Abstract
DTI-015 (BCNU in 100% ethanol) utilizes solvent-facilitated perfusion for the intratumoral treatment of gliomas. The water-miscible organic solvent vehicle, ethanol, facilitates a rapid and thorough saturation of the tumor with the dissolved anticancer agent, BCNU. Rats bearing established intracranial T9 gliosarcoma tumors received no treatment (group 1), a single intratumoral injection of ethanol vehicle (group 2) or DTI-015 (5 mg/kg BCNU) (group 3), or a single intratumoral injection of DTI-015 followed by systemic BCNU (group 4). Ethanol alone (n=13) had no effect on survival; MST=17 days compared to 18 days for untreated controls (n=35). DTI-015 (n=45) produced an ILS of 417% (MST=93) and 472% (MST=103) when combined with systemic BCNU (n=14). Overall, 24 of 59 rats receiving DTI-015 were judged to be cured, with 20 living a normal life span of 600 to 700 days, and 4 rats sacrificed healthy at 121, 135, 307, and 384 days post DTI-015 with no evidence of viable T9 tumor. Histology demonstrated that DTI-015 totally eradicated the T9 tumors in animals living a normal life span. The results demonstrate that a single injection of DTI-015 produces a 40% cure rate in rats bearing established intracranial T9 tumors.
Keywords: stereotactic injection, malignant glioma, intratumoral, DTI-015, T9 gliosarcoma
Introduction
Intravenously administered BCNU (carmustine) has been one of the leading chemotherapeutic agents used for treating gliomas for approximately 30 years now [15,35]. Although BCNU demonstrates a good activity against glioma cells in vitro [16,17], its clinical efficacy is limited by both acute toxic effects upon the bone marrow and gastrointestinal tract [7], and delayed toxic effects upon the liver and lungs [8,23,33], when administered intravenously.
Attempts have been made to achieve a higher tumor-to-systemic exposure ratio by administering BCNU intraarterially into the vascular region subtending the tumor. This approach for delivering BCNU, however, provides no increase in survival [30], a less than four-fold tumor-to-systemic exposure advantage [13,14], and results in unacceptable eye [18,20] and neurological toxicity [22,24,28].
More recently, locally applied slow-release polymeric implants (e.g., Gliadel® Wafer) have been employed in order to achieve a high tumor exposure to BCNU while minimizing overall systemic exposure [3]. When used as an adjunct to surgical resection for prolonging survival in patients with recurrent glioblastoma multiforme, Gliadel® demonstrates a small but significant clinical patient benefit [4]. However, the limitation of Gliadel® and other depot approaches is that the mechanism of drug movement into the tumor is by diffusion, which restricts penetration distances to a few millimeters.
We have recently developed the technology of solvent-facilitated perfusion (SFP) for the local/regional treatment of solid tumors [5,9,25,32]. Instead of commonly used aqueous delivery vehicles [1,19,21], SFP utilizes water-miscible organic solvent vehicles that move easily through both water and membranes to drive the penetration of solubilized anticancer drugs throughout tumors [26].
We now report on the efficacy of intratumorally administered DTI-015, a solution of BCNU in the water-miscible organic delivery solvent vehicle ethanol, in the rat T9 gliosarcoma intracranial tumor model.
Materials and Methods
Animals
Male CDF rats (Fischer 344; Taconic Farms, Germantown, NY), aged 60 to 80 days, were allowed to acclimate to laboratory conditions for 1 to 2 weeks prior to studies. They were given Purina rat chow and water ad libitum and maintained six per cage in a room with a light-dark cycle (12 hours on, 12 hours off). All experimental procedures were performed between 9 A.M. and 7 P.M. At the time of tumor cell implantation, the rats weighed between 120 and 240 g.
