-CCT ACA CAC ACA GCC TAT ATC (nts. 1042 to 1063; Forward) and 5′-CCT TCT CTT CAT CCT ACC C-3′ (nts. 1177 to 1196; Reverse), and GA3PDH (GenBank accession number: AF 261085): 5′-ATG AGA AGT ATG ACA ACA GCC-3′ (nts. 513 to 533; Forward) and 5′-TGA GTC CTT CCA CGA TAC C-3′ (nts. 608 to 626; Reverse). A low DNA mass ladder DNA molecular weight marker was used to determine the size of the test reaction.
To prepare the DIG-labeled nonradioactive probes for WISP-2 and GAPDH, 10 µl cDNA, prepared from total RNA of MCF-7 cells, was PCR amplified according to the manufacturer's instructions (PCR DIG probe synthesis kit, Roche Applied Science) in the presence of DIG-labeled dNTP mixture and WISP-2 or GAPDH specific primers.
In Situ Hybridization
In situ hybridization was performed essentially as described by the manufacturer (Innogenex, San Ramon, CA) with minor modifications. Five-micrometer paraffin sections were deparaffinized with xylene, hydrated in ethanol/water solutions and digested with proteinase K (ready to use vial) for 10 min followed by postfixation in 1% formaldehyde/1x PBS for 10 min at room temperature. The slides were washed with RNAse-free distilled water for 5 min. The DIG-labeled PCR generated WISP-2 probes were added to a slide at concentration of 250 ng/ml hybridization buffer (100 µl/section) and incubated overnight at 37°C in a humidified in situ hybridization chamber. Sections were washed with 2x PBS with 0.1% Tween-20 for 10 min and the following sequence with 3 min per step: 1x PBS, 0.1% Tween-20, 0.5x PBS and 0.2x PBS. The hybridized DIG-labeled probe was detected using alkaline phosphatase-conjugated anti-DIG antibody (Innogenex) and visualized with chromogen combination 5-bromo-4-chloro-3-indolyl phosphate and NBT. The sections were counterstained with nuclear first red.
Northern Blot Analysis
For Northern blot analysis, 10 µg of total RNA was fractionated by electrophoresis in 1.0% agarose gels containing formaldehyde and transferred to super charge nylon membrane (Schleicher & Schuell, Keene, NH). Membranes were hybridized with nonradioactive RT-PCR generated-DIG-labeled WISP-2 or GAPDH gene specific probe and washed according to the protocols provided by the manufacturer of the DIG high prime DNA labeling and detection kit.
Relative expressions of WISP-2 mRNAs were calculated by densitometric analyses using the Gelexpert software program (NeucleoTech, CA). The signal intensity of WISP-2 bands was normalized to that obtained with the GA3PDH bands.
Western Immunoblot Analysis
Cell extracts were prepared for immunoblotting according to our previous method [26]. Briefly, cells were lysed in 1 ml of RIPA buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate) containing 1 mM sodium orthovanadate, for phosphatase inhibition, 0.5 mM phenylmethylsulfonyl fluoride, 1 µM/ml leupeptin, 1 µM/ml aprotinin, and allowed to incubate on ice for 10 min. The lysate was cleared by centrifugation at 15,000 rpm for 60 min at 4°C and the supernatants were collected and frozen at -70°C.
Cell lysates corresponding to 50 µg protein [determined using Coomassie blue reagent assay (Bio-Rad, Richmond, CA)] were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the gel-fractionated proteins transferred to nitrocellulose membranes (Bio-Rad) for the detection of WISP-2 protein. A custom-made polyclonal WISP-2 antibody [22] was used as primary antibody for this assay.
WISP-2 Antisense Assays
Antisense (AS) and mismatch WISP-2 second generation chimeras (5′-7 phosphorothioates, 11 2′-O-methyl RNA, 1 sulfur linkage and inverted T) oligonucleotides were designed using a computer program (Vector NTI Suite, InforMax), synthesized and obtained from Oligos Etc. (Oligo Therapeutics Inc., Wilsonville, OR). The purity of the oligos was confirmed by polyacrylamide gel electrophoresis. The sequences of AS oligos were: WISP-2 AS1, 5′-CTT CGG TGT GCC TCT CAT-3′ (coding region), WISP-2 AS2, 5′-ATG GTC AAC TAA ATC GTC-3′ (untranslated region), and mismatch oligos, 5′-CTG CTA AAT CCA CTG TGA-3′. The mismatched oligos were used as a control.
For determining the effects of antisense oligonucleotides on WISP-2 expression and upon serum-induced cellular proliferation, MCF-7 cells were treated with different oligonucleotides. The treatment of oligos was performed by a previously described method with some modifications [27–29], Briefly, semiconfluent MCF-7 cells were trypsinized and resuspended in DMEM containing 10% FBS and were seeded in 96-well plates (Coster, Cambridge, MA) at the density of 2500 cells/well. After 48 h of culture, to minimize the endogenous WISP-2 protein levels and its signals stimulated by serum, cells were washed once with serum-free Opti-MEM medium (Life Technologies) and grown in the same medium for 72 h. After 72 h, cells were treated with various concentrations of antisense oligos dissolved in 7.5 µM lipofectamine (Life Technologies) for 24 h, washed and refed with DMEM containing 10% FBS. In this study, two sets of controls were used: 1) Cells unexposed to oligos were exposed to the 7.5-µM lipofectin to ensure lack of an effect from the reagent. These cells were considered the lipofectin control; 2) Negative controls consisted of cells that were exposed to neither oligos nor lipofectin.
To determine the effects of antisense oligonucleotides on serum-induced WISP-2 protein synthesis, MCF-7 cells (1x105/plate) were plated in six-well plates and allowed to grow for 48 h. After 48 h, the cells were grown in serum-free Opti-MEM medium for 72 h followed by incubation with 500 or 1000 nM AS1 WISP-2 antisense or 500 nM mismatch oligomers for 24 h. After this time, cells were washed and refed with DMEM containing 10% FBS. The culture was then continued for an additional day. The expression of WISP-2 protein in various cultures was evaluated by Western blot analysis. Each experiment was performed in triplicate.
[3H]-Thymidine Incorporation Assay
The radioactive thymidine incorporation assay was the same as previously described [30]. Briefly, cells treated with oligonucleotides or untreated, were pulsed for 6 h of final culture with 0.25 µCi [3H]-thymidine/well (NEN Research, Wilmington, DE). Then, the cells were precipitated with 10% trichloroacetic acid, filtered through glass fiber filters and dried. The amount of incorporated tritium was measured using a liquid scintillation counter (Beckman Instruments, Fullerton, CA).
