Abstract
The link between extrinsic signaling, progenitor cell specification and neuronal subtype identity is central to the developmental organization of the vertebrate central nervous system. In the hindbrain and spinal cord, distinctions in the rostrocaudal identity of progenitor cells are associated with the generation of different motor neuron subtypes. Two fundamental classes of motor neurons, those with dorsal (dMN) and ventral (vMN) exit points, are generated over largely non-overlapping rostrocaudal domains of the caudal neural tube. Cdx and Hox genes are important determinants of the rostrocaudal identity of neural progenitor cells, but the link between early patterning signals, neural Cdx and Hox gene expression, and the generation of dMN and vMN subtypes, is unclear. Using an in vitro assay of neural differentiation, we provide evidence that an early Wnt-based program is required to interact with a later retinoic acid- and fibroblast growth factor–mediated mechanism to generate a pattern of Cdx and Hox profiles characteristic of hindbrain and spinal cord progenitor cells that prefigure the generation of vMNs and dMNs.
Early Wnt signaling acts in combination with retinoic acid and/or FGF signals to induce the gene expression profiles forecasting differentiation of motor neurons characteristic of the hindbrain and spinal cord.
Introduction
During the early development of the vertebrate central nervous system, the position of generation of post-mitotic neurons depends on the patterning of progenitor cells along the dorsoventral and rostrocaudal axes of the neural tube [ 1– 3]. At many levels of the neuraxis, the dorsoventral pattern of progenitor cells, which later gives rise to motor, sensory, and local circuit neurons, is initiated by the opponent signaling activities of Sonic hedgehog (Shh) and bone morphogenetic proteins [ 2, 4, 5]. In contrast, the rostrocaudal pattern of neural progenitor cells that differentiate into distinct neuronal subtypes is imposed, in part, by opponent retinoid and fibroblast growth factor (FGF) signals [ 6– 9]. Within the hindbrain and spinal cord, the rostrocaudal positional identity of neurons is reflected most clearly by the generation of different motor neuron (MN) subtypes. One fundamental distinction in MN subtype identity is the emergence of two major classes of MNs that exhibit distinctive axonal trajections, ventral exiting motor neurons (vMNs) and dorsal exiting motor neurons (dMNs) [ 10]. vMNs include most spinal MNs as well as hypoglossal and abducens MNs of the caudal hindbrain [ 1, 10, 11], whereas dMNs are found throughout the hindbrain and at cervical levels of the spinal cord [ 10]. Each of the many subsequent distinctions in MN subtype identity emerge through the diversification of these two basic neuronal classes [ 2].
Despite many advances in defining the mechanisms of MN diversification [ 7, 8, 12– 14], it remains unclear how neural progenitors in the hindbrain and spinal cord acquire a rostrocaudal positional character that results in the generation of dMN and vMN classes. At both hindbrain and spinal levels, Hox genes are informative markers of the rostrocaudal positional identity of progenitor cells. Within the hindbrain, distinct rhombomeres are delineated by the nested expression of 3′ Hox genes [ 3, 15], whereas the spinal expression of 5′ Hox genes distinguishes progenitor cells and post-mitotic neurons at cervical, brachial, thoracic, and lumbar levels [ 1, 6– 8, 13, 16]. Moreover, Hox genes are determinants of MN subtype identity in both hindbrain and spinal cord. In the hindbrain, for example, the restricted expression of Hoxb1 helps to determine the identity of facial MNs [ 1, 17– 20], and in the spinal cord the restricted expression of Hox6, Hox9, and Hox10 proteins establishes MN columnar subtype [ 7, 16]. In addition, a more complex Hox transcriptional regulatory network specifies spinal MN pool identity and connectivity [ 21]. The neural pattern of Hox expression is, in turn, regulated by members of the Cdx homeobox gene family [ 6, 22– 25]. Cdx genes are transiently expressed in the caudal-most region of the neural plate prior to the onset of 5′ Hox gene expression [ 26– 28] and appear to be direct regulators of the expression of 5′ Hoxb genes [ 6, 23, 24, 27]. Thus, analysis of spatial profiles of Cdx and Hox gene expression may provide clues about the identity of signals that pattern MN subtypes in the hindbrain and spinal cord.
Several recent studies have provided insight into the signals that impose rostrocaudally-restricted patterns of neural Cdx and Hox expression. Retinoic acid (RA) and FGF signals appear to have opponent roles in the rostrocaudal patterning of Hox gene expression in the caudal hindbrain (cHB) and spinal cord [ 6, 8]. Mesodermal-derived RA signals promote the expression of Hox genes characteristic of the cHB and rostral spinal cord (rSC) [ 11, 29, 30], whereas FGF signals pattern the expression of Hox genes at more caudal levels of the spinal cord. At an earlier developmental stage, neural progenitors have been shown to acquire caudal forebrain, midbrain, and rostral hindbrain positional identities in response to graded Wnt signaling at the gastrula stage [ 31, 32]. It is unclear, however, whether an early phase of Wnt signaling is also required to establish Cdx and Hox gene expression profiles characteristic of the cHB and spinal cord, in turn specifying the generation of dMN and vMN subtypes.
This study uses in vitro assays of neural cell differentiation to obtain evidence that early Wnt signaling does indeed have a crucial role in specifying the identity of hindbrain and spinal cord progenitor cells as revealed by profiles of Cdx and Hox gene expression. This early influence of Wnt signaling is later refined by retinoid and FGF signals to impart additional rostrocaudal distinctions in Hox expression that correlate tightly with the generation of dMNs and vMNs. Our findings therefore define a crucial early role for Wnt signaling in inducing profiles of Cdx and Hox expression that prefigure the differentiation of dMN and vMN subtypes in the developing hindbrain and spinal cord.
Results
Transcriptional Markers of Progenitor Cell Position and MN Subtype
To explore how progenitor cells of different rostrocaudal regional identity differentiate into dMNs and vMNs, we analyzed a panel of transcription factors that are expressed in different temporal and spatial patterns in the developing hindbrain and spinal cord.
In the hindbrain, progenitor cells do not express Cdx genes [ 22, 26]. Cells in rhombomeres (r)3 and r5—here defined as the rostral hindbrain (rHB)—express Krox20 (Figure1D, [ 33]) and generate both dMNs defined by the expression of Tbx20 +/Isl + and vMNs defined by the expression of Hb9 +/Isl + ( Figure 1D, [ 34– 36]). In contrast, progenitor cells in r7 and r8—here defined as the cHB—express Hoxb4 but not Hoxb8 or Hoxc9 (termed Hoxb4 + /b8 − /c9 − cells and all monitored by in situ hybridization) ( Figure 1A, 1B, and 1D) and generate Tbx20 +/Isl + dMNs. ( Figure 1C, and 1D, [ 34– 36]).
Figure 1. Profiles of Hox Gene Expression, dMNs, and vMNs in the Hindbrain and Spinal Cord .
(A) Schematic figure of the caudal embryonic neural tube divided into four distinct regions along the rostrocaudal axis: rHB; r7 and r8 defined as cHB; the region of the spinal cord located at the level of somite 6–19 defined as the rSC; and the region located caudal to somite 19 defined as cSC.
(B) In a stage 17 (30-somite) chick embryo, the rostral borders of Hoxb4, Hoxb8, and Hoxc9 expression are located just caudal to the otic vesicle (OV) (at the level of the r6/7 border), at the level of somite 5/6, and at the level of somite 19/20, respectively. Hoxb4 expression in the absence of Hoxb8 and Hoxc9 is characteristic of cells in the cHB (r7 and r8). Expression of Hoxb4 and Hoxb8 in the absence of Hoxc9 is characteristic of the rSC domain, and expression of Hoxb4, Hoxb8, and Hoxc9 is characteristic of cells in the cSC domain.
(C) In stage 20 (42-somite) chick embryos, the rostral boundaries of expression of Hoxb4, Hoxb8, and Hoxc9 in the neural tube are maintained as in a stage 17 embryo (± 1-somite). Tbx20 +/Isl + dMNs are present at high numbers in the cHB and at lower numbers in the rSC. No Tbx20 +/Isl + dMNs are found in the cSC. In contrast, Hb9 +/Isl + vMNs are present at high numbers in both rSC and cSC, and at lower numbers in r8 of the cHB. Hoxc9 protein is expressed in a subset of Hb9 +/Isl + vMNs in the cSC and thus, distinguishes vMNs in the cSC from vMNs in the rSC.
(D) Horizontal bars represent rostrocaudal restrictions (applied to Figure 1A) of marker genes expressed by neural progenitor cells in the stage 17 neural tube, and by MNs in stage 20 embryos.
Cells that give rise to the spinal cord can, at Hamburger and Hamilton (HH) stages 6–8, be defined by their profile of CdxB and CdxC expression ( Figure 2 and [ 22, 26]). Later, at stage 17, a complex spatial pattern of Hox gene expression defines progenitor cells of different regional identities ( Figure 1). Spinal progenitor cells at prospective cervical/brachial levels adjacent to somites 6–19 (here termed “rostral” spinal cord [rSC])—express Hoxb4 and Hoxb8, but do not express Hoxc9 (termed Hoxb4 +/b8 +/ c9 − cells) ( Figure 1A, 1B, and 1D). MN progenitor cells at this level generate Hb9 +/Isl1 + vMNs, but only a few Tbx20 +/Isl + dMNs ( Figure 1C, 1D and [ 34– 36]). Progenitor cells at thoracic levels of the spinal cord—adjacent to somite 20–30 (here termed “caudal” spinal cord [cSC])—express Hoxb4, Hoxb8, and Hoxc9 (termed Hoxb4 +/b8 +/c9 + cells) ( Figure 1A, 1B, and 1D) and generate Hb9 +/Isl + vMNs that can be distinguished from those found at more rostral levels by their expression of Hoxc9 (Hb9 +/Isl +/Hoxc9 + MNs) ( Figure 1C and 1D, [ 7]). Since most of these markers are also expressed by non-neural cells, we used Sox proteins as general neural markers: Sox1 at stage 17 [ 37, 38] and Sox2 in combination with Sox3 at stage 8 as presumptive neural markers [ 39, 40]. In addition, we used Otx2 as a marker for neural cells located rostral to the midbrain/hindbrain boundary [ 41, 42].