T9 Gliosarcoma Cells
T9 gliosarcoma cells were maintained in a monolayer tissue culture in Falcon plastic flasks. Flasks contained Eagle's minimal essential medium (Gibco, Gaithersburg, MD) supplemented with 20% newborn calf serum, nonessential amino acids, l-glutamine, and an antibiotic-antimycotic solution containing penicillin, streptomycin, and amphotericin B. The cultures were incubated at 37°C in a high-humidity atmosphere containing 5% carbon dioxide. When cells formed a monolayer and became confluent, they were trypsinized (0.25% trypsin, 0.2% ethylenediaminetetraacetic acid), and subcultured. Aliquots of cells from various passages were kept frozen in liquid nitrogen with 10 vol.% glycerin.
Intracerebral Tumor Implantation
T9 cells were trypsinized, harvested from their flasks, and suspended in phosphate-buffered saline. Cells were counted in a hemocytometer and viability was determined by the trypan blue exclusion method. Immediately prior to implantation, cells were drawn up into a Hamilton microliter syringe (no. 702) equipped with a 25-gauge needle. Seven hundred thousand tumor cells in a volume of 10 µl were injected stereotactically into the right caudate hemisphere of each animal. For this tumor inoculation, animals were anesthesized on day 0 with pentobarbital sodium (25–30 mg/kg) intraperitoneally and placed into a stereotactic frame. The head was shaved, a midline longitudinal scalp incision was made, and the bregma was identified. Using a dental drill, a 1-mm burr hole was placed 3 mm to the right of the bregma and 1 mm posterior to the coronal suture. Next, the needle tip was lowered into the cerebrum to a depth of 5 mm below the dural surface and then retracted by 1 mm. The purpose of this retraction procedure was to create a small pocket into which the cells could be injected. The needle bevel was positioned to face laterally in order to mitigate against inadvertent intraventricular injection. Cells were injected slowly over a period of 2 minutes. The needle was then withdrawn and the burr hole was sealed with bone wax. The operative field was cleaned with povidone iodine solution and the scalp incision was closed with clips.
DTI-015
DTI-015 was prepared by dissolving 100 mg of BCNU [(1,3-bis(2-chloroethyl)-1-nitrosourea] (Bristol Laboratories, Syracuse, NY) in 1.5 ml of absolute ethanol. All intratumoral injections used this 66.7 mg/ml formulation directly. The dose delivered to each animal was 5 mg/kg body weight BCNU. Accordingly, a rat weighing 200 g received 15 µl, equivalent to a total dose of 1.0 mg of BCNU. For animals additionally receiving 5 mg/kg body weight BCNU intraperitoneally, this 66.7 mg/ml solution was further diluted to 5 mg/ml with sterile water immediately preceding its use. This is a relatively low dose, and approximates 40% of the LD10 determined for intraperitoneal BCNU in rats [33,34].
Intratumoral DTI-015 Injection
Rats were anesthesized with ether and pentobarbital sodium (25–30 mg/kg) and placed into the stereotactic frame. The clips and bone wax were carefully removed. DTI-015 was injected directly into the tumor (at a flow rate of 7.5 µl/min) through a Hamilton microsyringe set to the same coordinates and using the same technique as in the initial tumor implantation. As before, the operative field was cleaned with povidone iodine solution and the scalp was closed with clips.
Treatment
All animals received tumor implantation on day 0. Controls (group 1) received no treatment; vehicle controls (group 2) received a single intratumoral injection of absolute ethanol on day 7. One experimental group (group 3) received a single injection of intratumoral DTI-015 (BCNU dissolved in absolute ethanol at a dose of 5 mg/kg body weight) on day 7. A second experimental group (group 4) received a single intratumoral DTI-015 injection (BCNU dissolved in absolute ethanol at a dose of 5 mg/kg body weight) on day 7, followed by a second dose of BCNU in aqueous solution (5 mg/kg body weight) delivered intraperitoneally on day 12.