Statistical Analysis
For the statistical analysis of association with variables and WISP-2 expression in breast cancer samples, the variables were divided into two groups, which are categorical variables and quantitative variables. Categorical variables including ER, PR, p53 immunolabeling status, and grades were summarized by frequencies and percentages and quantitative variables, which included size of tumor, age, and lymph node metastasis, then summarized by median and range. Chi-square and Fisher's exact tests were utilized to determine an association of categorical variables with WISP status. The Wilcoxon rank sum test was used to determine if differed quantitative variables differed between WISP-2 positive or negative. Northern blot data are presented as mean±SE and analyzed by using either Student's t test. A type I error rate of 5% was used for all tests and an associated probability (P value) of <0.05 was considered significant.
Results
WISP-2 mRNA Levels and Cellular Localization of WISP-2 mRNA and Protein in Normal, Non-Neoplastic and Neoplastic Human Breast
The expression of WISP-2 mRNA was first confirmed in different breast samples using RT-PCR analysis with WISP-2 specific primers (Figure 1). The histologic distribution of WISP-2 mRNA and protein was localized in formalin-fixed, paraffin-embedded tissue sections using nonradioactive in situ hybridization and immunohistochemistry (Figure 2). A total of 98 breast samples, histologically classified according to the standard criteria [31,32], were used for these studies. These included 20 normal, 24 non-neoplastic, and 54 invasive ductal carcinoma specimens with or without preneoplastic lesions. For cellular localization of WISP-2 mRNA or protein, tissue samples were analyzed simultaneously in a multitissue format to avoid potential immunostaining artifacts including slide-to-slide and run-to-run variability.
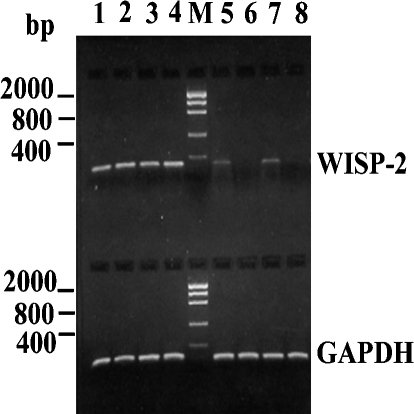
Figure 1.
RT-PCR analyses of WISP-2 mRNA expression in normal and tumor samples of human breast. Lanes 1–4, the representative biopsy samples of human breast tumor; Lanes 5 and 7, the representative biopsy samples of non-neoplastic breast adjacent to the tumors; Lanes 6 and 8, the representative biopsy samples of normal breast. Lane M, DNA markers. The representative RT-PCR of GAPDH mRNA exhibiting the quality of RNA. The predictive PCR-product size of WISP-2 is 155 bp and that of GAPDH is 114 bp.
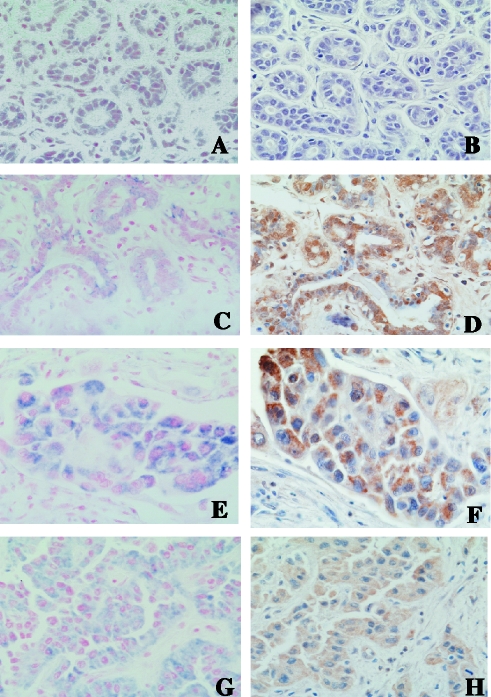
Figure 2.
Representative nonradioactive in situ hybridization and immunohistochemical photomicrographs exhibiting the distribution of WISP-2 mRNA (blue) and WISP-2 protein (brown) in ductal epithelial cells and tumor cells in human breast. (A and B) Ducts and lobules of normal breast; original magnification, x400. (C and D) Benign duct adjacent to the tumor; original magnification, x400. (E and F) Ductal carcinoma in situ; original magnification, x400. (G and H) Invasive ductal carcinoma; original magnification, x400.
Normal and non-neoplastic tissue samples In normal breast tissue, expression of WISP-2 mRNA was undetected or minimally detected by RT-PCR and in situ hybridization analyses as represented in Figures 1 and 2A, respectively. WISP-2 mRNA expression correlated well with their respective protein levels in the same adjacent tissues as determined by immunohistochemical labeling with WISP-2 antibodies (Figure 2B).
In non-neoplastic specimen, as was present in most of the tumor samples, WISP-2 mRNA expression was identified in about 46% (11 of 24) samples (Figure 1), whereas WISP-2 protein was detected in about 62% (15 of 24) samples by immunohistochemical labeling using antibodies to WISP-2 and proteins were mainly confined in the epithelial cells of the ducts and lobules. However, staining intensity for both mRNA and protein varies from weak to strong in this sample. Strong staining was found in three non-neoplastic samples.
Invasive carcinoma samples To determine the WISP-2 mRNA and protein status in invasive ductal carcinoma in breast as well as the association of this expression with other pathophysiological changes, 54 samples with pathological variables (Table 1) were studied. WISP-2 mRNA and protein expression were detected in 68.5% (37 of 54) breast tumor samples (Table 1). The level of expression was significantly higher in breast tumor samples when compared to normal or non-neoplastic breast samples (Figure 1). The WISP-2 mRNA and protein was mainly identified in the epithelial cells and tumor cells. The immunostaining with the WISP-2 antibody was predominantly cytoplasmic (Figure 2). Notably strong WISP-2 mRNA and protein expression were detected in the preneoplastic lesions (i.e., DCIS) that coexisted with invasive cancers (Figure 2, E and F).
Table 1.