Figure 2. Expression Pattern of CdxB and CdxC .
Whole mount in situ hybridization of CdxC and CdxB in HH stage 8 embryos (4-somite). Expression of CdxC and CdxB is limited to levels adjacent and caudal to the node. The node is indicated by the red arrowhead.
Hindbrain and Spinal Cord Progenitor Cells Acquire Rostrocaudal Regional Identity at Early Somite Stages
To study the patterning of Cdx and Hox genes by neural progenitor cells and its link to the differentiation of MN subtypes, we used in vitro differentiation assays that employed stage 4–8 prospective hindbrain and spinal cord explants, and stage 4 prospective forebrain (FB) explants. In stage 4 caudal (C) explants, cells have been exposed to caudalizing signals at the time of their isolation [ 31, 32], and these explants were used to examine the signals that specify hindbrain and spinal cord character. Cells in stage 4 FB explants have not been exposed to caudalizing signals at the time of their isolation [ 31, 32], and these explants were used in attempts to reconstitute more completely the events that direct the generation of dMNs and vMNs from “naive” neural cells.
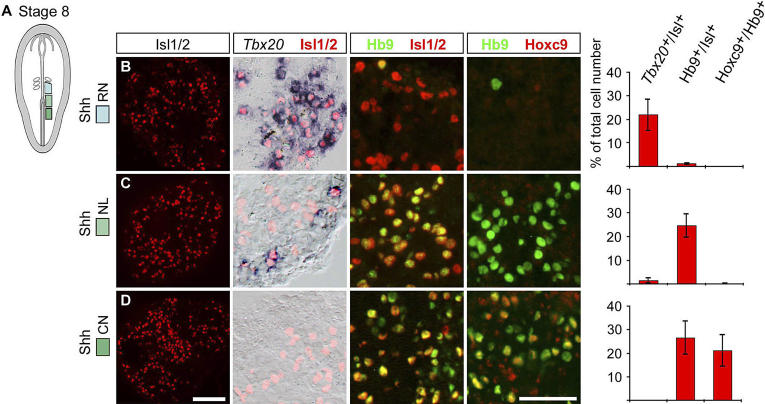
Prior to stage 8, the caudal neural plate is either specified as rSC or cSC (HH 5–7), but no explants generating cells of cHB character can be isolated (unpublished data). By stage 8, however, cells in explants isolated at a position just rostral to the regressing Hensen's node (RN explants) did not express CdxB and CdxC ( Figure 2) and generated cells characteristic of the cHB that expressed Hoxb4 alone ( Hoxb4 +/ b8 −/c9 − cells (85 ± 10% of total cell number) after 40 h in culture ( Figure 3B). In contrast, cells in stage 8 explants isolated at the level of the node (NL explants) expressed CdxB and CdxC ( Figure 2). These explants also generated cells that expressed Hoxb4 and Hoxb8 in the absence of Hoxc9 ( Hoxb4 +/b8 +/ c9 − cells; 96 ± 2% of total cell number), a marker profile characteristic of the rSC ( Figure 3C). Cells in stage 8 explants isolated caudal to the node (CN explants) also expressed CdxB and CdxC ( Figure 2), and generated Hoxb4 +/ b8 +/ c9 +cells (88 ± 7% of total cell number), a profile characteristic of the cSC ( Figure 3D). Thus by stage 8, prospective hindbrain and spinal cord progenitor cells appear to have acquired a coarse rostrocaudal regional identity.
Figure 3. Hindbrain and Spinal Cord Progenitor Cells Acquire Rostrocaudal Regional Identity at the Early Somite Stage.
(A) Schematic drawing of a stage 8 chick embryo. The boxed regions indicate neural plate explants cultured in vitro for 40 h.
(B–D) Sox1 was used as a general neural marker. Bars represent mean ± s.e.m. number of cells in Hoxb4 +/ b8 −/c9 −, Hoxb4 +/b8 +/ c9 −, and Hoxb4 +/b8 +/c9 + domains, respectively, as percentage of total cell number. Each row represents consecutive sections from a single explant.
(B) Explants isolated from the RN generated Hoxb4 +/ b8 −/c9 − cells and few Hoxb4 +/b8 +/ c9 − cells ( n = 8 explants).
(C) Explants isolated at the NL generated Hoxb4 +/b8 +/ c9 − cells ( n = 15 explants).
(D) Explants isolated from the CN generated Hoxb4 +/b8 +/c9 + and only a few Hoxb4 +/b8 +/ c9 − cells ( n = 12 explants). Scale bar represents 100 μm.
To determine whether the Hox gene profiles generated in stage 8 neural plate explants were correlated with the emergence of dMNs and vMNs, we exposed explants to the diffusible N-terminal fragment of the Shh protein (Shh-N) that exhibits MN-inducing activity [ 43, 44]. In the presence of Shh-N (15 nM) for ˜50 h, stage 8 RN explants generated many Tbx20 +/Isl + dMNs (22 ± 7% of total cell number) and very few Hb9 +/Isl + vMNs (1 ± 0.5% of total cell or number), a profile of MNs characteristic of the cHB (r7 and rostral r8) ( Figure 4B). Under these conditions, NL explants generated very few Tbx20 +/Isl + dMNs (1.5 ± 1% of total cell number) and many Hb9 +/Isl + vMNs (25 ± 4% of total cell number). Few, if any, of the induced Hb9 +/Isl + vMNs co-expressed Hoxc9 (0.3 ± 0.3% of total cell number), indicative of an rSC positional character ( Figure 4C). CN explants did not generate Tbx20 +/Isl + dMNs but did generate Hb9 +/Isl + vMNs (27 ± 7% of total cell number), most of which expressed Hoxc9 protein (80 ± 6% of Hb9 + cells)—a profile characteristic of the thoracic spinal cord ( Figure 4D). Thus, by stage 8, progenitor cells that occupy different rostrocaudal positions within the caudal neural plate, defined in part by their Cdx profiles, are specified as hindbrain and spinal cord cells of either rostral or caudal regional character, and have acquired sufficient positional information to differentiate into dMNs and vMNs in a position-appropriate manner.
Figure 4. Hindbrain and Spinal Cord Progenitor Cells Generate dMNs and vMNs as Predicted by Their Respective Hox Expression Profile When Exposed to Shh-N .
(A) Schematic of a stage 8 chick embryo. The boxed regions indicate isolated neural plate explants cultivated alone for 4 h and then exposed to Shh-N (15 nM) for an additional ˜50 h.
(B–D) Bars represent mean ± s.e.m. number of Tbx20 +/Isl +, Hb9 +/Isl +, and Hb9 +/Hoxc9 + cells, respectively, as percentage of total cell number. Each row represents consecutive sections from a single explant.
(B) Explants isolated from the RN generated Tbx20 +/Isl + cells and a few Hb9 +/Isl + cells ( n = 9 explants).
(C) Explants isolated at the NL generated Hb9 +/Isl + cells, only a few Tbx20 +/Isl + cells, and no Hb9 +/Isl +/Hoxc9 + cells ( n = 12 explants).
(D) Explants isolated from the CN generated Hb9 +/Isl + cells of which 80 ± 6% expressed Hoxc9 but no Tbx20 +/Isl + cells appeared ( n = 10 explants). Scale bars represent 100 μm (Isl) and 50 μm (double labels), respectively.
Spinal Cord Cells of Caudal Character Are Specified at the Late Gastrula Stage
To examine when the early pattern of Cdx and Hox profiles characteristic of hindbrain and spinal cord progenitor cells is established, we first monitored the generation of cells expressing CdxB and CdxC by in situ hybridization in stage 4 C explants cultured for 15 h in vitro, corresponding to a stage 8 embryo. We tracked the rostrocaudal orientation of these explants by labeling cells at the caudal margin with DiI crystals. Under these conditions, CdxB + and CdxC + cells (36 ± 11% of total cell number) were generated in a caudal domain of the explants ( Figure 5D), adjacent to the DiI labeling. To examine the rostrocaudal identity of the Cdx + cells, we monitored the generation of Hoxb4 +, Hoxb8 +, and Hoxc9 + cells by in situ hybridization in stage 4 C explants cultured for 44 h. Stage 4 C explants cultured for 44 h generated Krox20 + cells (45 ± 15% of total cell number), characteristic of the rHB away from the DiI label and, in a separate caudal domain (adjacent to the DiI label) Hoxb4 +/ b8 +/ c9 + cells (52 ± 12% of total cell number) characteristic of the cSC ( Figures 7B and 5E). Thus, the generation of CdxB +/C + cells and Hoxb4 +/ b8 +/ c9 + cells in a similar caudal domain of the explants supports the view that the expression of Cdx genes in neural plate cells is restricted to prospective spinal cord cells. In contrast, only a small domain of the explants generated Hoxb4 +/b8 +/ c9 − cells (12 ± 8% of total cell number), a marker profile characteristic of the rSC. Moreover, these explants lacked cells that expressed Hoxb4 alone ( Hoxb4 +/ b8 −/c9 − cells) ( Figures 5E and 7B), characteristic of the cHB. These results provide evidence that, at stage 4, prospective caudal neural plate cells are specified as cells of rHB and cSC character, and only later acquire cHB or rSC character.