Evaluation
Using length of survival as an endpoint, the efficacy of the two therapeutic regimens was compared. All animals were autopsied at their time of death. A gross inspection was made of the lungs, liver, and kidneys, and the brain was removed in toto and fixed in 10% buffered formalin for 3 days. A single coronal section was cut through the center of the tumor site and both halves were embedded in paraffin, serially sectioned, and stained with hematoxylin and eosin for blind histopathologic examination. Animals classified as cured were those that lived a normal life span, i.e., 600 to 700 days, and also four animals that were sacrificed healthy for histology on days 121, 136, 307, and 384, and demonstrated no observable viable T9 tumor.
Statistical Methods
Median survival rates were calculated for each group, and the data were analyzed nonparametrically with the Wilcoxon rank sum test.
Results
Survival
Operative mortality was 4.3% (4/93) for tumor-bearing animals following sodium pentobarbital administration but preceding intratumoral injection, 7.1% (1/14) following intratumoral ethanol vehicle injection, and 5.1% (4/79) following intratumoral DTI-015 injection. A subset of rats receiving intratumoral DTI-015 was sacrificed for histology on day 7 (n=2), day 10 (n=5), and day 14 (n=5), and was not included in the overall survival analysis. Survival data are summarized in Table 1.
Table 1.
Effect of Intratumoral DTI-015 on Survival.
| Group | Treatment | n | Median Survival Time (days) | % ILS* | Cures | % Cures | P† |
| 1 | Untreated control | 35 | 18 | 0 | 0 | 0 | |
| 2 | Intratumoral ethanol | 13 | 17 | -6 | 1 | 8 | NS |
| 3 | Intratumoral DTI-015 | 45 | 93 | 417 | 20‡ | 44 | < 1x10-6 |
| 4 | Intratumoral DTI-015+ IP BCNU | 14 | 103 | 472 | 4§ | 29 | < 1x10-6 |
| 5 | Groups 3 and 4 | 59 | 96 | 433 | 24 | 41 | < 1x10-6 |
Percent increase in life span=(median day of survival for treated group-median day of survival for control group/median day of survival for control group)x100.
Significantly different from group 1 untreated controls as determined by the Wilcoxon rank sum test.
Includes three animals, apparently healthy, euthanized on days 121, 136, and 384 for histology; no viable T9 tumor was found histologically.
Includes one animal, apparently healthy, euthanized on day 307 for histology; no viable T9 tumor was found histologically.
Control animals (n=35) had a median survival of 18 days. Ethanol vehicle controls (n=13) had a median survival of 17 days. One of the ethanol vehicle control animals lived a normal life span, apparently healthy, and was euthanized for histology on day 614.
Animals that received a single intratumoral injection of DTI-015 (n=45) had a median survival of 93 days, i.e., an increased life span of 417% (P<1x10-6). Twenty of the 45 rats (44%) were judged to be cured, with 17 living a normal life span and 3 animals sacrificed healthy at 121, 136, and 384 days posttreatment with no viable T9 tumor present.
Animals that received a single intratumoral injection of DTI-015 followed by intraperitoneal BCNU (n=14) lived a median of 103 days, i.e., an increased life span of 472% (P<1x10-6). Four of the 14 rats (29%) were judged to be cured, with three living a normal life span and one animal sacrificed healthy at 307 days posttreatment with no viable tumor present.
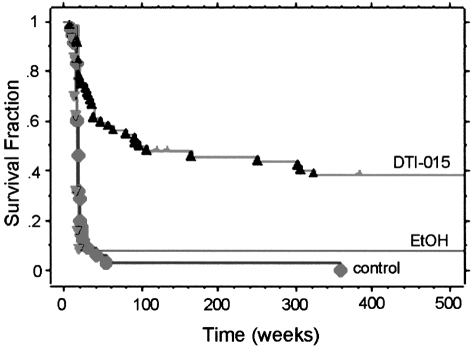
The addition of the intraperitoneal BCNU to the treatment regimen did not statistically enhance the therapeutic benefit of the primary intratumoral DTI-015. Considered as one group, the 59 rats that received intratumoral DTI-015 lived a median survival time of 96 days, i.e., an increase in life span of 433% (P<1x10-6). Twenty-four rats (41%) were judged to be cured. Survival curves are shown in Figure 1.