Pathological Information of the Human Female Breast Ductal Carcinoma Samples.
| Case no. | Age | Size | LN* | Grade | ER-α | PR | p53 | WISP-2 expression† |
| 1 | 65 | T2 | 3 | I | + | + | + | + |
| 2 | 34 | T1 | 1 | III | + | - | + | + |
| 3 | 39 | T2 | 2 | II | - | + | + | - |
| 4 | 38 | T2 | 2 | II | + | + | + | + |
| 5 | 67 | T3 | 2 | I | + | - | + | + |
| 6 | 50 | T2 | 0 | II | + | - | + | - |
| 7 | 37 | T2 | 10 | III | - | - | + | - |
| 8 | 52 | T2 | 14 | II | + | - | + | + |
| 9 | 84 | T3 | 0 | II | - | - | + | + |
| 10 | 40 | T2 | 0 | III | - | - | + | - |
| 11 | 64 | T2 | 0 | III | - | - | + | + |
| 12 | 61 | T4 | 0 | II | + | - | + | + |
| 13 | 35 | T1 | 0 | II | - | - | + | + |
| 14 | 66 | T2 | 0 | II | - | - | + | + |
| 15 | 58 | T3 | 4 | I | - | - | + | + |
| 16 | 49 | T3 | 5 | III | - | - | - | - |
| 17 | 75 | T1 | 0 | II | + | + | + | - |
| 18 | 44 | T2 | 1 | I | + | + | + | + |
| 19 | 40 | T2 | 0 | III | - | - | + | - |
| 20 | 49 | T2 | 2 | III | - | - | + | - |
| 21 | 50 | T2 | 0 | I | - | + | + | - |
| 22 | 71 | T3 | 3 | III | - | - | + | + |
| 23 | 50 | T3 | 25 | II | - | - | + | - |
| 24 | 42 | T3 | 4 | III | - | - | + | - |
| 25 | 64 | T1 | 5 | I | + | + | + | + |
| 26 | 32 | T3 | 28 | II | - | - | - | - |
| 27 | 60 | T2 | 0 | II | - | - | + | + |
| 28 | 53 | T2 | 32 | I | + | - | - | + |
| 29 | 73 | T2 | 0 | II | - | - | + | + |
| 30 | 50 | T3 | 5 | II | - | - | + | + |
| 31 | 46 | T3 | 4 | II | - | - | + | + |
| 32 | 51 | T2 | 0 | II | - | - | + | + |
| 33 | 59 | T1 | 0 | II | + | - | + | + |
| 34 | 36 | T2 | 0 | II | + | - | + | + |
| 35 | 53 | T3 | 31 | II | - | - | + | - |
| 36 | 69 | T2 | 0 | II | - | - | + | + |
| 37 | 34 | T2 | 6 | I | - | - | + | + |
| 38 | 36 | T3 | 6 | III | + | + | - | + |
| 39 | 60 | T3 | 6 | II | - | - | + | - |
| 40 | 49 | T3 | 12 | II | + | + | + | + |
| 41 | 48 | T3 | 0 | III | + | - | + | + |
| 42 | 36 | T2 | 0 | III | - | - | - | + |
| 43 | 34 | T1 | 0 | I | + | - | + | + |
| 44 | 54 | T3 | 0 | I | + | + | + | + |
| 45 | 41 | T1 | 0 | II | + | + | + | + |
| 46 | 67 | T2 | 4 | II | + | - | - | - |
| 47 | 43 | T1 | 0 | II | + | + | - | + |
| 48 | 31 | T2 | 1 | II | - | - | + | + |
| 49 | 41 | T3 | 0 | III | - | - | + | + |
| 50 | 42 | T1 | 0 | I | + | + | + | + |
| 51 | 55 | T2 | 5 | II | - | - | + | - |
| 52 | 59 | T1 | 0 | I | - | - | - | - |
| 53 | 52 | ‡ | 0 | II | + | + | - | + |
| 54 | 54 | T1 | 1 | II | + | - | - | + |
LN: axillary lymph node metastasis.
mRNA and protein expressions were determined using in situ hybridization and immunohistochemistry.
The tumor was multifocal involving many foci throughout the biopsy; therefore, tumor size cannot be evaluated.
The statistical analyses comparing categorical variables between WISP-2-positive and WISP-2-negative breast cancer samples showed that 20 of 37 (54%) of the positive specimens were ER-α-positive and 14 of 17 (82.4%) of the negative specimens were ER-α-negative. This suggests a significant association (P=0.0174) between WISP-2 and ER-α (Table 2). Actually, WISP-2 positivity increases the odds of being ER-positive by 5.5. There were no associations between WISP-2 expression and other categorical variables such as PR or p53 status and grades (Table 2).
Table 2.
Comparison of Categorical Variables between WISP-2-Positive and WISP-2-Negative Breast Cancer Samples.
| Variable* | WISP-2+ n (%) | WISP-2 - n (%) | P value |
| ER-α | |||
| Positive | 20 (54.0) | 3 (17.7) | 0.0174 |
| Negative | 17 (46.0) | 14 (82.3) | |
| PR | |||
| Positive | 11 (29.7) | 3 (17.7) | 0.5073 |
| Negative | 26 (70.3) | 14 (82.3) | |
| p53 | |||
| Positive | 31 (83.8) | 13 (76.5) | 0.7075 |
| Negative | 6 (16.2) | 4 (23.5) | |
| Grade† | |||
| 1 | 10 (27.0) | 2 (11.8) | 0.3676 |
| 2 | 19 (51.4) | 9 (52.9) | |
| 3 | 8 (21.6) | 6 (35.3) | |
n, number of breast cancer samples.
WISP-2 data were collected from in situ hybridization and immunohistochemical analyses; ER, PR, and p53 data were collected from immunohistochemical analyses.
1, well differentiated; 2, moderately differentiated; and 3, poorly differentiated.
The comparison of quantitative variables between WISP-2-positive and WISP-2-negative specimens showed that WISP-2 expression was independent of size, age, and LN status (Table 3). There were no significant statistical differences in the incidence of WISP-2 expression when compared with the grades (P<.3676), size (P<.5663), age (P<.7798), and LN (P<.1072).
Table 3.
Comparison of Quantitative Variables between WISP-2-Positive and WISP-2-Negative Breast Cancer Samples.
| Variable | WISP+Median (range) | WISP-Median (range) | P value |
| Size | 2 (1–4) | 2 (1–3) | 0.5663 |
| LN | 0 (0–32) | 4 (0–31) | 0.1072 |
| Age* | 51 (31–84) | 50 (32–75) | 0.7798 |
Age (year) at the diagnosis of the breast cancer.