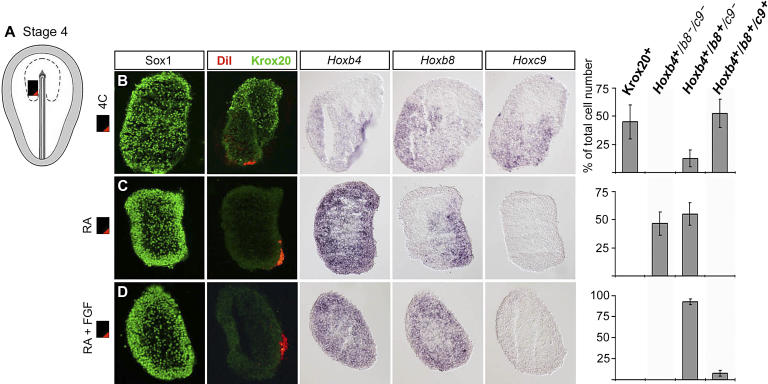
Figure 5. Cells of Spinal Cord Character Are Induced by Combinatorial Wnt and FGF Signaling at the Late Gastrula Stage.
(A and B) Caudal (C) neural plate tissue explants (black box) were isolated from HH stage 4 embryos and embedded in collagen matrix where their rostrocaudal orientation was maintained during in vitro cultivation for 15 h, corresponding to a stage 8 embryo (A, D, F, H, and J) or for 44 h, corresponding to a stage 17 ˜30-somite embryo (B, E, G, I, and K).
(C) Schematic drawing indicating the expression pattern of Wnt (red) and Fgf genes (green) in the primitive streak and caudal ectoderm, and in the node and primitive streak, respectively. Black dotted line indicates the presumptive neural plate.
(D–K) Each row represents consecutive sections from a single explant.
(D, F, H, and J) Sox2/3 was used as a presumptive neural marker.
(D) Stage 4 C explants were DiI-labeled and cultured alone. Cells in the caudal domain of the explants, close to the DiI-labeled cells, expressed CdxB and CdxC ( n = 12 explants). White arrowhead indicates the DiI-labeled cells.
(E, G, I, and K) Sox1 was used as a general neural marker.
(E) Cells in the rostral domain of 4 C explants cultured alone expressed Krox20, whereas Hoxb4 +/b8 +/c9 + cells appeared in the caudal domain of the explants. A small domain of Hoxb4 +/b8 +/ c9 − cells, but no domain of cells expressing Hoxb4 alone, were generated ( n = 30 explants).
(F) Explants cultured in the presence of mFrz8CRD-IgG conditioned medium (300 μl/ml culture medium) generated Sox2/3 + neural cells but no CdxB or CdxC positive caudal neural cells ( n = 11 explants).
(G) Explants cultured in the presence of mFrz8CRD-IgG conditioned medium (300 μl/ml culture medium) generated Otx2 + but no, or few, caudal neural cells ( n = 12 explants).
(H) Explants cultured in the presence of SU5402 (5 μM), an inhibitor of FGF signaling, generated Sox2/3 + neural cells but no CdxB or CdxC positive caudal neural cells ( n = 8 explants).
(I) Explants cultured in the presence of SU5402 (5 μM), an inhibitor of FGF signaling, generated Otx2 + but no, or few, caudal neural cells ( n = 9 explants).
(J) Simultaneous exposure to Wnt3A (˜75 ng/ml) and FGF4 (60 ng/ml) resulted in the generation of cells that expressed CdxB and CdxC in the entire explant ( n = 17 explants). Scale bar represents 100 μm.
(K) Exposure to Wnt3A (˜75 ng/ml) and FGF4 (60 ng/ml), in combination, almost completely blocked the generation of Krox20 + rHB cells, and only Hoxb4 +/b8 +/c9 + spinal cord cells were generated ( n = 17 explants). Scale bar represents 100 μm.
Figure 7. RA Induces cHB Cells, and RA and FGF, in Combination, Induce rSC Cells.
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Black box indicates caudal (C) neural plate explant isolated and cultured in vitro for 44 h. The red mark indicates the caudal margin of the explant labeled with DiI. Bars represent mean ± s.e.m. number of cells in Krox20 + Hoxb4 + /b8 −/c9 −, Hoxb4 +/b8 +/ c9 −, and Hoxb4 +/b8 +/c9 + domains, respectively, as percentage of total cell number.
(B–D) Each row represents consecutive sections from a single explant.
(B) Control stage 4 C explants generated Krox20 + cells in the rostral, and Hoxb4 +/b8 +/ c9 + cells were generated in the caudal region of the explant, adjacent to the DiI-labeled cells. A small domain of Hoxb4 +/b8 +/ c9 − cells was generated in the medial region but no domain of cells expressing Hoxb4 alone was generated ( n = 7 explants).
(C) RA (10 nM) blocked the generation of Krox20 + and induced Hoxb4 +/ b8 −/c9 −cells in the rostral region. Adjacent to the DiI-labeled cells in the caudal region of the explant, Hoxb4 +/b8 +/ c9 − cells, but no Hoxb4 +/b8 +/c9 + cells, were generated ( n = 6 explants).
(D) RA (10 nM) and FGF4 (30 ng/ml), in combination, generated Hoxb4 +/b8 + /c9 − cells in both the rostral and caudal regions. No, or a few, Hoxb4 +/b8 +/ c9 + cells appeared, and no Krox20 + or Hoxb4 + /b8 −/c9 − cells were generated ( n = 5 explants). Scale bar represents 100 μm.
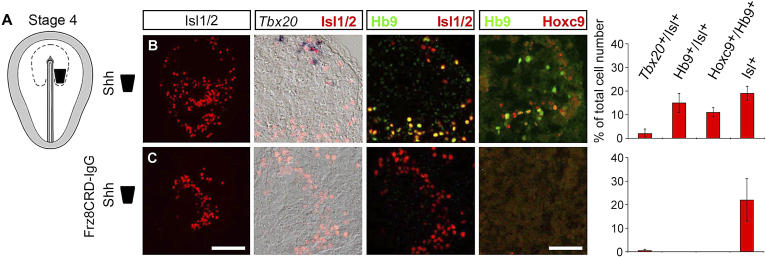
We next examined whether prospective rHB and cSC cells have acquired sufficient rostrocaudal positional information by stage 4 to permit them to differentiate into dMNs or vMNs when exposed to Shh-N. To test this possibility, we cultured stage 4 C explants for 28 h to allow cells to acquire a stable rostrocaudal positional identity, and then for an additional 38 h in the presence of Shh-N (15 nM) (combined culture time corresponding to a stage 20, ˜40-somite embryo). In the presence of Shh-N, Tbx20 +/Isl + dMNs (1 ± 1% of total cell number) and Hb9 +/Isl + vMNs (16 ± 2% of total cell number) were generated in separate domains of the explants ( Figure 6B). A majority of the vMNs expressed Hoxc9 (10 ± 2% of total cell number)—a profile characteristic of vMNs at thoracic levels of the spinal cord ( Figure 6B). These results indicate that by stage 4, cells in the prospective caudal neural plate have acquired a positional character that permits them to differentiate into dMNs and vMNs.
Figure 6. Wnt Signaling Is Required for the Generation of dMNs and vMNs in the Hindbrain and Spinal Cord.
(A) Caudal (C) neural plate tissue explants (black box) were isolated from HH stage 4 embryos. Explants were cultured alone or in the presence of mFrz8CRD-IgG for 28 h and then exposed to Shh-N (15 nM) for an additional 38 h.
(B–C) Bars represent mean ± s.e.m. number of Tbx20 +/Isl +, Hb9 +/Isl +, Hb9 +/Hoxc9 +, and Isl1 + cells, respectively, as percentage of total cell number. Each row represents consecutive sections from a single explant.
(B) Stage 4 C explants cultured with Shh-N alone generated Tbx20 +/Isl + cells in the rostral domain of the explant and Hb9 +/Isl + cells and Hb9 +/Hoxc9 + cells in the caudal domain of the explant ( n = 18 explants).
(C) Explants cultured in the presence of mFrz8CRD-IgG conditioned medium (500 μl/ml culture medium) and Shh-N generated Isl1/2 + cells but no Tbx20 +, Hb9 +, or Hoxc9 + cells ( n = 7 explants). Scale bars represent 100 μm (Isl1/2) and 50 μm (double labels), respectively.
Joint Wnt and FGF Signaling at Late Gastrula Stages Specifies Spinal Cord Character
We next addressed the identity of the secreted signals that might be involved in the early specification of cells of spinal cord character. Several Wnt and Fgf genes are expressed in and around the prospective caudal neural plate [ 45– 47], and the specification of rHB cells at stage 4 requires convergent Wnt and FGF signaling [ 32, 48]. To examine whether combined Wnt and FGF signaling is required for the specification of cells of spinal cord positional character, we cultured stage 4 C explants in the presence of a soluble fragment of the Frizzled receptor 8 protein (Frz8CRD-IgG), an antagonist of Wnt signals [ 32, 49, 50], or with SU5402, an antagonist of FGF receptor signaling [ 49, 51, 52] and monitored the expression of Cdx and Hox genes.
In the presence of Frz8CRD-IgG or SU5402 (5 μM) for 15 h, the expression of both CdxB and CdxC was blocked ( Figure 5F and 5H). After 44 h under these conditions, the generation of cells of both rHB (Krox20 +) and of spinal cord ( Hoxb4 +/b8 +/ c9 − and Hoxb4 +/b8 +/c9 + ) character was almost completely blocked (3 ± 3% caudal cells remaining versus 64 ± 10% in the controls) ( Figure 5G and 5I). Instead, Otx2 + forebrain-like cells were generated (79 ± 9% of total cell number versus 0% in the controls) ( Figure 5G and 5I). These results support the idea that the specification of cells of spinal cord positional character also involves convergent Wnt and FGF signaling.