Figure 1.
Survival of rats with intracranial T9 gliosarcoma following either intratumoral DTI-015 (n=59), or ethanol vehicle (n=13), or no treatment (n=35).
Both control and vehicle-treated groups demonstrated essentially one steep phase, with 90% of the animals dying between days 9 and 24. In contrast, the DTI-015-treated group demonstrated four phases: an initial steep phase in which animals died between days 9 and 24; a second less steep phase in which animals died between days 25 and 166; a third shallow phase in which animals died between days 251 and 325; and a fourth phase in which animals lived a normal life span.
Histopathology
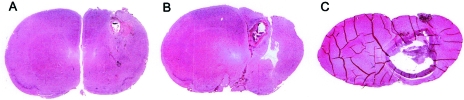
Following implantation of tumor cells, the tumors sometimes appeared as single foci but often grew in multifocal fashion demonstrating both upper and lower foci (Figure 2, A and B). The tumors were very healthy and densely cellular with many mitoses. The average size of the tumor and the fact that animals began dying as early as day 9 indicate the severe therapeutic challenge posed by this model. Day 7 following tumor implantation was chosen for treatment. Figure 2 demonstrates the tumor growth at 7, 9, and 17 days postimplantation.
Figure 2.
T9 gliosarcoma 7 days (A), 9 days (B), and 17 days (C) postimplantation in untreated rats (hematoxylin and eosin-stained).
If untreated, tumors grew within 12 to 24 days into very large masses typically replacing an entire hemisphere in cross section (Figure 2C). The bulk of the tumors consisted of viable and rapidly dividing cells, although small focal areas of necrosis were occasionally observed.
Ethanol vehicle-treated controls also demonstrated tumors occupying most of a hemisphere at death (Figure 3). However, small cysts were usually found — often accompanied by necrosis, polymorphonuclear leukocytes, and some hemorrhage — presumably marking the site of injection. Apart from the small areas of necrosis, these ethanol-treated tumors were indistinguishable in size and viability from untreated control tumors. An exception was the one ethanol-treated animal that lived a normal life span, which demonstrated histology similar to the DTI-015-treated animals that lived a normal life span as described below.
Figure 3.
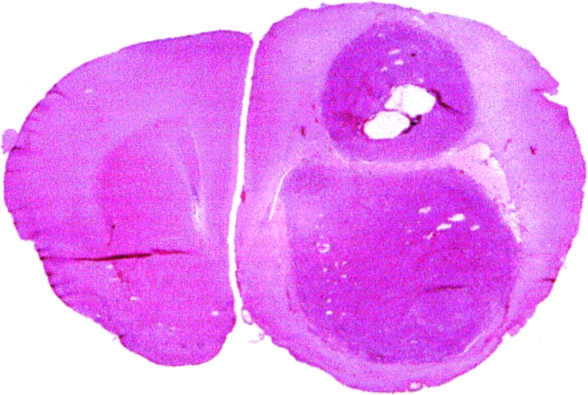
T9 gliosarcoma 14 days postintratumoral ethanol vehicle injection (hematoxylin and eosin-stained).
DTI-015-treated animals that died during the first phase, i.e., up to 24 days, displayed large, healthy tumors. However, substantial tumor cell necrosis with cysts, polymorphonuclear leukocyte infiltration, and sometimes hemorrhage was also observed usually at the top of the tumor, whereas the bottom grew out aggressively.
Most DTI-015-treated animals that died during the second phase (25–166 days posttreatment) displayed large tumors containing prominent bands of central necrosis. However, three animals that lived relatively long (94, 108, and 98 days) did not. Two of these histologically presented with acute hemorrhage and no tumor, while the third displayed a large cyst with polymorphonuclear leukocytes and some very unhealthy T9 tumor.