Time- and Dose-Dependent Effects of Estradiol on WISP-2 mRNA Expression in MCF-7 Breast Tumor Cells
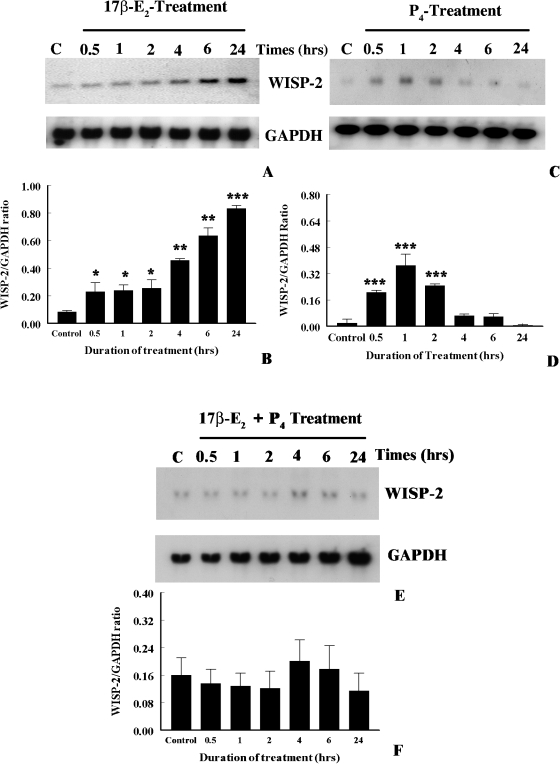
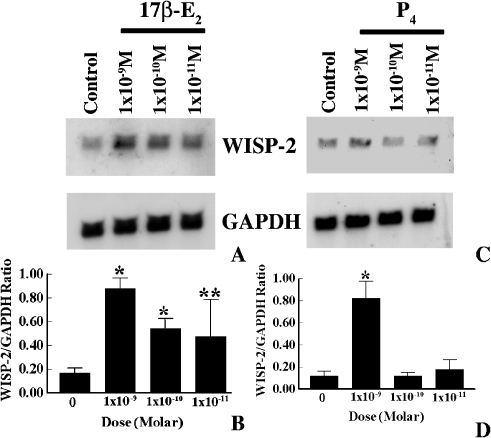
Previous studies, by our laboratory and others, have shown that WISP-2 mRNA expression was upregulated by a natural estrogen, 17β-estradiol (17β-E2), in MCF-7 breast tumor cells [33,34]. In the present studies, time- and dose-dependent effects of estrogen on WISP-2 mRNA expression were evaluated. For this experiment, MCF-7 cells were grown in phenol red-free and serum-free medium for 3 days to make the complete estrogen-free environment and minimize the WISP-2 expression levels. After 3 days, cells were exposed for various lengths of time to 17β-E2 (1x10-8 M), and WISP-2 mRNA levels were determined by nonradioactive Northern blot analysis. As shown in Figure 3, WISP-2 mRNA expression was induced by 2.5-fold as compared to untreated control after 30 min of 17β-E2 exposure and it steadily increased by 9.9-fold until 24 h (Figure 3, A and B). The levels were slightly reduced after 48 h of exposure (data not shown). Because at 24 h of 17β-E2 (1x10-8 M) exposure exhibits maximum induction of WISP-2 mRNA, next we determined the impact of different doses of 17β-E2 (i.e., 1x10-9, 1x10-10, and 1x10-11 M) on WISP-2 mRNA expression in MCF-7 cells for identical time frame. The upregulation of WISP-2 mRNA levels by 17β-E2 was first detected at 1x10-11 M concentration, which was 2.8-fold higher than untreated controls (Figure 4, A and B). The expression levels were improved correspondingly as the dose of estradiol increased (Figure 4, A and B). No impact was perceived when cells were exposed to 1x10-12 M or less concentration of 17β-E2 (data not shown). Collectively, these findings show that estrogen transiently and steadily induced WISP-2 mRNA expression and this upregulation is dose dependent in MCF-7 breast tumor cells.
Figure 3.
Effects of estrogen and progesterone on WISP-2 mRNA expression in a MCF-7 cell line. (A, C, and E) Exponentially growing MCF-7 ER+ breast cancer cells in phenol-red-free DMEM containing 10% FBS were serum starved for 3 days before exposure to 17β-E2 (1x10-8 M) or P4 (1x10-8 M) or in combination for different times. The total RNA was extracted from cells harvested at indicated times and analyzed by Northern blotting using nonradioactive DIG-labeled PCR generated probes for WISP-2 and GAPDH. (B, D, and F) The arbitrary values indicate WISP-2 mRNA concentrations. Data displayed as mean±SD from three separate experiments. P value was determined by Student's t test. *P <.01 versus control; **P < .005 versus control; ***P <.0001 versus control.
Figure 4.
Dose-dependent effects of estrogen and progesterone on WISP-2 mRNA expression in MCF-7 cells. (A and C) Exponentially growing MCF-7 ER+ breast cancer cells in phenol-red-free DMEM containing 10% FBS were serum starved for 3 days before exposure to different doses of 17β-E2 and P4 for 24 and 1 h, respectively. Total RNA was extracted and analyzed by RNA blotting using nonradioactive DIG-labeled PCR generated probes for WISP-2 and GAPDH. (B and D) The arbitrary values indicate WISP-2 mRNA concentrations. Data displayed as mean±SD from three separate experiments. P value was determined by Student's t test. *P <.001 versus control; **P<.01 versus control.
Estradiol-Induced Upregulation of WISP-2 mRNA Expression in Tumor Cells is ER-α Mediated
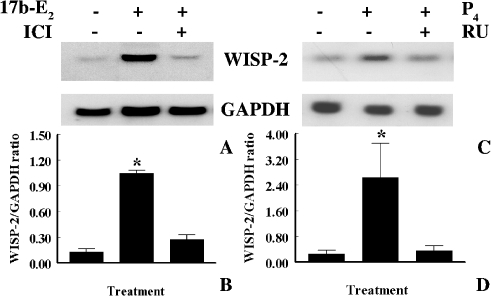
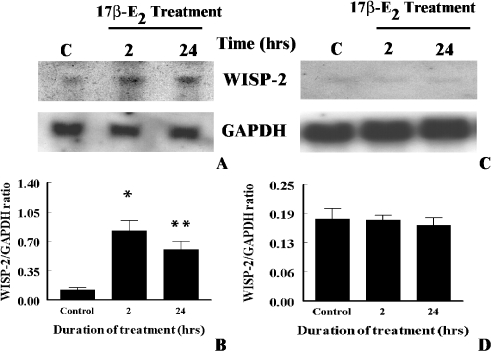
To determine whether estrogen-induced upregulation of WISP-2 mRNA expression is mediated through ER, first the impact of 17β-E2, and a pure antagonist of ER ICI 182,780 on estrogen-responsive human breast tumor derived MCF-7 tumor cells was explored. Cells were exposed 17β-E2 (1x10-8 M) alone or in combination with ICI 182,780 (1x10-6 M) for 24 h, and then WISP-2 mRNA levels were evaluated by Northern blot analysis using nonradioactive DIG-labeled PCR generated probe. As shown above, 17β-E2 alone increased WISP-2 mRNA levels in MCF-7 cells by eight-fold or more as compared to untreated control (Figure 5, A and B). Whereas cells were exposed to 17β-E2 along with ICI 182,780, estrogen-induced upregulation of WISP-2 expression was inhibited significantly (Figure 5B). Taken together, studies indicate that the induction was mediated through ER. To confirm this conclusion, non-transformed and estrogen-non-responsive HMEC with or without stably transfected with ER-α were grown in serum-free medium for 3 days and then exposed to 17β-E2 (1x10-8 M) for 2 and 24 h. WISP-2 mRNA levels were increased significantly in ER-α-transfected HMEC by 6.7- and 4.9-fold after 2 and 24 h of 17β-E2 exposure, respectively, as compared to untreated cells (Figure 6, A and B). However, unlike tumor cells, the level of expression was significantly higher at 2 h as compared to 24 h (Figure 6, A and B). The estrogenic effect on WISP-2 mRNA expression was undetected in HMEC transfected with vector only (Figure 6, C and D). This study supports the previous data (Figure 5) and indicates that ER-α is required to mediate the 17β-E2 action on WISP-2 mRNA expression.