To test whether Wnt signaling in prospective rHB and cSC cells is required for the generation of vMNs and dMNs, stage 4 C explants were cultured in the presence of mFrz8CRD-IgG, and after 28 h Shh-N (15 nM) was added for a further 38 h. Under these conditions, the generation of Tbx20 +/Isl + dMNs and Hb9 +/Isl + and Hoxc9 +/Hb9 + vMNs was blocked (0% of total cell number), and instead Isl +/ Tbx20 −/Hb9 −/Hoxc9 − neurons, characteristic of the ventral forebrain [ 53, 54], were generated (18 ± 3% of total cell number) ( Figure 6C). Thus, exposure of prospective caudal neural plate cells to Wnt signals is required for the generation of vMNs and dMNs.
High-Level Wnt Signaling Promotes Spinal Cord Character
We next examined whether differences in the level or duration of exposure to Wnt and FGF signals contribute to the early distinction in hindbrain and spinal cord character. To test this possibility, we exposed stage 4 C explants to exogenous Wnt and FGF signals for 15 h or 44 h in vitro. In explants exposed to Wnt3A (75 ng/ml) and FGF4 (60 ng/ml) simultaneously for 15 h, CdxB + and CdxC + cells were generated throughout the entire explant ( Figure 5J). After 44 h of culture under these conditions, the generation of Krox20 + cells was largely suppressed (3 ± 2% of total cell number versus 45 ± 15% in the controls) and most cells acquired a Hoxb4 +/b8 +/c9 + cSC character (96 ± 2% of total cell number versus 52 ± 12% in the controls) ( Figure 5K). To examine whether mesodermal cells were generated under these conditions, we monitored the expression of Mox1[ 55], which is expressed in caudal paraxial mesoderm and of Brachyury (Bra) [ 56], which at caudal levels is expressed in both the mesoderm and in cells of the forming caudal neural plate ( Figure S3G). No Mox1 cells and no, or very few, Bra + cells were induced ( Figure S3D), indicating that the few Bra + cells represent caudal neural cells and not mesodermal cells. Exposure to FGF4 (60–120 ng/ml), or Wnt3A (150 ng/ml) alone did not increase the number of cells expressing Cdx genes, nor did it change the ratio of Krox20 + and Hoxb4 +/b8 +/c9 + cells ( Figure S1 and unpublished data). These results are consistent with the view that exposure of cells to prolonged or higher level Wnt and FGF signaling promotes the specification of cells of spinal cord rather than midbrain or hindbrain character.
RA Imparts Caudal Character to Hindbrain Cells and Rostral Character to Spinal Cord Cells
How then, are rostral and caudal sub-domains of the hindbrain and spinal cord established? Since Wnt signaling contributes to the distinction in specification of prospective rHB and cSC cells at gastrulation stages, we examined first whether exposure of prospective hindbrain and spinal cord cells to Wnt signals beyond stage 8 was required for the later specification of cHB and rSC cells. Stage 8 caudal neural plate explants exposed to Frz8CRD-IgG still generated cells of cHB and spinal cord character (unpublished data); indicating that prolonged exposure to Wnt signals is not required for the generation of these two sub-domains of the caudal neural tube.
By stage 8, the retinoid acid synthesizing enzyme retinaldehyde dehydrogenase 2 (RALDH2) is expressed in the paraxial mesoderm adjacent to the prospective cHB and rSC [ 57– 59]. RA signaling might therefore promote the generation of cells of cHB and rSC character by acting on neural cells that have already acquired an initial caudal character, through convergent Wnt and FGF signaling. To test this idea, we exposed stage 4 C explants to RA, and used the combinatorial expression of Hoxb4, Hoxb8, and Hoxc9 to distinguish cells of cHB, and rSC or cSC character. Since Cdx expression does not distinguish between cells of rSC and cSC character, we did not monitor Cdx expression in these experiments. To map prospective rHB and cSC cells in these explants, we tracked their rostrocaudal orientation by labeling cells at the caudal margin with DiI crystals. We found that Hoxb4 +/ b8 +/ c9 + cells derive from the caudal-most region of the explants, whereas Krox20 + cells derive from a rostral domain of the explants ( Figure 7B).
In the presence of RA (10 nM), no Krox20 + cells of rHB character were generated in the rostral domain of the explants—the domain lacking DiI-labeled cells. Instead, Hoxb4 +/ b8 −/c9 − cells characteristic of the cHB (47 ± 10% of total cell number versus 0% in the controls) were generated in this domain ( Figure 7C). In the caudal domain of the explants—the domain adjacent to DiI-labeled cells—the presence of RA (10 nM) blocked the generation of Hoxb4 +/b8 +/c9 + cells characteristic of the cSC (0% of total cell number versus 52 ± 12% in controls), and instead, Hoxb4 +/b8 +/ c9 − cells characteristic of the rSC (55 ± 10% of total cell number versus 12 ± 8% in controls) were generated ( Figure 7C). Furthermore, RA was unable to induce caudal character in stage 4 prospective FB cells that had not been exposed to caudalizing signals [ 31] ( Figure S2E). These results provide evidence that RA promotes the generation of Hoxb4 +/ b8 −/c9 − cHB cells by blocking Krox20 and inducing Hoxb4 expression in caudalized prospective Krox20 + rHB cells. In addition, they show that RA induces Hoxb4 +/b8 +/ c9 − rSC cells by preventing Hoxc9 expression in prospective cSC cells.
FGF Signaling Contributes to the Distinction between Cells of cHB and rSC Character
We next examined how the distinction between cHB and rSC cells is established. RA promotes the generation of cHB cells, and FGFs promote the expression of Hox genes characteristic of the caudal region of the spinal cord [ 8, 46]. Moreover, RA and FGF signals act in an opponent manner during rostrocaudal patterning of Hox gene expression and MN progenitor cell specification [ 6, 8]. These observations led us to examine the possibility that FGF and RA signals converge during the initial assignment of cHB and rSC positional character.
We exposed stage 4 C explants to both RA (10 nM) and FGF4 (30 ng/ml) and assayed their Hox profile after 44 h. The generation of Hoxb4 +/ b8 −/c9 − cHB cells was suppressed (1 ± 1% of total cell number versus 47 ± 10% in explants cultured with RA alone), and most cells (92 ± 3% of total cell number versus 55 ± 10% in explants cultured with RA alone) expressed Hoxb4 and Hoxb8, a profile indicative of rSC character. A few Hoxb4 +/ b8 +/ c9 + cells (7 ± 4% of total cell number versus 0% in explants cultured with RA alone), characteristic of cSC character were also detected ( Figure 7D). Thus, in the presence of RA, prolonged exposure to FGF signals promotes the generation of cells of rSC at the expense of cHB character. This finding supports the idea that the status of FGF signaling biases whether RA exposure induces Hox gene profiles characteristic of cHB or rSC.
Combinatorial Wnt, RA, and FGF Signals Impose Hindbrain and Spinal Cord Character in Naive Neural Cells
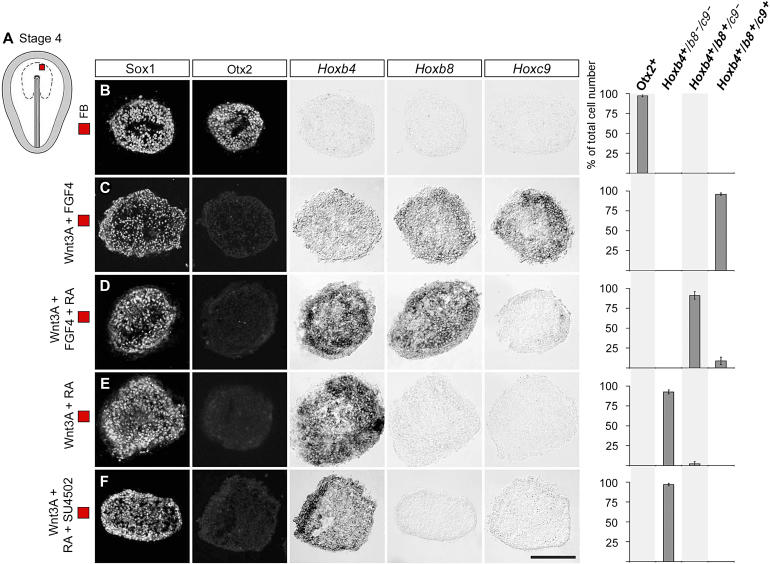
As a further test of the sufficiency of Wnt, FGF, and RA signals in establishing hindbrain and spinal cord pattern, we examined whether a combination of these factors can establish appropriate Cdx and Hox gene profiles in naive rostral forebrain cells that appear not to have been exposed to caudalizing signals in ovo.
Stage 4 FB explants cultured alone for 44 h generated Sox1 +/Otx2 + cells (94 ± 4% of total cell number) ( Figures 9B and S2B). Wnt3A, FGF4, or RA added separately, or FGF4 and RA added in combination, did not induce cells of caudal neural character ( Figure S2; see also [ 31, 32]). Stage 4 FB explants exposed to Wnt3A and FGF4 in combination for 15 h, corresponding to stage 8, generated CdxB + and CdxC + cells (95 ± 4 % of total cell number) ( Figure 8C). After 44 h of culture, Hoxb4 +/b8 +/c9 + cSC cells (96 ± 2% of total cell number) and only a few Krox20 + cells (4 ± 1% of total cell number) were generated ( Figure 9C). Thus, combined Wnt and FGF signals establish Cdx and Hox gene profiles indicative of cSC identity in prospective FB cells.
Figure 9. Combinatorial Wnt, RA, and FGF Signaling Reconstruct Hox Gene Profiles Characteristic of the cHB and Spinal Cord .
