Abstract
Objective To estimate the effectiveness of delivering an anthelmintic through a community child health programme on the weight gain of preschool children in Uganda.
Design Cluster randomised controlled trial.
Setting Eastern Uganda.
Participants 48 parishes participating in a new programme for child health: 24 offered children an additional service of anthelmintic treatment. The outcome is based on measurements from 27 995 children.
Intervention Treatment of children aged between 1 and 7 years with 400 mg albendazole added to standard services offered during child health days over a three year period.
Main outcome measure Weight gain.
Results The provision of periodic anthelmintic treatment as a part of child health services in Uganda resulted in an increase in weight gain of about 10% (166 g per child per year, 95% confidence interval 16 to 316) above expected weight gain when treatments were given twice a year, and an increase of 5% when the treatment was given annually.
Conclusion Deworming of preschool children in Uganda as part of regularly scheduled health services seems practical and associated with increased weight gain.
Introduction
Many children in low income countries are commonly infected with parasitic helminths and this can have important consequences for their development.1 Infections are easily treated with inexpensive, single dose oral drugs.2-4 A recent study of deworming children during home visits in rural Zanzibar indicated that the youngest children, who are most at risk of growth retardation, showed the greatest increase in growth rate after treatment with anthelmintics.5 Home delivery of treatments may not be a practical and sustainable intervention, however, and periodic mass deworming without diagnosis, as recommended by the World Health Organization in areas with more than 50% of children infected, may be needed to have an effect on growth during a programme or community health project.
The Ugandan government has established a community based programme of regular child health days during which simple health and nutrition interventions are delivered to preschool children. We assessed whether including anthelmintic treatment in such a programme at scale could result in additional weight gain in young children.
Participants and methods
As a part of a nutrition and early child development project in 25 districts of Uganda, the parents of all children aged less than 7 years were offered a range of health services at child health days, including vaccinations, vitamin A supplements, growth monitoring and promotion, and demonstrations of complementary feeding. These services were delivered by community organisations that were supervised by non-governmental organisations acting as subcontractors in each district.
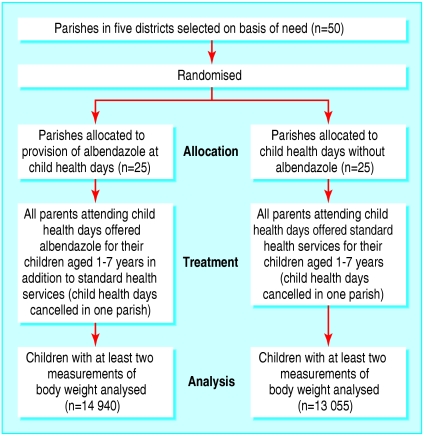
The study was a cluster randomised effectiveness trial in which the unit of randomisation was the parish, the administrative unit at which child health days were organised within each district. Five districts in the eastern region of Uganda were selected (Busia, Iganga, Mbale, Palissa, and Tororo) because a survey had indicated that about 60% of children aged 5-10 years were infected with intestinal nematodes, most commonly hookworm.6 Fifty parishes selected by the local governments were randomly allocated into two groups (figure): The randomisation was done by a member of the research team (HA) by assigning numbers to all of the parishes and converting these to base two and then determining which of the parishes were to be in the treatment by coin flips. Twenty five were assigned to standard services and 25 to standard services plus treatment with albendazole. One parish from each group was subsequently removed from the project by district officials and was not replaced. Albendazole was offered as one 400 mg chewable tablet (Zentel: GlaxoSmithKline) to all healthy children aged 1-7 years who attended any child health day. Sick children were not treated. Parents could refuse albendazole or any other service. As anthelmintics were not then a standard treatment offered by the government, they were not provided in the other parishes, which thus constituted the control group. We included a stopping rule in the study design; if a statistically significant extra weight gain occurred by the third round of child health days, albendazole would be given to all children.
Figure 1.