Figure 5.
Effects of pure antiestrogen ICI 182,780 and antiprogesterone RU38486 on estrogen and progesterone-induced WISP-2 mRNA expression. (A and C) Serum starved MCF-7 ER+ breast cancer cells were exposed to either 1x10-8 M 17β-E2 in the absence (control) or presence of ICI 182,780 (1x10-6 M) for 24 h or 1x10-8 M P4 alone or in combination with RU38486 (1x10-6 M) for 1 h. Total RNA was extracted and analyzed by RNA blotting using nonradioactive DIG-labeled PCR generated probes for WISP-2 and GAPDH. (B and D) The arbitrary values indicate WISP-2 mRNA concentrations. Data displayed as mean±SD from three separate experiments.
Figure 6.
Effects of estrogen on ER-α-transfected and non-transfected normal human mammary epithelial cells. (A) Serum-starved ER-transfected or (C) only vector-transfected cells were exposed to 1x10-8 M 17β-estradiol for 2 or 24 h. Total RNA was extracted and analyzed by RNA blotting using nonradioactive DIG-labeled PCR generated probes for WISP-2 and GAPDH. (B and D) The arbitrary values indicate WISP-2 mRNA concentrations. Data displayed as mean±SD from three separate experiments. P value was determined by Student's ttest. *P <.001 versus control; **P <.004 versus control.
Effects of Estradiol on WISP-2 mRNA Transcription and Stability in MCF-7 Breast Tumor Cells
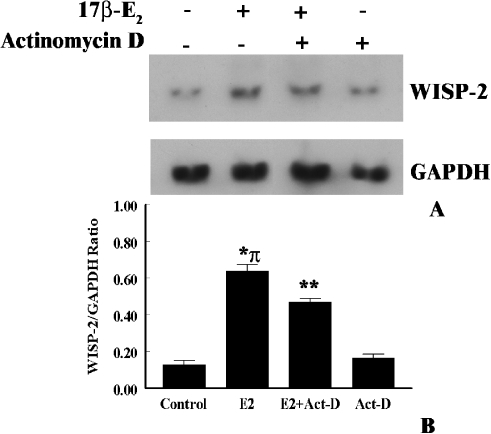
Because 17β-E2 steadily upregulated WISP-2 mRNA expression for 24 h, we determined whether this was at the level of transcription or increased stability of mRNA in the tumor cells. MCF-7 cells were left untreated or exposed to 17β-E2 (1x10-8 M) for 2 h before the addition of the RNA synthesis inhibitor, actinomycin-D (3 µg/ml). As shown in Figure 7, 17β-E2 treatment showed a marked induction of WISP-2 mRNA partially abrogated in the presence of actinomycin-D. On the contrary, actinomycin-D had no impact on WISP-2 mRNA expression in estrogen-untreated cells (Figure 7). Longer exposure (24 h or more) of actinomycin-D abolishes the constitutive (untreated) and estrogen-induced WISP-2 mRNA expression (data not included), which is in agreement with previous work [33]. These results conclude that estrogen may regulate the WISP-2 gene at the transcription level as well as by increased stability of WISP-2 mRNA.
Figure 7.
Effects of estrogen on mRNA stability and transcriptional activation of WISP-2 gene in MCF-7 cells. (A) Exponentially growing MCF-7 ER+ breast cancer cells were serum starved for 3 days, and then exposed to 17β-E2 (1x10-8 M) or vehicle (ethanol) alone for 2 h before actinomycin-D (3 µg/ml) was added into the media for 2 h. Total RNA was extracted and assessed for nonradioactive Northern blotting analysis using DIG-labeled PCR generated probes for WISP-2 and GAPDH. (B) The arbitrary values indicate WISP-2 mRNA concentrations. Data displayed as mean±SD from three separate experiments. P value was determined by Student's t test. *P < .01 versus control; **P <.005 versus control; π, P <.05 versus actinomycin-D treated.
Modulation of WISP-2 mRNA Expression by Progesterone in MCF-7 Breast Tumor Cells
To determine the impact of progesterone (P4) on WISP-2 mRNA expression, MCF-7 cells were grown in a serum-free environment for 3 days, and then cells were either exposed to P4 (1x10-8 M) for different times or different doses of P4 for single time point that has been selected from the result of different time-dependent effect studies. WISP-2 mRNA expression was significantly elevated after 30 min of P4 exposure and reached maximum levels at 1 h (Figure 3, C and D). The basal level of expression was obtained at 4 h, or longer, exposure. Moreover, WISP-2 mRNA level was increased in MCF-7 cells by nanomolar dose of P4 (Figure 4, C and D). The impact was undetected at picomolar levels.
To determine if progesterone receptor (PR) can mediate rapid induction of WISP-2 mRNA by P4 in MCF-7 cells, we tested the ability of the progesterone receptor antagonist RU38486 (mifepristone) to block P4-induced upregulation of WISP-2 mRNA expression. As shown in Figure 5, C and D, RU38486 (1 µM) significantly inhibited the increase in WISP-2 mRNA expression by P4 in MCF-7 cells. These data together with the above studies indicate that transiently, upregulation of WISP-2 expression by P4 can be mediated through a progesterone receptor.