The DTI-015-treated animals that died during the third phase (days 251–325) demonstrated cysts, necrosis, scar tissue, and no viable T9 tumor. The cause(s) of death was unknown.
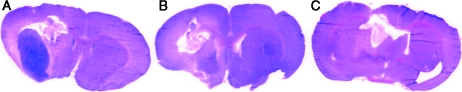
DTI-015-treated animals that lived a normal life span (600–700 days) displayed large cysts always surrounded by dense connective tissue capsules, some containing polymorphonuclear leukocytes (Figure 4, A–C). Although no viable T9 tumor was found, tumor cell necrosis was usually present, as was hemosiderin pigment. Some animals also presented with extensive infiltrates of lymphocytes and plasma cells.
Figure 4.
T9 gliosarcoma-implanted rat brain sections 700 (A), 535 (B), and 616 days (C) post DTI-015 injection (hematoxylin and eosin-stained).
In order to study the acute effects of DTI-015, a group of animals implanted with T9 gliosarcoma and treated with intratumoral DTI-015 was sacrificed healthy on days 7, 10, and 14 (n=2, 5, and 5, respectively, at each time point), as shown Figure 5. Histology demonstrated that these animals could be placed into one of three groups. One group displayed tumor destruction at the top, with the majority of the tumor left untouched (Figure 5A). In a second group, most of the tumor was successfully treated, but small amounts of viable tumor remained (Figure 5B). In a third group, the tumor was totally eradicated by the therapy and was replaced with a cyst containing necrotic tumor tissues (Figure 5C).
Figure 5.
T9 gliosarcoma-implanted rat brain sections from animals sacrificed healthy at 14 (A), 10 (B), and 14 (C) days post DTI-015 injection (hematoxylin and eosin-stained).
Discussion
This study was undertaken to investigate the efficacy of a single intratumoral injection of DTI-015 in established T9 gliosarcoma intracranial tumors. The results demonstrate that DTI-015 is a highly effective treatment in this model, producing an increase in life span in excess of 400% with 40% of the treated animals being cured.
The degree of efficacy obtained is striking considering the aggressiveness of this tumor, the multifocal tumor growth exhibited, the inability to precisely target the intratumoral injection, and the otherwise poor efficacy of BCNU in T9. In the latter regard, we previously demonstrated in this same model that the equivalent BCNU dose administered systemically produced no significant efficacy and only a 57% increase in life span when delivered intraarterially [6].
The lack of efficacy exhibited by the ethanol vehicle administered alone is not surprising given that intratumoral ethanol has never been demonstrated to be effective in brain tumors and its well-known activity in primary liver tumors is based upon the delivery of ethanol volumes in great excess of the tumors being treated [31]. In contrast, the volume of ethanol vehicle used to deliver BCNU in DTI-015 is a fraction of the tumor volume (Bodell WJ, in preparation) [2,9,11,12,25,32], estimated in the current study at 25% to 50% of the treated tumor volume. That the ethanol, however, is necessary to deliver the solubilized BCNU throughout the tumor effectively has been demonstrated in the subcutaneous Walker 256 model, where substitution of the ethanol vehicle for an aqueous delivery vehicle results in loss of efficacy [32].
The histology studies demonstrated that the direct intratumoral treatment totally eradicated the tumors in rats that lived a normal life span. A near total absence of normal tissue necrosis was observed, suggesting that DTI-015 selectively eliminated the T9 tumors without producing significant collateral damage to the surrounding normal brain. Consistent with this finding, autoradiography studies demonstrate that DTI-015 selectively perfuses high levels of 14C-BCNU throughout 9L intracranial tumors with minimal penetration occurring into surrounding normal brain [26]. Recent pharmacokinetic studies also support these findings demonstrating that DTI-015 produces a selective BCNU exposure to the tumor that is two to three logs higher than the exposure to normal brain and peripheral tissues, and results in orders-of-magnitude increases in the efficacy/safety ratio compared to intravenous BCNU [29].