Flow chart of study design
We obtained the date of birth from the growth monitoring card of each child or from the caregiver. Weight was measured at each child health day to a precision of 100 g using Unicef scales. Height was measured to a precision of 1 cm for children older than 2 years. This information was recorded on a parish register by staff as part of their normal duties and later transcribed by research staff, who gave each child a unique identification code to track them over time. This separation of the programme from research served to minimise the influence of the study on child health days. To increase compliance the children were not visited at home.
It was not possible for us to carry out a double blind trial because of the scale of the programme and because we aimed to assess the effectiveness of giving albendazole—the efficacy of which was already established—during standard child health days without any study specific inputs. We considered the possibility of a Hawthorne effect unlikely as the child health days were new for both treatment and control groups and the primary outcomes were determined objectively for all children as a part of normal growth monitoring by programme staff.
To assess programme coverage and the use of anthelmintics not given by the programme, we carried out cluster surveys of households in all parishes during the first quarter of 2000 and 2003. Two villages were randomly selected in each parish, a household census was done, and 1500 households (750 in each group) containing at least one child aged less than 6 years, were randomly selected. Each household was located with a satellite global positioning system to facilitate follow up. We administered a questionnaire between January and March 2000. Each caregiver was asked if the child had been treated for worms, from where the treatment had been obtained, and how much the treatment had cost. Each household was revisited between January and March 2003 and the questionnaire was repeated. Non-compliance was less than 2%.
The main outcome measure was weight gain, calculated as the difference in weight between the first and last child health day for each child. We excluded from the analysis any measurement six or more standard deviations above or below reference weight for age. This is a more inclusive definition of outliers than recommended by WHO7 and reduced the sample size by 1.8% (260 children in the control group and 253 in the treatment group). We used EpiInfo to calculate Z scores of weight for age and height for age, which uses the National Center for Health Statistics reference values recommended by WHO.
As the effect of treatment with albendazole on weight gain is likely to be related to personal and environmental factors, we controlled for these by using multivariate regression models in Stata. These factors were the number of albendazole treatments received, so we included in the model the number of visits to child health days by children in both groups; the sex of the child; the age, weight, and initial nutritional status of the child at recruitment, as these all can influence subsequent weight gain; the period between the first and last measurements, as this will affect total weight gained; the parish, as the local environment can affect food production and disease transmission; the district in which the parish was located, and, finally, the round, to control for seasonal food production and disease transmission. We also examined alternative models. In one, initial weight was replaced by initial weight predicted from height, sex, and age, because initial weight was correlated with the outcome measure, weight gain. In another model, we examined weight gain per month as an alternative to absolute weight gain. (See bmj.com for further details of the statistical models and sensitivity analysis.) As some children attended more child health days than others and received more treatments with albendazole, in a portion of the analysis we divided the treatment group into three based on intervals between attendance: 7.5 months or less, 7.5-13 months, and more than 13 months. Although this is not an equal division of the sample (it represents 18%, 67%, and 15% of the treatment group, respectively) these intervals corresponded to practical targets of biannual, annual, or less frequent treatment during a programme, with slight delays typical of programmes, and were used to indicate the potential benefit of treatments given at those frequencies.
Results
The study was undertaken between November 2000 and June 2003 during which five child health days occurred in each parish. Because of a delay in funding, almost 12 months, rather than 6 months, elapsed between the first two child health days. Table 1 presents descriptive statistics on the child health days and the percentage of children classified as underweight at each round. The absence of any apparent difference in the percentage of underweight children or in the mean Z scores between the treatment and control groups does not indicate the absence of an effect, as new children entered the project and study at each round, whereas others did not always attend.
Table 1.