P4 usually antagonizes estrogen action and inhibit estrogen-induced cell proliferation as well as the growth of estrogen-dependent cancer [35,36]. Therefore, the effect of P4 on 17β-E2-induced WISP-2 upregulation was determined. P4 exhibited an antagonizing effect on WISP-2 mRNA expression in the presence of 17β-E2 and also inhibited 17β-E2-induced WISP-2 expression as shown in Figure 3, E and F.
Effect of WISP-2 Antisense Oligos on Serum-Induced Tumor Cell Proliferation and WISP-2 Protein Synthesis
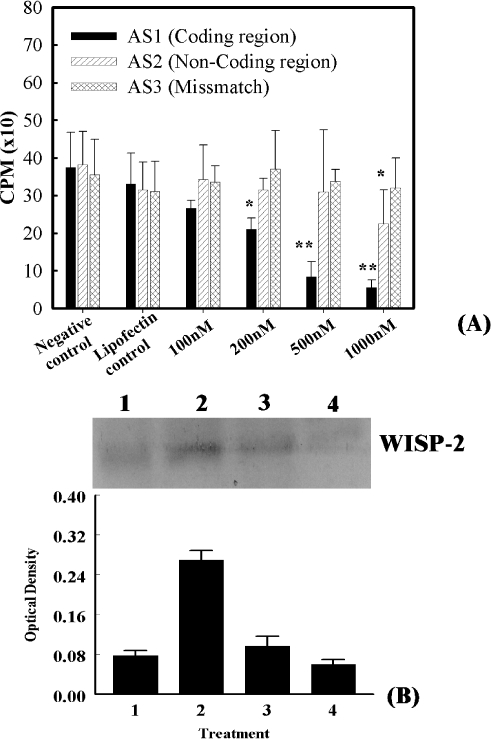
To determine the functional role of WISP-2 on tumor cell proliferation, the impact of two different WISP-2 antisense oligonucleotides on MCF-7 cell proliferation was examined. Cells were treated with various concentrations (100, 200, and 500 nM or 1 µM), of antisense oligos and cellular proliferation determined using [3H]-thymidine incorporation assays. The results obtained at 24 h after antisense treatments are depicted in Figure 8. The degree of impact of two different antisenses on MCF-7 cell proliferation was not identical. The antisense oligos AS1 (5′-CTT CGG TGT GCC TCT CAT-3) exhibited a higher inhibitory effect at a dose of 500 nM, or higher, as compared to AS2. At a concentration of 500 nM, AS1 oligos inhibited the [3H]-thymidine incorporation by 77%, whereas no significant inhibition was observed at 100 nM. Cells treated with 7.5 µM lipofectamine alone or exposed to mismatch oligos at various concentrations ranging from 100 nM to 1 µM underwent logarithmic cell growth (Figure 8A). The inhibitory effects of WISP-2 AS1 oligos on MCF-7 cells were further confirmed using BrdU-ELISA assays, in which BrdU incorporation was consistently and significantly reduced when cells were exposed to 500 nM AS-1 oligos as compared to unexposed cells (data not shown). Lipofectamine and mismatch oligos exhibit no significant changes in radioactive thymidine incorporation.
Figure 8.
Effects of antisense oligos treatment on the serum-induced [3H]-thymidine incorporation and WISP-2 protein synthesis in MCF-7 cells. (A) Semi-confluent MCF-7 cells were seeded in 96-well plates at the density of 2500 cells/well. After 48 h of culture, cells were washed one time with serum-free Opti-MEM medium and grown in the same medium for 72 h. After 72 h, cells were treated with indicated concentrations of antisense oligos dissolved in 7.5 µM lipofectamine for 24 h. After this time, cells were washed and refed with DMEM containing 10% FBS. The culture was continued for one additional day. Cells were assayed for [3H]-thymidine incorporation. In this experiment, two sets of controls were used. Results are displayed as mean±SD from three separate experiments performed in triplicate. *P < .02 versus control; **P <.05. (B) MCF-7 cells (1x105/plate) were plated in six-well plates and allowed them to grow for 48 h, and then cells were grown in serum-free Opti-MEM medium for 72 h followed by incubation with 500 or 1000 nM AS1 WISP-2 antisense or 500 nM mismatch oligomers for 24 h. After this time, cells were washed and refed with DMEM containing 10% FBS for 24 h. The expression of WISP-2 protein was evaluated by Western blot analysis in the lysates obtained from cells grown in serum-free medium (Lane 1), cells treated with mismatch oligomers before serum addition (Lane 2), cells treated with 500 nM AS1 antisense before serum addition (Lane 3), and cells treated with 1000 nM AS1 antisense before serum addition. The intensity of the bands was quantitated using NucleoVision image analysis system and arbitrary values indicate WISP-2 concentration in different samples. Results are displayed as mean±SD from three separate experiments.
To validate that the AS1-antisense-induced inhibition of cell proliferation was mediated through inhibition of protein expression in MCF-7 cells, WISP-2 protein synthesis was evaluated using Western blot analysis. Preincubation of cells for 24 h with 500 or 1000 nM AS1 antisense oligos significantly reduced the impact of serum on WISP-2 protein synthesis when compared to the cells treated with nonsense oligomers (Figure 8B). The relative intensity of WISP-2 protein after 500 nM AS1 antisense treatment was 2.8-fold less after serum stimulation.
Discussion
WISP-2 is Overexpressed in Preneoplastic and Neoplastic Breast Diseases and is Correlated with ER Positivity
Our recent data have shown by differential display and RT-PCR analyses that WISP-2 mRNA was overexpressed in different breast tumor cell lines such as MCF-7, ZR-75, SKBR-3, and T-47D, but the expression was virtually undetected in normal mammary epithelial cells (HMEC) [21]. Further studies have demonstrated that WISP-2 is a serum-induced early response gene, and cellular content of mRNA and protein was maximal during the process of serum-induced tumor cell proliferation [22]. Moreover, these studies implied a major role for WISP-2 signaling in tumor cell proliferation [22]. The findings presented in this study demonstrated that WISP-2 was expressed in 68% ductal carcinoma tissue samples (Table 1) and undetectable or barely detectable in normal breast tissue samples as shown by RT-PCR, in situ hybridization, and immunohistochemical analyses (Figures 1 and 2). The WISP-2 was also detected in preneoplastic lesions [i.e. atypical ductal hyperplasia (ADH) and DCIS] in tumor samples. WISP-2 protein was primarily localized in the cytoplasm of ductal epithelial cells or tumor cells, and was undetected in stromal cells and blood vessels by conventional in situ hybridization and immunohistochemical analyses. Moreover, the statistical evaluations indicate that WISP-2 expression was frequently associated with ER positivity. No statistical correlation was found with other variability including age, size, and grade of the tumors, LN, PR, or P53 status. Taken together, the studies suggest that WISP-2 expression may be an early genetic lesion that is closely associated with the development of breast cancer. Thus, WISP-2 may serve as a possible marker of the potential progression of the breast cancers, which are often ER-positive.