For most animals that did not live a normal life span, the single intratumoral injection missed a portion of the tumor. These geographic misses are not surprising given the multifocal tumor growth exhibited in this model and the inability to precisely target the single injection of DTI-015 by simply using the same stereotactic coordinates that were used for implanting the tumor. In this regard, the tumor often did not grow exclusively at the site of drug injection, but instead grew as multiple foci. Histology indicated that often an upper tumor foci was eliminated by the treatment, whereas the injection missed a bottom foci. The use of MR guidance should improve injection targeting. In fact, diffusion MRI is currently being evaluated for its potential to guide targeted delivery by mapping the spatial distribution of DTI-015 antitumor activity in gliomas.
Although rat intracranial tumors comprise standard models for preclinical efficacy evaluation, their accuracy in predicting activity in humans is limited by their small size, and substantial differences in biology and growth pattern (e.g., lack of infiltration). The issue of size is a particularly troublesome problem in predicting the potential of local and intratumoral delivery technologies because a major limitation of these methods has been their inability to deliver efficacious drug levels over clinically relevant distances.
Our approach to solving the problem of limited perfusion distance following local / intratumoral injection has been to develop the technology of SFP. In SFP, water-miscible organic solvent vehicles (possessing no electrical charge, molecular weight of less than 1000 Da, and partition coefficient of at least 0.1) are utilized to drive the penetration of anticancer agents rather than standard aqueous vehicles. Such vehicles move readily through both the aqueous and membranous components of the tumor providing a thorough saturation of all tumor compartments by the solubilized anticancer agent. That DTI-015 produces efficacious levels of BCNU over centimeter distances following a single injection is supported by its efficacy in large Walker subcutaneous tumors [32], its activity in human brain and liver tumors [9,11,27], and by measurements of DNA adducts in biopsy specimens taken from human brain tumors treated with DTI-015 (Bodell, in preparation) [2,12].
Conclusion
We have demonstrated that BCNU, when formulated into DTI-015 and delivered intratumorally, produces a 40% cure rate in T9 intracranial gliosarcoma. We hypothesize that the remarkable efficacy demonstrated in this model, coupled with the activity profile demonstrated in early clinical testing of DTI-015 [2,9,10,11], signals the beginning of a significant advance in the treatment of brain tumors. Of course, this hypothesis can only be definitively tested by employing adequate and well-controlled randomized prospective clinical trials.
Acknowledgements
We would like to thank Gleb Budzilovich, MD (New York University School of Medicine), for the histological readings, and Julie Carter, PhD (Direct Therapeutics), for help in preparing this manuscript.
Footnotes
This work is dedicated to Joseph Ransohoff, MD, whose inspiration, leadership, and support will always be remembered.
References