Dates of child health days in rural Uganda, sample sizes, proportion of children classified as underweight (−2 SD below reference vales), and mean Z scores of weight for age of children in treatment and control parishes
|
Variable
|
Round
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Start of child health days | 2 Nov 2000 | 14 Aug 2001 | 18 Feb 2002 | 2 Sep 2002 | 18 Mar 2003 |
| End of child health days | 8 Dec 2000 | 30 Nov 2001 | 29 Jun 2002 | 19 Oct 2002 | 26 Jun 2003 |
| No of children weighed | 37 165 | 33 711 | 21 124 | 20 787 | 20 443 |
| Percentage of girls | 50.0 | 50.3 | 50.6 | 51.1 | 50.7 |
| Percentage receiving treatment | 50.7 | 51.2 | 51.9 | 53.8 | 56.8 |
| Mean (SD) age (years) | 3.69 (1.66) | 3.64 (1.80) | 3.69 (1.77) | 3.63 (1.63) | 3.54 (1.54) |
| Proportion underweight: | |||||
| Treatment parishes | 0.26 | 0.24 | 0.23 | 0.23 | 0.24 |
| Control parishes | 0.26 | 0.25 | 0.25 | 0.23 | 0.24 |
| Mean (SD) Z scores of weight for age: | |||||
| Treatment parishes | −1.14 (1.48) | −1.06 (1.52) | −1.07 (1.45) | −1.14 (1.32) | −1.23 (1.25) |
| Control parishes | −1.17 (1.45) | −1.11 (1.52) | −1.14 (1.44) | −1.17 (1.29) | −1.20 (1.25) |
Table 2 shows that at least two measurements of body weight were made on 14 940 treated children and 13 055 control children, and that there was a statistically significant difference in extra weight gained of 154 g (95% confidence interval 96 to 214, P < 0.01). This is equivalent to an extra 166 g per year (16 to 316 g) or nearly 10% of average initial body weight.
Table 2.
Average weight gain, months in programme, and number of visits to child health days in Uganda for all children with two or more measurements
| No of children with repeated measurements | Treatment parishes (n=14 940) | Control parishes (n=13 055) | All (n=27 995) |
|---|---|---|---|
| Mean (SD) weight gain (g) | 2413 (2536) | 2259 (2474) | 2341 (2508) |
| Mean (SD) months in programme | 16.9 (7.7) | 16.2 (7.5) | 16.6 (7.6) |
| Mean (SD) visits to child health days | 2.7 (0.9) | 2.6 (0.8) | 2.7 (0.9) |
Table 3 shows the results of six regression models, five using total weight gain and the sixth using weight gain per month. Model 1 indicates that weight gain was greater in children who attended more child health days but that children in the parishes where albendazole was given gained 55 g (9 to 104 g) more weight per visit than children in control parishes. The robustness of this model was then tested in other models.
Table 3.
Variables generated by regression models examining effect on weight gain of attendance at child health days in rural Uganda where albendazole was provided. 95% confidence intervals are corrected for cluster sampling effects
|
Outcome variable
|
Model 1
|
Model 2
|
Model 3
|
Model 4
|
Model 5
|
Model 6
|
|---|---|---|---|---|---|---|
| Total weight gain (g) (95% CI) | Total weight gain (g) (95% CI) | Total weight gain (g) (95% CI) | Total weight gain (g) (95% CI) | Total weight gain (g) (95% CI) | Weight gain per month (g) (95% CI) | |
| No of visits to treatment site | 705.0** (648 to 762) | 143.7** (87 to 201) | — | — | — | — |
| No of visits to control site | 649.6** (592 to 707) | 92.5** (25 to 160) | — | — | — | — |
| Initial age (months) | −689.4** (−790 to −589) | −693.9** (−791 to −597) | −694.2** (−797 to −597) | −519.9** (−603 to −510) | −838.23** (−955 to −721) | −41.0** (−48.9 to −33.0) |
| Age2 (months) | 56.5** (43 to 70) | 66.3** (54 to 78) | 66.3** (54.2 to 78.4) | 41.7** (29.8 to 53.6) | 78.5** (64.0 to 93.0) | 3.8** (2.8 to 4.8) |
| Interval between first and final measurement | — | 99.0** (89 to 109) | 111.6** (104.6 to 118.7) | 102.8** (95.6 to 109.9) | 102.0** (93.3 to 110.7) | −2.0* (−2.4 to −1.5) |
| Girls | 49.6* (1 to 98) | 48.5 (−0.8 to 98) | 49.1 (−0.7 to 100) | 125.2** (73.0 to 177.3) | 101.4** (50 to 153) | 1.9 (−2.5 to 6.3) |
| Treatment if time between visits ≤7.5 months | — | — | 158.2** (59.0 to 262.3) | 169.5** (52.4 to 286.6) | 178.0* (37.3 to 318.6) | 13.8* (1.3 to 26.3) |