(A) Schematic drawing of a stage 4 chick embryo. Dotted line indicates the presumptive neural plate. Red box indicates FB explants isolated and cultured in vitro for 44 h.
(B–F) Sox1 was used as a general neural marker. Bars represent mean ± s.e.m. number of cells in Otx2 +, Hoxb4 +/ b8 −/c9 −, Hoxb4 +/b8 +/ c9 −, and Hoxb4 +/b8 +/c9 + domains, respectively, as percentage of total cell number. Each row represents consecutive sections from a single explant.
(B) Control stage 4 FB explants generated Sox1 +/Otx2 + but no caudal neural cells ( n = 24 explants).
(C) Stage 4 FB explants cultured in the presence of Wnt (˜150 ng/ml) and FGF (60 ng/ml) generated Hoxb4 +/b8 +/c9 + cells and only a few Krox20 + cells ( n = 24 explants).
(D) Cultivation in the presence of Wnt3A (˜150 ng/ml), RA (10 nM), and FGF (30 ng/ml) generated Hoxb4 +/b8 +/ c9 − cells and a few Hoxb4 +/b8 +/c9 + cells ( n = 18 explants).
(E) Cultivation in the presence of Wnt3A (˜150 ng/ml) and RA (10 nM) generated Hoxb4 +/ b8 −/c9 − cells and no, or only a few, Hoxb4 +/b8 +/ c9 − cells ( n = 28 explants).
(F) Exposure to Wnt3A (˜150 ng/ml) and RA (10 nM) in the presence of SU5402 (3 μM), an inhibitor of FGF signaling, generated Hoxb4 +/ b8 −/c9 − cells ( n = 12 explants). Scale bar represents 100 μm.
Figure 8. Wnt and FGF, in Combination, Induce Cdx Gene Expression in Prospective FB Cells .
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Red box indicates prospective FB explants used for in vitro studies.
(B–D) Sox2 and Sox3, in combination, were used as general presumptive neural markers. Each row represents consecutive sections from a single explant.
(B) Control stage 4 FB explants generated Sox2 +/3 + but no CdxC +/CdxB + presumptive neural cells ( n = 24 explants).
(C) Stage 4 FB explants cultured in the presence of Wnt (˜150 ng/ml) and FGF (60 ng/ml) simultaneously generated CdxC + /CdxB + presumptive caudal neural cells ( n = 26 explants).
(D) 4 FB explants cultivated in the presence of Wnt3A (˜150 ng/ml), RA (10 nM), and FGF (30 ng/ml) generated CdxC + /CdxB + presumptive caudal neural cells ( n = 18 explants).
(E) 4 FB explants cultivated in the presence of Wnt3A (˜150 ng/ml) and RA (10 nM) did not generate CdxC + /CdxB + presumptive caudal neural cells ( n =14 explants).
We next examined whether, in the presence of Wnt and FGF signals, RA can induce the generation of cHB or rSC cells. In the combined presence of Wnt3A (150 ng/ml), FGF4 (30 ng/ml), and RA (10 nM) for 15 h, stage 4 FB explants generated CdxB + and CdxC + cells (97 ± 3% of total cell number) ( Figure 8D). After 44 h of culture, Hoxb4 +/b8 +/ c9 − rSC cells (91 ± 5% of total cell number) and some Hoxb4 +/ b8 +/ c9 + cSC cells (9 ± 5% of total cell number), but no Hoxb4 +/ b8 −/c9 − cells of cHB character, were generated ( Figure 9D). Thus, Wnt signals in combination with RA and FGF signals predominantly induce a Hox gene profile characteristic of the rSC.
Next, we tested whether combined exposure to Wnt3A (150 ng/ml) and RA (10 nM) induces cHB cells. No CdxB + and CdxC + cells were generated after 15 h of culture ( Figure 8E). After 44 h of culture, many Hoxb4 +/ b8 −/c9 − cHB cells (92 ± 3% of total cell number versus 0% in control explants), few (3 ± 3% of total cell number) Hoxb4 +/b8 +/ c9 −, and no Hoxb4 +/ b8 +/ c9 + cells characteristic of the rSC and cSC, were generated ( Figure 9E). Moreover, in the presence of SU5402 (3 μM), Wnt3A (150 ng/ml) and RA (10 nM) still induced Hoxb4 +/ b8 −/c9 −/ cHB cells (97 ± 2% of total cell number) but no Hoxb4 +/b8 +/ c9 − cells, suggesting that the convergent activities of Wnt and RA signaling are sufficient to induce cHB cells ( Figure 9F). No combination of Wnt3A-, RA-, and FGF4-induced Mox1 + cells characteristic of caudal paraxial mesoderm, and only Wnt3A and FGF4 in combination, induced a few Bra + cells ( Figure S3B and S3C, unpublished data). These results provide evidence that Wnt signals, in combination with RA and/or FGF, are sufficient to reconstruct Cdx and Hox gene profiles indicative of cHB and both rSC and cSC in naive prospective FB explants.
We also addressed whether Hox gene profiles induced by Wnt, RA, and/or FGF signals in naive prospective FB cells predict the later generation of dMNs and vMNs. We cultivated stage 4 FB explants in the presence of Wnt, RA, and/or FGF signals for 44 h, to allow hindbrain and spinal cord progenitor cells to acquire their rostrocaudal positional identity, and for an additional 22 h in the absence or presence of Shh-N (15 nM) to induce MN differentiation. Stage 4 FB explants, grown both in the absence or presence of Shh-N (15 nM), generated Otx2 + neural progenitor cells and Isl +/ Tbx20 −/Hb9 −/Hoxc9 − ventral FB neurons ( Figure 10B and Table 1, [ 54]). Stage 4 FB cells exposed to FGF4 (60 ng/ml) and Wnt3A (150 ng/ml), which generate Hoxb4 +/b8 +/c9 + cSC progenitor cells, generated Hb9 +/Isl + and Hoxc9 +/Hb9 + vMNs in the presence of Shh-N (15nM) ( Figure 10E and Table 1). We also determined which MN subtypes are generated from the Hoxb4 +/b8 +/ c9 − rSC progenitor cells, induced by Wnt3A (150 ng/ml), FGF4 (30 ng/ml), and RA (10 nM). Addition of Shh-N (15 nM) induced a large number of Hb9 +/Isl + MNs, but few Hb9 +/Hoxc9 + or Tbx20 +/Isl + MNs—a MN profile characteristic of the rSC ( Figure 10D and Table 1). Finally, we assessed the MN subtype generated from stage 4 FB cells which generate Hoxb4 +/ b8 −/c9 − cHB progenitor cells, upon Wnt3A (150 ng/ml) and RA (10 nM) exposure. Addition of Shh-N (15 nM) induced Tbx20 +/Isl +, but only a few Hb9 +/Isl + MNs and no Hb9 +/Hoxc9 + MNs ( Figure 10C and Table 1)—a profile characteristic of cHB (r7 and r8) MNs ( Figure 1C and 1D).
Figure 10. Hox Gene Profiles Induced by Wnt, RA, and/or FGF Signals Predict Later MN Subtype.
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Red box indicates prospective FB explants used for in vitro studies.
(B–E) Explants were cultured alone or exposed to Wnt, RA, and/or FGF4 for 44 h, then washed and exposed to Shh-N (15 nM) for an additional 22 h. Bars represent mean ± s.e.m. number of Tbx20 +/Isl +, Hb9 +/Isl +, and Hb9 +/Hoxc9 + cells, respectively, as percentage of total cell number.
(B–E) Each row represents consecutive sections from a single explant.
(B) Stage 4 FB explants cultured alone, before exposure of Shh-N, generated Isl + cells but no Tbx20 +, Hb9 +, or Hoxc9 + cells ( n = 6 explants).
(C) Stage 4 FB explants cultured in the presence of Wnt3A (˜150 ng/ml) and RA (10 nM), before exposure of Shh-N, generated Tbx20 +/Isl + cells but no, or very few, Hb9 +/Isl + cells and no Hoxc9 + cells ( n = 12 explants).
(D) Cultivation in the presence of Wnt3A (˜150 ng/ml), RA (10 nM), and FGF4 (30 ng/ml), before exposure of Shh-N, generated Hb9 +/Isl + cells and only a few Tbx20 +/Isl + and Hb9 +/Hoxc9 + cells ( n = 14 explants).
(E) Cultivation in the presence of Wnt3A (˜150 ng/ml) and FGF4 (60 ng/ml), before exposure of Shh-N, generated Hb9 +/Isl + and Hb9 +/Hoxc9 + cells but no Tbx20 + cells ( n = 9 explants). Scale bars represent 100 μm (Isl) and 50 μm (double labels), respectively.
Table 1.
Quantification of Transcription Factor Expression in Neural Plate Explants
Collectively, these findings provide evidence that Wnt signals in combination with RA and/or FGF exposure, induce Hox profiles in neural cells that predict the later position-specific emergence of dMNs and vMNs characteristic of the hindbrain and spinal cord. These observations support the idea that early exposure to Wnt signals, together with later RA and FGF signals imposes Hox profiles that anticipate the patterned generation of dMN and vMN subclasses in the developing hindbrain and spinal cord.
Discussion
This study has examined the link between extrinsic patterning signals, regionally restricted profiles of transcription factor expression in neural progenitor cells, and the specification of MN subtype along the rostrocaudal axis of the hindbrain and spinal cord. Our results support four main conclusions: (i) Wnt signaling is required to specify cells of spinal cord character, (ii) the initial specification of spinal cord progenitor cells appears to require prolonged, or higher level Wnt signaling than does the specification of cells of hindbrain character, (iii) early Wnt signaling provides a positional context for the later actions of RA and FGF signals in specifying the rostrocaudal regional identity of hindbrain and spinal cord cells, and (iv) the interplay of Wnt, retinoid, and FGF signals establish distinction in progenitor cell Cdx and Hox profiles that anticipate the rostrocaudal position of generation of dMNs and vMNs in the hindbrain and spinal cord. Below, we discuss the evidence that supports each of these conclusions.