- 1.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodell WJ, Giannini DD, Hassenbusch S, Levin VA. Levels of N7-(2-hydroxyethyl)guanine as a molecular dosimeter of drug delivery to human brain tumors. Neurooncology. 2001;3:241–245. doi: 10.1093/neuonc/3.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Mahaley MS, Jr., Vick NA, Black KL, Schold SC, Jr., Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, Kenealy JN. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group [see comments] Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Carter J, Singh S, Leipold M, Pietronigro D. Increased antitumor efficacy of cisplatinum and 4-hydroperoxycyclophosphamide by solvent-facilitated perfusion. Proc Am Assoc Cancer Res. 2002:A381. [Google Scholar]
- 6.Cohen AR, Pietronigro DD, Cravioto H, Flamm ES. Effect of difluoromethylornithine on the antiglioma therapeutic efficacy of intra-arterial BCNU. J Neurosurg. 1986;65:671–678. doi: 10.3171/jns.1986.65.5.0671. [DOI] [PubMed] [Google Scholar]
- 7.Dorr R, Hoff DV. Carmustine. In: Dorr R, Hoff DV, editors. Cancer Chemotherapy Handbook. Norwalk: Appleton and Lange; 1994. pp. 267–275. [Google Scholar]
- 8.Hasleton PS, O'Driscoll BR, Lynch P, Webster A, Kalra SJ, Gattamaneini HR, Woodcock AA, Poulter LW. Late BCNU lung: a light and ultrastructural study on the delayed effect of BCNU on the lung parenchyma. J Pathol. 1991;164:31–36. doi: 10.1002/path.1711640106. [DOI] [PubMed] [Google Scholar]
- 9.Hassenbusch SJ, Nardone EM, Levin VA, Leeds N, Pietronigro D. Stereotactic injection of DTI-015 into recurrent malignant gliomas: phase I/II trial. Neoplasia. 2003;5:9–16. doi: 10.1016/s1476-5586(03)80012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassenbusch S, Levin V, Sawaya R, Pietronigro D. Intratumoral DTI-015 for inoperable recurrent malignant gliomas. Neurooncology. 2000;2:A93. [Google Scholar]
- 11.Hassenbusch S, Levin V, Smith D, Phuphanich S, Chen T, Chamberlain M, Cozzens J, Paleologos N, Hariharan S, Zappulla R, Pietronigro D. Multi-center Phase I/II trial of stereotactic injection of DTI-015 into inoperable recurrent malignant gliomas; American Association of Neurological Surgeons Brain Tumor Satellite Symposium; 2002. p. A20. [Google Scholar]
- 12.Hassenbusch S, Levin VA, Bodell WJ. DTI-015 in human glioma: evidence for a large treatment volume by analysis of DNA adduct formation; Congress of Neurological Surgeons Poster Program Book; 2001. p. A189. [Google Scholar]
- 13.Hassenbusch SJ, Anderson JH, Colvin OM. Predicted and actual BCNU concentrations in normal rabbit brain during intraarterial and intravenous infusions. J Neurooncol. 1996;30:7–18. doi: 10.1007/BF00177438. [DOI] [PubMed] [Google Scholar]
- 14.Hassenbusch SJ, Anderson JH, Whiting DM. Intra-arterial chemotherapy for brain tumors. Clevel Clin J Med. 1990;57:513–520. doi: 10.3949/ccjm.57.6.513. [DOI] [PubMed] [Google Scholar]
- 15.Jones RB, Matthes SM, Dufton C, Shpall E, Bearman S, Ross M, Cagnoni P. Nitrosoureas. In: Grochow LB, Ames MM, editors. Clinician's Guide to Chemotherapy Pharmacokinetics and Pharmacodynamics. Baltimore, MD: Williams and Wilkins; 1998. pp. 331–344. [Google Scholar]
- 16.Kornblith PL, Smith BH, Leonard LA. Response of cultured human brain tumors to nitrosoureas: correlation with clinical data. Cancer. 1981;47:255–265. doi: 10.1002/1097-0142(19810115)47:2<255::aid-cncr2820470209>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Kornblith PL, Szypko PE. Variations in response of human brain tumors to BCNU in vitro. J Neurosurg. 1978;48:580–586. doi: 10.3171/jns.1978.48.4.0580. [DOI] [PubMed] [Google Scholar]
- 18.Kupersmith MJ, Frohman LP, Choi IS, Hiesinger E, Berenstein A, Wise A, Carr RE, Ransohoff J. Visual system toxicity following intra-arterial chemotherapy. Neurology. 1988;38:284–289. doi: 10.1212/wnl.38.2.284. [DOI] [PubMed] [Google Scholar]