| Treatment if time between visits >7.5 and ≤13 months | — | — | 106.6* (23.2 to 190.0) | 127.7* (30.0 to 225.5) | 128.8* (14.8 to 242.8) | 6.6* (0.2 to 12.9) |
| Treatment if time between visits ≥13 months | — | — | −125.4 (−304.7 to 53.9) | −127.7 to 307.5 to 52.1) | −150.6 (−142.5 to 41.0) | −4.3 (−13.4 to 4.8) |
| Initial weight (g) | — | — | — | −555.2** (−600.1 to −510.3) | — | — |
| Initial weight (g, predicted) | — | — | — | — | −273.3** (−333.8 to −212.8) | — |
| Constant | 2684** (2452 to 2915) | 1853** (1581 to 2124) | 1801** (1524 to 2078) | 911** (668 to 1154) | 1917** (1625 to 2208) | 257** (235 to 280 |
| No of children | 27 995 | 27 995 | 27 995 | 27 995 | 21 134 | 27 995 |
| R2 | 0.146 | 0.178 | 0.178 | 0.283 | 0.183 | 0.018 |
Constant is average of fixed effects.
Coefficient differs from zero (two tailed test) with P≤0.05.
Coefficient differs from zero (two tailed test) with P≤0.01.
Model 2 shows that the interval between the first and last measurements was highly correlated with total weight gain, as expected, but the difference in total weight gain per visit between study groups did not change although the magnitude of both did change.
Model 3 shows the effect of attending child health days in which the treatment effect is divided into three groups: about twice a year, annually, or at a longer interval. The children treated twice a year gained more weight than children treated less often.
Model 4 controls for initial weight. No biological interpretation can be assigned to the coefficient of initial weight in this model since any measurement error in the initial weight is also in the dependent variable, total weight gain, leading to a bias towards minus one for that coefficient. This initial weight variable, however, picks up unexplained variance without biasing the variable of interest. It indicates that there was no bias.
Model 5 repeats model 4 but uses a value for initial weight predicted from the height of children aged more than 2 years, their sex and age, and fixed effects of the parish. This was done because the initial weight used in model 4 is correlated with the outcome, gain in weight. The prediction equation had an R2 value of 0.782. As recumbent length was not recorded for children less than two years old, not every child weighed had height recorded, so the sample size was reduced. Neither models 4 nor 5 led to an appreciable change in variable estimates.
Model 6 uses the variables in model 3, but standardises the outcome per unit time by using weight gain per month as the outcome variable. It shows an average additional weight gain of 13.8 g per month among children treated about twice a year, but it was not significant if the interval between treatments was a year or more. In model 6 the interval between the first and last measurement was negatively associated with monthly weight gain. This probably reflects the fact that weight gain declines with age so that, as the interval between the first and last measurements increases the longer participants were studied, the velocity of weight gain decreases. The length of time each child was studied has a strong correlation with the total weight gain, but not with weight gain per month.
Table 4 shows that the percentage of households who reported deworming their children before the programme started in 2000 was similar in both treatment and control parishes. By 2003 three times as many children in the treatment parishes had been dewormed, mostly at the child health days, but this practice had also increased by 50% in the control parishes. Most treatments for the control group were obtained from private clinics or shops, where the average cost was Ugandan shillings (USH) (SD 705) 748 or about £0.20 (€0.30, $0.40). No significant difference was found in the average number of child health days attended by children in the treatment and control parishes.