Interplay between Early Wnt and Later FGF and RA Signals in the Assignment of Caudal and Neural Fates
The generation of different subclasses of MNs along the rostrocaudal axis of the hindbrain and spinal cord depends on two crucial early steps of caudal neural development: first, the early specification of cells of hindbrain and spinal cord character, and second, the subsequent refinement of rostrocaudal regional character of hindbrain and spinal cord progenitor cells. Wnt signaling has been implicated in the generation of caudal neural cells [ 48, 60– 69], and results in chick have provided evidence that FGF and graded Wnt signaling in neural cells specify cells of caudal forebrain, midbrain, and rostral hindbrain character [ 32, 66]. The present study provides evidence that early Wnt signaling is also essential to impose caudal hindbrain and spinal cord character on neural progenitor cells. The results also support the view that the specification of spinal cord progenitor cells requires prolonged, or higher level Wnt signaling than is required for the specification of cells of hindbrain character, and they are consistent with previous findings that Cdx genes respond to Wnt signals and act upstream of 5′ Hox genes in neural progenitor cells [ 23, 70– 75]. Thus, taken together, our results support the idea that in the presence of FGF signals, graded Wnt signaling imposes midbrain, hindbrain, and spinal cord character on prospective caudal neural plate cells.
The signals and mechanisms that act in the subsequent step to impose rostrocaudal regional identity on hindbrain and spinal cord progenitor cells have been examined previously. From early somite stages, RA supplied by the paraxial mesoderm and newly formed somites, promotes the expression of Hox genes characteristic of the cHB and rostral levels of the spinal cord [ 6, 8, 29, 31, 67, 76, 77]. FGF signals derived from the regressing primitive streak promote the expression of progressively more caudal Hox-c proteins in a concentration-dependent manner [ 7, 8]. Thus, RA and FGF signals act in an opponent manner to impose rostrocaudal regional identity on hindbrain and spinal cord progenitor cells [ 6, 78]. Our findings extend these results by showing that early Wnt signaling establishes a positional context for the later actions of RA and FGF signals in specifying hindbrain and spinal cord cells of rostral and caudal regional identity.
Collectively, our results suggest a model of how hindbrain and spinal cord cells of early rostrocaudal regional identity are generated ( Figure 11). At gastrula stages, prospective caudal neural plate cells are exposed to Wnt signals derived from the emerging caudal paraxial mesoderm and from epiblast cells; and to FGF signals derived from the primitive streak [ 32, 46, 47, 79, 80]. In response to convergent Wnt and FGF signaling, prospective caudal neural plate cells are initially specified either as cells of rHB character or as Hoxb4 +/b8 +/ c9 + cells characteristic of caudal/thoracic spinal cord. At early somite stages, caudal paraxial mesoderm and newly formed somites located adjacent to the prospective cHB and rSC, express high levels of Raldh2, providing a local source of RA [ 59, 81]. Our results suggest that RA specifies cells of r7/r8 cHB character by inducing Hoxb4 expression in prospective rHB cells and cells of rSC identity by preventing the expression of Hoxc9 in prospective cSC cells. At these stages, several Fgfs are expressed in the regressing Hensen's node and primitive streak adjacent to the developing spinal cord [ 8, 46, 79]. We find that FGF signals maintain the specification of cSC cells, and in the presence of RA, promote the generation of rSC cells. This model is strengthened by our data providing evidence that distinct combinations of Wnt, RA, and/or FGF signals can reconstitute rostral and caudal hindbrain and spinal cord character in naive prospective FB cells in a predictable manner.
Figure 11. Combinatorial Wnt, RA, and FGF Signals Specify Progenitor Cell Identity That Prefigure MN Subtype in the Developing Hindbrain and Spinal Cord.
Combinatorial actions of Wnt, FGF, and RA signals specify neural progenitor cells expressing Hox gene profiles characteristic of the cHB, rSC, and cSC that generate patterns of differentiated MNs, with dMN or vMN exit points, characteristic of hindbrain and spinal cord, in response to Shh signaling.
Genetic analyses have provided evidence that inactivation of Wnt genes, expressed in the caudal regions of gastrula stage mouse and zebrafish embryos, perturbs the development of the caudal neural plate [ 60, 62, 63, 69, 82]. However, the formation of paraxial mesoderm, which serves as a local source of neural caudalizing signals [ 63, 83– 85], is also impaired in these mutant embryos [ 45, 69, 86]. Thus, these genetic studies left unresolved the issue whether the effect of perturbed Wnt signaling on caudal neural development reflects the impaired formation of paraxial mesoderm or reflects direct Wnt signaling in neural cells. Our in vitro studies establish direct effects of Wnt signals on neural tissue in the absence of other tissues and clarify the integrative mechanisms that control the early development of the hindbrain and spinal cord.
FGF and retinoid signals also regulate the temporal pattern of differentiation of caudal neural progenitor cells. FGF has been shown to keep cells in a stem zone–like state [ 14, 78, 87], whereas RA promotes the differentiation of neural cells [ 14, 78, 88]. Consistent with these roles of FGF and RA, exposure of naive neural explants, cultured with Wnt and FGF to low levels of RA (2 nM), does not change the rostrocaudal identity of neural progenitor cells but results in an increased number of differentiated MNs (unpublished data), whereas under these conditions, increased levels of FGFs greatly reduce MN differentiation (unpublished data). These findings fit well with the suggested opponent activities for FGF and RA in deciding the balance between neural cell proliferation and differentiation [ 87].
Hox Gene Profiles of Hindbrain and Spinal Cord Progenitor Cells Predict the Pattern of dMNs and vMNs
The patterned expression of Hox genes in neural progenitor cells appears to be a major determinant of the identity of different MN populations [ 1, 7, 11, 16, 18]. Earlier studies have provided evidence that RA and FGF act in an opponent manner on caudal neural cells to establish the rostrocaudal pattern of distinct subclasses of differentiated MNs [ 7– 9]. dMNs and vMNs represent two major MN subclasses that are generated in distinct rostrocaudal patterns in the hindbrain and spinal cord [ 10, 89]. Our findings provide evidence that Wnt signaling in neural progenitor cells is required for the generation of both vMNs and dMNs. We also show that Wnt, RA, and/or FGF signals can induce cells with Hox gene profiles characteristic of cHB, rSC, and cSC progenitor cells that differentiate into corresponding dMNs and vMNs when exposed to Shh-N. These findings therefore reveal a tight link between Wnt, RA, and FGF signals, profiles of progenitor cell Hox expression, and the rostrocaudal pattern of dMN and vMN generation in the hindbrain and spinal cord.
Other recent studies have revealed a determinative role of Hox genes in MN subtype specification. In the hindbrain, Hoxb1 is expressed throughout r4, and has been shown to be required for the specification of facial branchiomotor neurons [ 17– 19]. Similarly, targeted expression of Hoxa3 in the rHB leads to the generation of ectopic somatic MNs [ 11]. In the spinal cord, the rostrocaudal profile of genes of the Hox6 to Hox9 paralog group have been shown to establish distinctions in MN columnar and pool subtype [ 7, 16]. It seems likely, therefore, that the initial profiles of Hox expression, shown here to depend on early Wnt signaling, are involved in establishing domains of dMN and vMN formation. Furthermore, Hox genes appear to represent a common regulatory target for the three classes of signaling factors—Wnts, FGFs, and RA [ 90]—that conspire to regulate the position of generation of dMNs and vMNs.
Thus, our results reveal that an early Wnt-based program is required to interact with a later RA- and FGF-mediated mechanism to generate a pattern of neural progenitor cells with Cdx and Hox profiles that prefigures the generation of two major subclasses of MNs in the developing hindbrain and spinal cord. Further studies will reveal how these three signals are integrated at the molecular level to regulate Cdx and Hox gene profiles, leading to the subsequent differentiation of dMN and vMN classes.
Materials and Methods
Embryos.
Fertilized white leghorn chicken eggs were obtained from Agrisera AB, Umeå, Sweden. Chick embryos were staged according to Hamburger and Hamilton (HH) [ 91].
Isolation of tissue explants.
Prospective neural plate explants were isolated from HH stage 4, 5, 6, 7 (1-somite), and 8 (3–4-somites) chick embryos. For stage 5–8 embryos, Dispase I (Roche Diagnostics) was used to facilitate removal of the underlying mesoderm.
Culture of tissue explants.
Explants were cultured in vitro as previously described [ 32]. To enable tracing the rostrocaudal orientation of the dissected explants, rostrocaudally asymmetrical explants were isolated and then placed in a defined orientation in collagen matrix, where their orientation was maintained during cultivation, fixation, and cryo-sectioning (see Figure 5A); or the caudal margin of the explants was labeled with DiI crystals (Molecular Probes) during the dissection process ( Figures 5D, 7B, 7C, and 7D). Recombinant human FGF4 (R & D Systems, Minneapolis, Minnesota, United States) was used at 30 and 60 ng/ml. The FGF receptor inhibitor SU5402 (Calbiochem, EMD Biosciences, San Diego, California, United States) was used at 3 and 5 μM. All- trans retinoic acid (RA) (Sigma-Aldrich, St. Louis, Missouri, United States) was used at 10–40 nM. Purified recombinant mouse Wnt3A (R & D Systems) was used at 150 ng/ml. Soluble Wnt3A and control-conditioned media [ 92] were obtained as described [ 32] and used at 50–100 μl/ml, which for Wnt3A conditioned medium, mimicked the activity 75–150 ng/ml of Wnt3A protein (R & D Systems). Soluble mouse Frizzled 8 (mFrz8CRD-IgG) [ 50] and control-conditioned medium were generated as described [ 32] and used at 300–500 μl/ml of culture medium. Explants cultured with control-conditioned medium behaved like explants cultured alone. In MN differentiation studies, Shh-N (R & D Systems) was used at 15 nM, and cultivated explants were washed to remove Wnt, RA, and/or FGF4 from the medium before addition of Shh-N.