- 19.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 20.Miller DF, Bay JW, Lederman RJ, Purvis JD, Rogers LR, Tomsak RT. Ocular and orbital toxicity following intracarotid injection of BCNU (carmustine) and cisplatinum for malignant gliomas. Ophthalmology. 1985;92:402–406. doi: 10.1016/s0161-6420(85)34036-8. [DOI] [PubMed] [Google Scholar]
- 21.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266:R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 22.Nagahiro S, Yamamoto YL, Diksic M, Mitsuka S, Sugimoto S, Feindel W. Neurotoxicity after intracarotid 1,3-bis(2-chloroethyl)-1-nitrosourea administration in the rat: hemodynamic changes studied by double-tracer autoradiography. Neurosurgery. 1991;29:19–25. doi: 10.1097/00006123-199107000-00004. discussion, p. 26. [DOI] [PubMed] [Google Scholar]
- 23.O'Driscoll BR, Hasleton PS, Taylor PM, Poulter LW, Gattamaneni HR, Woodcock AA. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 24.Omojola MF, Fox AJ, Auer RN, Vinuela FV. Hemorrhagic encephalitis produced by selective non-occlusive intracarotid BCNU injection in dogs. J Neurosurg. 1982;57:791–796. doi: 10.3171/jns.1982.57.6.0791. [DOI] [PubMed] [Google Scholar]
- 25.Pietronigro D, Drnovsky F, Cravioto H, Ransohoff J. Stereotactic intratumoral injection of DTI-015 in a rat intracranial T9 gliosarcoma model. Proc Am Assoc Cancer Res. 1999;40:583. [Google Scholar]
- 26.Pietronigro D, Frey K, Desmond T, Carter J, Ross BD. Rapid facilitated distribution of high 14C-BCNU concentrations following intratumoral injection of DTI-015 in rat 9L brain tumors. Proc Am Assoc Cancer Res. 2000;41:523. [Google Scholar]
- 27.Roh M, Charnsangavej C, Fornage B, Pietronigro D. Intratumoral injection of DTI-136 for inoperable liver tumors. Proc Am Soc Clin Oncol. 1998;17:A692. [Google Scholar]
- 28.Rosenblum MK, Delattre J-Y, Walker RW, Shapiro W. Fatal necrotizing encephalopathy complicating treatment of malignant gliomas with intra-arterial BCNU and irradiation: a pathological study. J Neurooncol. 1989;7:269–281. doi: 10.1007/BF00172921. [DOI] [PubMed] [Google Scholar]
- 29.Ross BD, Frey K, Desmond T, Pietronigro D. Rapid and selective tumor distribution of DTI-015; American Association of Neurological Surgeons, Brain Tumor Satellite Symposium; 2002. p. A60. [Google Scholar]
- 30.Shapiro WR, Green SB, Burger PC, Selker RG, VanGilder JC, Robertson JT, Mahaley MS. A randomized comparison of intra-arterial versus intravenous BCNU, with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg. 1992;76:772–781. doi: 10.3171/jns.1992.76.5.0772. [DOI] [PubMed] [Google Scholar]
- 31.Shiina S, Tagawa K, Unuma T, Terano A. Percutaneous ethanol injection therapy for the treatment of hepatocellular carcinoma. Am J Roentgenol. 1990;154:947–951. doi: 10.2214/ajr.154.5.2157329. [DOI] [PubMed] [Google Scholar]
- 32.Simpson-Herren L, Pietronigro D. Intratumoral injection of DTI-015 in the Walker 256 subcutaneous model. Proc Am Assoc Cancer Res. 1999;40:583. [Google Scholar]
- 33.Thompson GR, Larson RE. The hepatotoxicity of 1,3-bis (2-chloroethyl)-1-nitrosurea (BCNU) in rats. J Pharmacol Exp Ther. 1969;166:104–112. [PubMed] [Google Scholar]
- 34.Thompson GR, Larson RE. A toxicologic comparison of the potency and activity of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) in mice and rats. Toxicol Appl Pharmacol. 1972;21:405–413. doi: 10.1016/0041-008x(72)90160-3. [DOI] [PubMed] [Google Scholar]
- 35.Walker MD, Green SB, Byar DP, Alexander E, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Mealey J, Owens G, Ransohoff J, Robertson JT, Shapiro WR, Smith KR, Wilson CB, Strike TA. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]