Table 4.
Proportion of children aged 1 year or older who were reported to have been dewormed in samples of households in two surveys three years apart in parishes in rural Uganda in which deworming was being provided (treatment) at child health days and in parishes where no deworming was provided (control)
|
Round
|
Treatment parishes
|
Control parishes
|
||
|---|---|---|---|---|
| Jan-Mar 2000 | Jan-Mar 2003 | Jan-Mar 2000 | Jan-Mar 2003 | |
| No of children | 1392 | 1296 | 1393 | 1314 |
| % of children dewormed from any source in past six months | 21.7 | 65.8 | 23.9 | 34.6 |
| % getting treatment from private or non-governmental organisation sources | NA | 11.4 | NA | 15.2 |
| Cost (USH) at private providers | NA | 748 | NA | 776 |
| Attended child health day in past two years (yes or no) | NA | 0.735 | NA | 0.730 |
| No of child health days attended | NA | 1.738 | NA | 1.764 |
NA=question not asked in first round (child health days not organised in region at time of initial survey). USH=Ugandan shillings.
Discussion
Periodic treatment with albendazole given twice a year as a part of child health services in Uganda led to a 10% extra gain in weight of about 166 g per child per year compared with untreated controls, or an extra weight gain of around 5% if children were treated annually. This study involved nearly 30 000 children taking part in a national programme, and the data were collected as a part of routine growth monitoring to minimise the effect of the study on implementation. The effect of treatment may have been underestimated because about a third of children in the control parishes were also dewormed by their parents (table 4). As the predominant intestinal helminth in the districts was hookworm, and infections in young children in the same districts were generally light,8 a greater effect of treatment may be achieved elsewhere.
The research design did not permit determining if other health services complement the provision of albendazole. Moreover, as compliance was estimated to be 73%, it is not possible to generalise these results to the total population of children.
A meta-analysis of deworming children and young people aged up to 16 years9 found a similar magnitude of extra weight gain, but the lack of consistency in trial design and follow-up period in these studies makes it premature to conclude that this is a general finding. A review of randomised trials of anthelmintic treatment given to preschool children—the age group in our study—found that growth was improved in four trials, was unaffected in four, and had an inverse relation to treatment in one.5 Although only one study reviewed showed an increase in linear growth, all four of the trials with positive results reported benefits in ponderal growth, as in our study. The meta-analysis pointed out that most studies have been done in populations infected with Ascaris lumbricoides, but the largest effect on weight gain in preschool children has been in populations infected predominantly with Trichuris or hookworm, as in our study.
Deworming has benefits beyond those measured in our study. These include effects on anaemia, cognitive development, and possible improvements of vitamin A status,10,11 as well as spillover outside the treated group as a result of reduced transmission. Treating children alone can reduce the prevalence of worms among untreated people12 and provide measurable benefits for untreated populations.13
On average, child health days in Uganda cost between $500 and $600 per event, exclusive of the value of the time of community volunteers, and slightly more when days included a demonstration of how to prepare food. On average each event reaches 450 children, suggesting a cost for services delivered of about $1 to $1.33 per child. The direct resource cost per child is similar to delivering vitamin A with polio inoculations in other countries14 and similar to programmes to treat helminths.1
A single dose of albendazole added $0.21 (USH 385) to the costs of materials at child health days at 2002 prices but added little to the costs of staff since they were already dispensing vitamin A. This cost could be substantially reduced, to as low as $0.03, by bulk purchase of the drug. But even when applying the actual prices in 2002 and ignoring the other known benefits of deworming, the 10% increase in weight gain with twice yearly treatment at a cost of $0.42, or a 5% increase at a cost of $0.21, represents an attractive return. Shortly after completing this study the government of Uganda purchased eight million doses of mebendazole to extend deworming at child health days to the whole nation.