In situ hybridization, immunohistochemistry, and quantification of cells.
Chick Hoxb4, Hoxb8, Hoxc9, CdxC, CdxB, Tbx20, and Mox1 (see Figure S3, [ 55]) and Brachyury ( Bra) (see Figure S3, [ 56]) expression was detected using in situ RNA hybridization on consecutive cryo-sections using digoxigenin-labeled probes and was carried out essentially as described [ 93], before being labeled with DAPI for quantification. Hoxc9 protein and all further markers were detected using fluorescent immunohistochemistry. Neural tissue was detected with rabbit anti-Sox1 antiserum, kindly provided by S. Wilson. Rabbit anti-Otx2 was kindly provided by G. Corte. Rabbit anti-Krox20 was obtained from Babco. Rabbit anti-Isl1/2, mouse anti-Hb9, and rabbit anti-Hoxc9 were used as described [ 8, 94]. Co-localization of Tbx20/Isl was determined by double labeling with RNA probe and antibody, and co-localization of Hb9/Isl and Hoxc9/Hb9 was determined by double labeling with antibodies. Images of consecutive sections were collected using a Nikon (Tokyo, Japan) E800 light/epi-flourescent microscope. Images presented are representative of the number of explants indicated, for each experiment, in the figure legends. For each experiment, all explants were sectioned, and cells from consecutive sections were counted from 3–5 representative explants using nuclear Dapi staining. Each explant usually generated ˜10–18 8-μm sections, and the cell counts in Figures 3, 7, and 9 indicate the mean percentage of total cell number ± s.e.m. per section in Hox-gene + domains that were compared by overlay of consecutive sections. The number of Isl1/2 + cells, Tbx20 +/ Isl1/2 + cells, Hb9 +/ Isl1/2 + cells, and Hb9 +/ Hoxc9 + cells in each experiment was acquired from two to four explants (two sections from each explant) as described above ( Figures 4, 6, and 10).
Supporting Information
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Boxed region indicates caudal (C) neural plate explants used for in vitro studies.
(B–D) Stage 4 C explants cultured alone (B), in the presence of FGF4 (120 ng/ml) ( n = 18 explants) (C), or Wnt3A (150 ng/ml) ( n = 9 explants) (D) for 44 h generated a domain of Krox20 + cells (30%–60% of total cell number) and a domain of Hoxb4 + /Hoxb8 + /Hoxc9 + cells (40%–70% of the total cell number). Each row represents consecutive sections from a single explant. Scale bar represents 100 μm.
(3.2 MB TIF)
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Red box indicates prospective FB explants that were used for in vitro studies. (B–F) Sox1 was used as a general neural marker. Each row represents consecutive sections from a single explant.
(B–F) Stage 4 FB explants cultured alone ( n = 24 explants) (B), or in the presence of Wnt3A (75 ng/ml) ( n = 12 explants) (C), FGF4 (60 ng/ml) ( n = 9 explants) (D), RA (10 nM) ( n = 17 explants) (E), or FGF (60 ng/ml) and RA (10 nM) in combination ( n = 12 explants) (F) for 44 h generated Sox1 +/ Otx2 + cells (75%–98% of total cell number), but no caudal neural cells. Scale bar represents 100 μm.
(3.1 MB TIF)
(A) Schematic drawing of a stage 4 chick embryo. Dotted line indicates the presumptive neural plate. Red box indicates FB explants and black box indicates caudal neural plate tissue explants (4C) that were used for in vitro studies.
(B–D) All explants were cultured for 24 h (˜ corresponding to a stage 10 embryo)
(B) Stage 4 FB explants exposed to Wnt3A (150 ng/ml), FGF4 (20ng/ml), and RA (10 nM) generated Sox1 + and Sox2 + cells, but no Mox1- or Bra-expressing cells.
(C) Stage 4 FB explants exposed to Wnt3A (150 ng/ml) and FGF4 (60 ng/ml) generated Sox1 + and Sox2 + cells and few Bra + cells, but no Mox1-expressing cells.
(D) Stage 4C explants grown in the presence of Wnt3A (˜75 ng/ml) and FGF4 (60 ng/ml), in combination, generated Sox1 + and Sox2 + cells and few Bra + cells, but no Mox1-expressing cells.
(E–G) Transversal sections of a stage 10 embryo.
(E) Schematic drawing of a stage 10 embryo. The lines indicate the level of the transverse sections shown in the corresponding panels (F and G).
(F) Sox1 and Sox2 are expressed in the neural tube. Mox1 is expressed in the adjacent somites (s), whereas Bra is expressed in the notochord (NC).
(G) Sox1 and Sox2 are expressed in the neural plate (NP). Mox1 is expressed in the presomitic mesoderm (PSM) at the neural plate level and Brachyury is expressed in the neural plate and in the mesendoderm.
(3.1 MB TIF)
Acknowledgments
We are grateful to R. Nusse for providing Wnt3A-expressing cells and to J. Nathans for the mFrzCRD-IgG plasmid. We thank C. Tabin for kindly providing cHoxb4 and cHoxb8, J. S. Dasen for cHoxc9, J. Ericson for cTbx20, J. P. Liu for CdxC, and K. E. Yutzey for CdxB. S. I. Wilson, G. Corte, and J. Gilthorpe are acknowledged for kindly providing antibodies. We thank members of the Edlund lab for helpful discussions.
Competing interests. The authors have declared that no competing interests exist.
Abbreviations
- C
caudal
- cHB
caudal hindbrain
- CN
caudal to the node
- cSC
caudal spinal cord
- dMN
dorsal exiting motor neuron
- FB
forebrain
- FGF
fibroblast growth factor
- HH
Hamburger and Hamilton
- MN
motor neuron
- NL
node level
- r
rhombomere
- RA
retinoic acid
- RALDH2
retinaldehyde dehydrogenase 2
- rHB
rostral hindbrain
- RN
rostral to the node
- rSC
rostral spinal cord
- Shh
sonic hedgehog
- Shh-N
N-terminal fragment of the sonic hedgehog protein
- vMN
ventral exiting motor neuron
Author contributions. All authors conceived and designed the experiments. UN and EM performed the experiments. All authors analyzed the data. TMJ and TE contributed reagents/materials/analysis tools. All authors wrote the paper.
Citation: Nordström U, Maier E, Jessell TM, Edlund T (2006) An early role for Wnt signaling in specifying neural patterns of Cdx and Hox gene expression and motor neuron subtype identity. PLoS Biol 4(8): e252. DOI: 10.1371/journal.pbio.0040252
Funding. TE is supported by the Swedish Medical Research Council and by the Foundation for Strategic Research. TMJ is supported by grants from NINDS and is an Investigator of the Howard Hughes Medical Institute.