Regulation by Sex Steroids of WISP-2 mRNA Expression
The present in vivo studies, which demonstrate a close association between WISP-2 expression and ER positivity, and previous studies from our lab and others have suggested the possible role of estrogen in regulation of the WISP-2 gene [22,33,34]. In the present studies, therefore, the impact of estrogen was evaluated more precisely. The results presented herein indicate that natural estrogen, 17β-E2, rapidly induces WISP-2 mRNA expression in MCF-7 breast tumor cell line as well as in ER-α-transfected non-transformed mammary epithelial cells. The mRNA expression was progressively increased, and the effect of estrogen on WISP-2 activation lasted for >24 h or more. Both physiological and supraphysiological doses of estrogen increase WISP-2 mRNA expression in MCF-7 cells. The inhibition of this action of estrogen by a pure anti-estrogen ICI 182,780 indicated that the effect was mediated through the action of ER-α. Moreover, partial inhibition of estrogen-induced WISP-2 mRNA expression by actinomycin-D suggested multiple actions of estrogen on WISP-2. It may regulate at the transcription level as well as potentiates the stabilization of WISP-2 mRNA. Further studies are warranted to confirm these findings.
Despite controversy, it is well accepted that progesterone (P4) is required for normal mammary gland development and also plays a critical role in regulation of breast cancer growth and progression [37,38]. P4 has both proliferative and antiproliferative effects on breast epithelial cells and cancerous breast cells [37–40]. Additionally, antagonistic actions of progesterone on estrogen effects in normal and breast cancer cells have been well documented [37]. This distinctive feature of P4 persuaded us to evaluate its impact on WISP-2 expression in the presence or absence of natural estrogen 17β-E2. The studies indicated a dual action of progesterone. Alone, P4, with different doses, rapidly upregulated WISP-2 mRNA expression in MCF-7 cells, but unlike that of estrogen, this action was transient and decreased within 4 h when cells were exposed to 10 nM of P4. The prolonged exposure to P4 did not alter the WISP-2 mRNA level in MCF-7 breast cancer cells, as previously reported [41]. Moreover, studies indicate that progesterone act through a PR-dependent mechanism to increase WISP-2 mRNA expression.
When breast tumor cells were exposed concurrently to estrogen and progesterone, the antagonistic action of progesterone was apparent. These data strongly suggest that WISP-2 is an estrogen or progesterone-induced early response gene and may participate in the cascade of events underlying the progesterone regulation of estradiol action in breast cancer cells. The mechanisms of interactions are still to be determined.
Role of WISP-2 Expression in Cellular Proliferation
Data presented in this report and previous studies [21,22,33,34] have shown that the transcriptional and translational activation of WISP-2 gene is a characteristic of breast cancer cells and suggesting that WISP-2 expression may be critical for the growth and survivability of breast tumor cells. To test the hypothesis, the impact of WISP-2 antisense oligos on serum-induced breast tumor derived MCF-7 cell proliferation was determined. Antisense approaches have been used to inhibit the function of various genes by blocking the information conveyed from DNA to protein through RNA by binding a small complementary nucleotide sequence to the target (see the review) [42]. Our results indicate that the suppression of endogenous serum-induced WISP-2 synthesis by antisense oligos (complementary to WISP-2 cDNA, i.e., mRNA, at the coding region) inhibited radioactive thymidine or BrdU incorporation into the MCF-7 cells exposed to serum. In contrast, incorporation of radioactive thymidine or BrdU into normal breast epithelial cells was unaltered when WISP-2 antisense oligonucleotides were incorporated into these cells. Therefore, it appears that WISP-2 signaling is closely correlated with the serum (mitogen)-induced proliferation in MCF-7 breast tumor cell line, and indicates a role for WISP-2 in tumor cell proliferation. Moreover, these novel findings appear to indicate that WISP-2 is not functioning as a tumor suppressor gene in breast cancer cells, although WISP-2 is homologous to a recently identified tumor suppressor gene, rCop-1 [43,44].
Conclusions
The findings presented in this study lead to the conclusion that WISP-2, a member of CCN family of growth factor, is overexpressed in human breast tumor samples and exhibits a close association with ER positivity. Moreover, in vitro studies demonstrated that the WISP-2 gene is a target of both estrogen and progesterone and mediated through estrogen and progesterone receptors. The action of progesterone was biphasic. Progesterone alone transiently induced WISP-2 expression in MCF-7 cells, but in the presence of estrogen, it exhibits anti-estrogenic effects and inhibited estrogen-induced WISP-2 mRNA expression. Moreover, WISP-2 protein content and mitogen-induced MCF-7 cell proliferation were reduced by WISP-2 antisense oligos pretreatment, suggesting a major role for WISP-2 signaling in tumor cell proliferation.
Acknowledgements
We thank Donald Johnson for fruitful collaborations in the reviewing of the manuscript. The authors also thank Kimberly Collins for editorial comments. This work is dedicated to the memory of J.N. Medda, PhD.
Abbreviations
- WISP-2
Wnt-1 induced signaling protein-2
- RT-PCR
reverse transcription-polymerase chain reaction
- DIG
digoxigenin
- ER
estrogen receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HMEC
human mammary epithelial cells
- 17β-E2
17β-estradiol
- P4
progesterone
- CCN
the connective tissue growth factor/cysteine-rich 61/neuroblastoma overexpressed family of growth factors
- ADH
atypical ductal hyperplasia
- DCIS
ductal carcinoma in situ
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
Footnotes
This work was supported by V.A. Merit Review grant, NIHNCI CA87680, the Midwest Biomedical research Foundation, Kansas City, MO, and Research Service Funds, Veterans Affairs Medical Center, Kansas City, MO.