What is already known on this topic
Clinical trials of anthelmintic treatments have shown increased linear growth in young people up to age 16 years, whereas in younger people the increase is mainly in weight
Little evidence exists of the benefits of giving periodic mass treatment through public health programmes, and none is targeted at preschool children
What this study adds
Giving anthelmintic treatments routinely as a part of periodic child health days can lead to extra weight gain in preschool children in Uganda
The weight gain of children who attended child health days every six months was 10% greater than in untreated controls
Supplementary Material
 Additional information on statistical analysis is on bmj.comWe thank Shally Awasthi and Lani Stephenson for comments on an earlier draft.
Additional information on statistical analysis is on bmj.comWe thank Shally Awasthi and Lani Stephenson for comments on an earlier draft.
Contributors: HA, JK-L, DB, and AH conceived and designed the study and drafted the manuscript. HA and IS were involved in the data analysis. HA is guarantor.
Funding: The research was funded by the nutrition and early child development project, government of Uganda, which contracted the Institute of Public Health and the research committee of the World Bank. The World Bank employs HA and DB and contracted AH.
Competing interests: None declared.
Ethical approval: This study was approved by the ethics review committee of the Institute of Public Health and conforms to human study protocols for the government of Uganda.
References
- 1.Awasthi S, Bundy DAP, Savioli L. Helminthic infections. BMJ 2003;327: 431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundy DAP, Guyatt HL. Schools for health: focus on health, education and the schoolaged child. Parasitol Today 1996;12: 1-16. [DOI] [PubMed] [Google Scholar]
- 3.Hotez P, Bundy DAP, Beegle K, Brooker S, Drake L, De Silva N, et al. Helminth infections: soil-transmitted helminth infections and schistosomiasis. Chapter 24. Disease control priorities for developing countries. In: Jamison D, Claeson M, Breman J, Meacham A, eds. Oxford: Oxford University Press, 2005.
- 4.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once- or twice-yearly treatment with albendazole. J Nutr 1993;123: 656-65. [DOI] [PubMed] [Google Scholar]
- 5.Stoltzfus RJ, Chway H, Montresor A, Tielsch JM, Jape JK, Albonico M, et al. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr 2004;134: 348-56. [DOI] [PubMed] [Google Scholar]
- 6.Kabatereine N, Tukahebwa E. Brooker S, Alderman H, Hall A. Epidemiology of intestinal helminth infections among schoolchildren in southern Uganda. East Afr Med J 2001;78: 283-6. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Physical status: the use and interpretation of anthropometry. WHO technical report series 854. Geneva: WHO, 1995. [PubMed]
- 8.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. Report of a WHO expert committee. WHO technical report series 912. Geneva: WHO, 2002. [PubMed]
- 9.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effect of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomized trials. BMJ 2000;320: 1697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bundy DAP, Shaeffer S, Jukes M, Beegle K, Gillespie A, Drake L, et al. School based health and nutrition programs. Chapter 61. Disease control priorities for developing countries. In: Jamison D, Claeson M, Breman J, Meacham A, eds. Oxford: Oxford University Press, 2005.
- 11.Jalal F, Neisheim M, Agus Z, Sanjur D, Habicht J-P. Serum retinol concentrations in children are affected by food sources of β-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr 1998;68: 623-9. [DOI] [PubMed] [Google Scholar]
- 12.Bundy DAP, Wong MS, Horton J. Control of geohelminths by delivery of targeted chemotherapy through schools. Trans R Soc Trop Med Hyg 1990;84: 115-20. [DOI] [PubMed] [Google Scholar]
- 13.Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 1004;72: 159-217. [Google Scholar]
- 14.Goodman T, Dalmiya N, de Benoist B, Schultink W. Polio as a platform: using national immunization days to deliver vitamin A supplements. Bull World Health Organ 2000;78: 305-14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.