References
- Guthrie S. Neuronal development: Putting motor neurons in their place. Curr Biol. 2004;14:R166–168. [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signaling. Semin Cell Dev Biol. 1999;10:353–362. doi: 10.1006/scdb.1999.0295. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Itasaki N, Krumlauf R. Initiating Hox gene expression: In the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups . Development. 2002;129:5103–5115. doi: 10.1242/dev.129.22.5103. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity . Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons. Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids . Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in post-mitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, et al. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Guidato S, Prin F, Guthrie S. Somatic motoneurone specification in the hindbrain: The influence of somite-derived signals, retinoic acid, and Hoxa3. Development. 2003;130:2981–2996. doi: 10.1242/dev.00496. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Carpenter EM. Hox genes and spinal cord development . Dev Neurosci. 2002;24:24–34. doi: 10.1159/000064943. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R. Hox genes, neural crest cells, and branchial arch patterning . Curr Opin Cell Biol. 2001;13:698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- Shah V, Drill E, Lance-Jones C. Ectopic expression of Hoxd10 in thoracic spinal segments induces motoneurons with a lumbosacral molecular profile and axon projections to the limb. Dev Dyn. 2004;231:43–56. doi: 10.1002/dvdy.20103. [DOI] [PubMed] [Google Scholar]
- Bell E, Wingate RJ, Lumsden A. Homeotic transformation of rhombomere identity after localized Hoxb1 misexpression . Science. 1999;284:2168–2171. doi: 10.1126/science.284.5423.2168. [DOI] [PubMed] [Google Scholar]
- Jungbluth S, Bell E, Lumsden A. Specification of distinct motor neuron identities by the singular activities of individual Hox genes . Development. 1999;126:2751–2758. doi: 10.1242/dev.126.12.2751. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Kheirbek MA, Prince VE. Knockdown of duplicated zebrafish hoxb1 genes reveals distinct roles in hindbrain patterning and a novel mechanism of duplicate gene retention . Development. 2002;129:2339–2354. doi: 10.1242/dev.129.10.2339. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Wilkinson DG. Establishing neuronal circuitry: Hox genes make the connection . Genes Dev. 2004;18:1643–1648. doi: 10.1101/gad.1227004. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity . Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Yutzey KE. Anterior expression of the caudal homolog cCdx-B activates a posterior genetic program in avian embryos. Dev Dyn. 2001;221:412–421. doi: 10.1002/dvdy.1151. [DOI] [PubMed] [Google Scholar]
- Charite J, de Graaff W, Consten D, Reijnen MJ, Korving J, et al. Transducing positional information to the Hox genes: Critical interaction of cdx gene products with position-sensitive regulatory elements . Development. 1998;125:4349–4358. doi: 10.1242/dev.125.22.4349. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. Regulation of Hox gene expression and posterior development by the Xenopus caudal homolog Xcad3 . Embo J. 1998;17:3413–3427. doi: 10.1093/emboj/17.12.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation . Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- Marom K, Shapira E, Fainsod A. The chicken caudal genes establish an anterior-posterior gradient by partially overlapping temporal and spatial patterns of expression. Mech Dev. 1997;64:41–52. doi: 10.1016/s0925-4773(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Deschamps J, van den Akker E, Forlani S, De Graaff W, Oosterveen T, et al. Initiation, establishment, and maintenance of Hox gene expression patterns in the mouse . Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Dupe V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signaling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Muhr J, Graziano E, Wilson S, Jessell TM, Edlund T. Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron. 1999;23:689–702. doi: 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Bradley LC, Wilkinson DG. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Development Suppl. 1991;2:59–62. [PubMed] [Google Scholar]
- Ahn DG, Ruvinsky I, Oates AC, Silver LM, Ho RK. tbx20 a new vertebrate T-box gene expressed in the cranial motor neurons and developing cardiovascular structures in zebrafish . Mech Dev. 2000;95:253–258. doi: 10.1016/s0925-4773(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Iio A, Koide M, Hidaka K, Morisaki T. Expression pattern of novel chick T-box gene, tbx20 . Dev Genes Evol. 2001;211:559–562. doi: 10.1007/s00427-001-0187-y. [DOI] [PubMed] [Google Scholar]
- Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene tbx20 . Mech Dev. 2001;100:87–91. doi: 10.1016/s0925-4773(00)00499-8. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: Their expression during embryonic organogenesis of the chicken . Mech Dev. 1999;84:103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, et al. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development . Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, et al. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue . Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Simeone A, Alvarado-Mallart RM. Fgf8 and Gbx2 induction concomitant with Otx2 repression is correlated with midbrain-hindbrain fate of caudal prosencephalon. Development. 1999;126:3191–3203. doi: 10.1242/dev.126.14.3191. [DOI] [PubMed] [Google Scholar]
- Millet S, Campbell K, Epstein DJ, Losos K, Harris E, et al. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature. 1999;401:161–164. doi: 10.1038/43664. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signaling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, et al. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC. Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol (Berl) 2002;205:365–370. doi: 10.1007/s00429-002-0264-7. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus . Mech Dev. 1997;69:105–114. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Wilson S, Rydstrom A, Trimborn T, Willert K, Nusse R, et al. The status of Wnt signaling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Moreno TA, Kintner C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis . Dev Cell. 2004;6:205–218. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, et al. Sonic hedgehog induces the differentiation of ventral forebrain neurons: A common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Jessell TM, Edlund T. Sonic hedgehog signaling at gastrula stages specifies ventral telencephalic cells in the chick embryo. Development. 2000;127:3283–3293. doi: 10.1242/dev.127.15.3283. [DOI] [PubMed] [Google Scholar]
- Candia AF, Wright CV. Differential localization of Mox-1 and Mox-2 proteins indicates distinct roles during development. Int J Dev Biol. 1996;40:1179–1184. [PubMed] [Google Scholar]
- Kispert A, Ortner H, Cooke J, Herrmann BG. The chick Brachyury gene: Developmental expression pattern and response to axial induction by localized activin . Dev Biol. 1995;168:406–415. doi: 10.1006/dbio.1995.1090. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Thaller C, Sockanathan S, Petkovich M, Jessell TM, et al. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev Biol. 1999;216:282–296. doi: 10.1006/dbio.1999.9487. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Berggren K, McCaffery P, Drager U, Forehand CJ. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol. 1999;210:288–304. doi: 10.1006/dbio.1999.9286. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekany-Lee K, Gonzalez E, Miller-Bertoglio V, Solnica-Krezel L. The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways . Development. 2000;127:2333–2345. doi: 10.1242/dev.127.11.2333. [DOI] [PubMed] [Google Scholar]
- Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Itasaki N, Jones CM, Mercurio S, Sargent MC, et al. The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signaling . Dev Biol. 2001;239:148–160. doi: 10.1006/dbio.2001.0431. [DOI] [PubMed] [Google Scholar]
- Fredieu JR, Cui Y, Maier D, Danilchik MV, Christian JL. Xwnt-8 and lithium can act upon either dorsal mesodermal or neurectodermal cells to cause a loss of forebrain in Xenopus embryos . Dev Biol. 1997;186:100–114. doi: 10.1006/dbio.1997.8566. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signaling regulates anteroposterior neural patterning in Xenopus . Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt, and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–4346. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, et al. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. doi: 10.1242/dev.124.15.2997. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, et al. Cdx4 is a direct target of the canonical Wnt pathway . Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lickert H, Domon C, Huls G, Wehrle C, Duluc I, et al. Wnt/beta-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine . Development. 2000;127:3805–3813. doi: 10.1242/dev.127.17.3805. [DOI] [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, et al. Multiple pathways governing Cdx1 expression during murine development . Dev Biol. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of Cdx1 expression . Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- Taylor JK, Levy T, Suh ER, Traber PG. Activation of enhancer elements by the homeobox gene Cdx2 is cell- line specific . Nucleic Acids Res. 1997;25:2293–2300. doi: 10.1093/nar/25.12.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes . Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Kolm PJ, Apekin V, Sive H. Xenopus hindbrain patterning requires retinoid signaling . Dev Biol. 1997;192:1–16. doi: 10.1006/dbio.1997.8754. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, et al. Retinoic acid signaling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Storey KG. Opposing FGF and retinoid pathways: A signaling switch that controls differentiation and patterning onset in the extending vertebrate body axis. Bioessays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo . Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Hume CR, Dodd J. Cwnt-8C A novel Wnt gene with a potential role in primitive streak formation and hindbrain organization . Development. 1993;119:1147–1160. doi: 10.1242/dev.119.4.1147. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Eichele G. Retinoid metabolizing enzymes in development. Biofactors. 1999;10:85–89. doi: 10.1002/biof.5520100201. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Muhr J, Jessell TM, Edlund T. Assignment of early caudal identity to neural plate cells by a signal from caudal paraxial mesoderm. Neuron. 1997;19:487–502. doi: 10.1016/s0896-6273(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Ensini M, Tsuchida TN, Belting HG, Jessell TM. The control of rostrocaudal pattern in the developing spinal cord: Specification of motor neuron subtype identity is initiated by signals from paraxial mesoderm. Development. 1998;125:969–982. doi: 10.1242/dev.125.6.969. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- Delfino-Machin M, Lunn JS, Breitkreuz DN, Akai J, Storey KG. Specification and maintenance of the spinal cord stem zone. Development. 2005;132:4273–4283. doi: 10.1242/dev.02009. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, et al. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Lohnes D. The Cdx1 homeodomain protein: An integrator of posterior signaling in the mouse. Bioessays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, et al. Cytoskeletal reorganization by soluble Wnt-3a protein signaling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: In situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes . Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Boxed region indicates caudal (C) neural plate explants used for in vitro studies.
(B–D) Stage 4 C explants cultured alone (B), in the presence of FGF4 (120 ng/ml) ( n = 18 explants) (C), or Wnt3A (150 ng/ml) ( n = 9 explants) (D) for 44 h generated a domain of Krox20 + cells (30%–60% of total cell number) and a domain of Hoxb4 + /Hoxb8 + /Hoxc9 + cells (40%–70% of the total cell number). Each row represents consecutive sections from a single explant. Scale bar represents 100 μm.
(3.2 MB TIF)
(A) Schematic drawing of a stage 4 embryo. Dotted line indicates the presumptive neural plate. Red box indicates prospective FB explants that were used for in vitro studies. (B–F) Sox1 was used as a general neural marker. Each row represents consecutive sections from a single explant.
(B–F) Stage 4 FB explants cultured alone ( n = 24 explants) (B), or in the presence of Wnt3A (75 ng/ml) ( n = 12 explants) (C), FGF4 (60 ng/ml) ( n = 9 explants) (D), RA (10 nM) ( n = 17 explants) (E), or FGF (60 ng/ml) and RA (10 nM) in combination ( n = 12 explants) (F) for 44 h generated Sox1 +/ Otx2 + cells (75%–98% of total cell number), but no caudal neural cells. Scale bar represents 100 μm.
(3.1 MB TIF)
(A) Schematic drawing of a stage 4 chick embryo. Dotted line indicates the presumptive neural plate. Red box indicates FB explants and black box indicates caudal neural plate tissue explants (4C) that were used for in vitro studies.
(B–D) All explants were cultured for 24 h (˜ corresponding to a stage 10 embryo)
(B) Stage 4 FB explants exposed to Wnt3A (150 ng/ml), FGF4 (20ng/ml), and RA (10 nM) generated Sox1 + and Sox2 + cells, but no Mox1- or Bra-expressing cells.
(C) Stage 4 FB explants exposed to Wnt3A (150 ng/ml) and FGF4 (60 ng/ml) generated Sox1 + and Sox2 + cells and few Bra + cells, but no Mox1-expressing cells.
(D) Stage 4C explants grown in the presence of Wnt3A (˜75 ng/ml) and FGF4 (60 ng/ml), in combination, generated Sox1 + and Sox2 + cells and few Bra + cells, but no Mox1-expressing cells.
(E–G) Transversal sections of a stage 10 embryo.
(E) Schematic drawing of a stage 10 embryo. The lines indicate the level of the transverse sections shown in the corresponding panels (F and G).
(F) Sox1 and Sox2 are expressed in the neural tube. Mox1 is expressed in the adjacent somites (s), whereas Bra is expressed in the notochord (NC).
(G) Sox1 and Sox2 are expressed in the neural plate (NP). Mox1 is expressed in the presomitic mesoderm (PSM) at the neural plate level and Brachyury is expressed in the neural plate and in the mesendoderm.
(3.1 MB TIF)