References
- 1.Parkin DM. The global burden of cancer. Semin Cancer Biol. 1998;8:219–235. doi: 10.1006/scbi.1998.0080. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49(2):33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AG, Jemal A, Thun MJ. Figures 2001–2002. USA: American Cancer Society; 2002. Breast Cancer Facts and; pp. 1–20. [Google Scholar]
- 5.Bryant HE, Brasher PM. Risks and probabilities of breast cancer: short-term versus lifetime probabilities. CMAJ. 1994;150:211–216. [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society, author. Cancer Facts and Figures 1999. Atlanta, GA: American Cancer Society Inc.; 1999. pp. 1–37. [Google Scholar]
- 7.American Cancer Society, author. Cancer Facts and Figures 1994. Atlanta, GA: American Cancer Society; 1994. pp. 13–60. [Google Scholar]
- 8.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49(1):8–31. doi: 10.3322/canjclin.49.1.8. [see comments] [DOI] [PubMed] [Google Scholar]
- 9.Bergstein I, Brown AM. WNT genes and breast cancer. In: Bowcock AM, editor. Breast Cancer: Molecular Genetics, Pathogenesis and Therapeutics. New Jersey: Humana Press; 1999. pp. 181–198. [Google Scholar]
- 10.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 11.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis — a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 12.Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Wendy Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- 13.Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001;3:351–355. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalley MJ, Dale TC. Wnt signaling and mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:37–52. doi: 10.1023/a:1009564431268. [DOI] [PubMed] [Google Scholar]
- 15.Lee FS, Lane TF, Kuo A, Shackleford GM, Leder P. Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci USA. 1995;92:2268–2272. doi: 10.1073/pnas.92.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 17.Roelink H, Wagenaar E, Lopes DS, Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci USA. 1990;87:4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 20.Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 21.Saxena N, Banerjee S, Sengupta K, Zoubine MN, Banerjee SK. Differential expression of WISP-1 and WISP-2 genes in normal and transformed human breast cell lines. Mol Cell Biochem. 2001;228:99–104. doi: 10.1023/a:1013338912642. [DOI] [PubMed] [Google Scholar]
- 22.Zoubine MN, Banerjee S, Saxena NK, Campbell DR, Banerjee SK. WISP-2: a serum-inducible gene differentially expressed in human normal breast epithelial cells and in MCF-7 breast tumor cells. Biochem Biophys Res Commun. 2001;282:421–425. doi: 10.1006/bbrc.2001.4584. [DOI] [PubMed] [Google Scholar]
- 23.Zajchowski DA, Sager R, Webster L. Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res. 1993;53:5004–5011. [PubMed] [Google Scholar]
- 24.Zajchowski DA, Sager R. Induction of estrogen-regulated genes differs in immortal and tumorigenic human mammary epithelial cells expressing a recombinant estrogen receptor. Mol Endocrinol. 1991;5:1613–1623. doi: 10.1210/mend-5-11-1613. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee SK, Sarkar DK, Weston AP, De A, Campbell DR. Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumors may mediate estrogen-initiated tumor angiogenesis. Carcinogenesis. 1997;18:1155–1161. doi: 10.1093/carcin/18.6.1155. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee SK, Zoubine MN, Tran TM, Weston AP, Campbell DR. Overexpression of vascular endothelial growth factor164 and its co-receptor neuropilin-1 in estrogen-induced rat pituitary tumors and GH3 rat pituitary tumor cells. Int J Oncol. 2000;16:253–260. doi: 10.3892/ijo.16.2.253. [DOI] [PubMed] [Google Scholar]
- 27.Momiyama N, Shimada H, Mitsuhashi M. Suppression of c-jun by antisense oligonucleotides inhibits cell adhesion but not respiratory burst during phorbol ester-induced differentiation of U937 human monoblastic cells. Cell Growth Differ. 1996;7:1005–1012. [PubMed] [Google Scholar]
- 28.Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-cdk2 complex formation: receptor overexpression does not determine growth dependency [In Process Citation] Mol Cell Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witters L, Kumar R, Mandal M, Bennett CF, Miraglia L, Lipton A. Antisense oligonucleotides to the epidermal growth factor receptor. Breast Cancer Res Treat. 1999;53:41–50. doi: 10.1023/a:1006127527107. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee SK, Campbell DR, Weston AP, Banerjee DK. Biphasic estrogen response on bovine adrenal medulla capillary endothelial cell adhesion, proliferation and tube formation. Mol Cell Biochem. 1997;177:97–105. doi: 10.1023/a:1006888020596. [DOI] [PubMed] [Google Scholar]
- 31.Lininger RA, Tavassoli FA. Breast. In: Henson DE, Albores-Saavedra J, editors. Pathology of Incipient Neoplasia. 3rd ed. New York: Oxford University Press; 2001. pp. 319–375. [Google Scholar]
- 32.Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. New York: Churchill Livingstone; 1987. [Google Scholar]
- 33.Inadera H, Hashimoto S, Dong HY, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. WISP-2 as a novel estrogen-responsive gene in human breast cancer cells. Biochem Biophys Res Commun. 2000;275:108–114. doi: 10.1006/bbrc.2000.3276. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee SK. CCN family genes in breast cancer: impact of estrogen. Int J Mol Med. 2000;6:S74. (Spandidos DA, Lychnia, Athens, Greece) [Google Scholar]
- 35.Henderson BE, Ross RK, Pike MC. Hormonal chemoprevention of cancer in women. Science. 1993;259:633–638. doi: 10.1126/science.8381558. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Kyo S, Takakura M, Tanaka M, Yatabe N, Maida Y, Fujiwara M, Hayakawa J, Ohmichi M, Koike K, Inoue M. Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res. 2000;60:5376–5381. [PubMed] [Google Scholar]
- 37.Plu-Bureau G, Touraine P, Mauvais-Jarvis P. Interactions between estradiol and progesterone in normal breast. In: Manni A, editor. Endocrinology of Breast Cancer. New Jersey: Humana Press; 1999. pp. 21–37. [Google Scholar]
- 38.Lange CA, Richer JK, Horwitz KB. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 39.Musgrove EA, Lee CS, Sutherland RL. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991;11:5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musgrove EA, Sutherland RL. Steroids, growth factors, and cell cycle controls in breast cancer. Cancer Treat Res. 1991;53:305–331. doi: 10.1007/978-1-4615-3940-7_15. [DOI] [PubMed] [Google Scholar]
- 41.Inadera H, Dong HY, Matsushima K. WISP-2 is a secreted protein and can be a marker of estrogen exposure in MCF-7 cells. Biochem Biophys Res Commun. 2002;294:602–608. doi: 10.1016/S0006-291X(02)00530-2. [DOI] [PubMed] [Google Scholar]
- 42.Stein CA. Anti-sense oligodeoxynucleotides — promises and pitfalls. Leukemia. 1992;6:967–974. [PubMed] [Google Scholar]
- 43.Perbal B, Martinerie C, Sainson R, Werner M, He B, Roizman B. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci USA. 1999;96:869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131–6141. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]