Abstract
Characterization of defects in a variant subline of RBL mast cells has revealed a biochemical event proximal to IgE receptor (FcεRI)-stimulated tyrosine phosphorylation that is required for multiple functional responses. This cell line, designated B6A4C1, is deficient in both FcεRI-mediated degranulation and biosynthesis of several lipid raft components. Agents that bypass receptor-mediated Ca2+ influx stimulate strong degranulation responses in these variant cells. Cross-linking of IgE-FcεRI on these cells stimulates robust tyrosine phosphorylation but fails to mobilize a sustained Ca2+ response. FcεRI-mediated inositol phosphate production is not detectable in these cells, and failure of adenosine receptors to mobilize Ca2+ suggests a general deficiency in stimulated phospholipase C activity. Antigen stimulation of phospholipases A2 and D is also defective. Infection of B6A4C1 cells with vaccinia virus constructs expressing constitutively active Rho family members Cdc42 and Rac restores antigen-stimulated degranulation, and active Cdc42 (but not active Rac) restores ganglioside and GPI expression. The results support the hypothesis that activation of Cdc42 and/or Rac is critical for FcεRI-mediated signaling that leads to Ca2+ mobilization and degranulation. Furthermore, they suggest that Cdc42 plays an important role in the biosynthesis and expression of certain components of lipid rafts.
INTRODUCTION
Immune cell receptor activation triggers cascades of biochemical pathways that lead to diverse cellular responses such as stimulated exocytosis, production of lipid mediators, and transcriptional activation. For the multichain immune recognition receptors (MIRR) which include FcεRI and other Fc receptors (Daëron, 1997), T cell receptors (Davis et al., 1998), and B cell receptors (Reth and Wienands, 1997), critical roles for nonreceptor tyrosine kinases in initiating these signaling cascades are well-established, and the mechanisms by which stimulated tyrosine phosphorylation leads to the activation of downstream signaling events are understood in some detail (Weiss and Littman, 1994; Kinet, 1999).
MIRR-stimulated Ca2+ responses are central to the functional responses elicited by these receptors, and much is known about the mechanism by which this process is activated. For most of the receptors in this family, cross-linking initiates tyrosine phosphorylation of receptor-containing ITAM sequences by Src family tyrosine kinases, and detergent-resistant, glycolipid-enriched membrane rafts have been implicated in this process (Field et al., 1997; Sheets et al., 1999; Xavier and Seed, 1999). ITAM phosphorylation allows recruitment and activation of Syk or Zap-70 tyrosine kinases which in turn phosphorylate multiple protein substrates, including the phospholipase Cγ (PLCγ) subfamily that hydrolyze phosphatidylinositol-4,5-bisphosphate (PIP2). Additionally, there is evidence for the involvement of a second family of tyrosine kinases, the Tec family, in activation of PLCγ (Kurosaki, 1999).
Previous studies demonstrated that Syk-dependent tyrosine phosphorylation of PLCγ1 and PLCγ2 is necessary for antigen-stimulated production of IP3 via FcεRI on RBL mast cells (Zhang et al., 1996). Recent studies have suggested that MIRR-stimulated tyrosine phosphorylation of PLCγ is not sufficient for stimulated inositol-1,4,5-trisphosphate (IP3) production. For example, molecular genetic studies identified Vav, a guanine nucleotide exchange factor for Rac1, a Rho family GTPase, as an essential protein for T cell receptor-mediated activation of IP3 production (Costello et al., 1999). In Vav-negative cells, cross-linking of T cell receptors caused tyrosine phosphorylation of PLCγ1 similar to wild-type cells, but failed to stimulate IP3 production. In RBL-2H3 mast cells, evidence for the involvement of Rac1 and/or Cdc42 in FcεRI-mediated IP3 production and Ca2+ mobilization was recently described (Hong-Geller and Cerione, 2000).
In the present study, we have characterized signaling deficiencies in an RBL mast cell subline that was selected following chemical mutagenesis because of a deficiency in the expression of a mast cell-specific ganglioside (Stracke et al., 1987; Oliver et al., 1992). Our results identify a defect in FcεRI signaling downstream of tyrosine phosphorylation but upstream of phospholipase activation that can be overcome by expression of constitutively active mutants of the Rho family members Cdc42 and Rac. Furthermore, the capacity of activated Cdc42 (but not wild-type Cdc42) to restore ganglioside biosynthesis as well as FcεRI signaling reveal activation of this Rho family GTPase as a critical defect in these mutant cells.
MATERIALS AND METHODS
Cell Lines
Mutant RBL-2H3 cells designated B6A4C1 were generated by exposure to ethyl methane sulfonate followed by subcloning and identification of a subline deficient in IgE-mediated degranulation and, initially, in binding of the monoclonal antibody AA4 (Stracke et al., 1987) that is specific for α-galactosyl GD1b gangliosides (Guo et al., 1989). The wild-type RBL-2H3 cells used for these experiments were previously characterized (Barsumian et al., 1981). Both cell lines were maintained as previously described for the RBL-2H3 cells (Pierini et al., 1996).
Fluorescence Microscopy
Cells were labeled and analyzed by fluorescence confocal microscopy as previously described (Pierini et al., 1996). Suspended cells sensitized with FITC-IgE were fixed and permeabilized by cold methanol for labeling with anti-Lyn and Cy3-conjugated secondary antibody. For Cy3-AA4 mAb, Cy3-OX7 mAb (anti-Thy-1), and FITC-cholera toxin B (Sigma Chemical Co., St. Louis, MO), cells were either fixed with 3.7% formaldehyde and permeabilized with 0.1% Triton X-100 before labeling (as for Figure 1), or else labeled with these antibodies at 4°C for 1 h, followed by washing and formaldehyde fixation (as in Figure 10). For some experiments, FITC-cholera toxin B-labeled cells sensitized with anti-DNP IgE were also labeled with Cy3-conjugated DNP-BSA (Xu et al., 1998) post fixation.
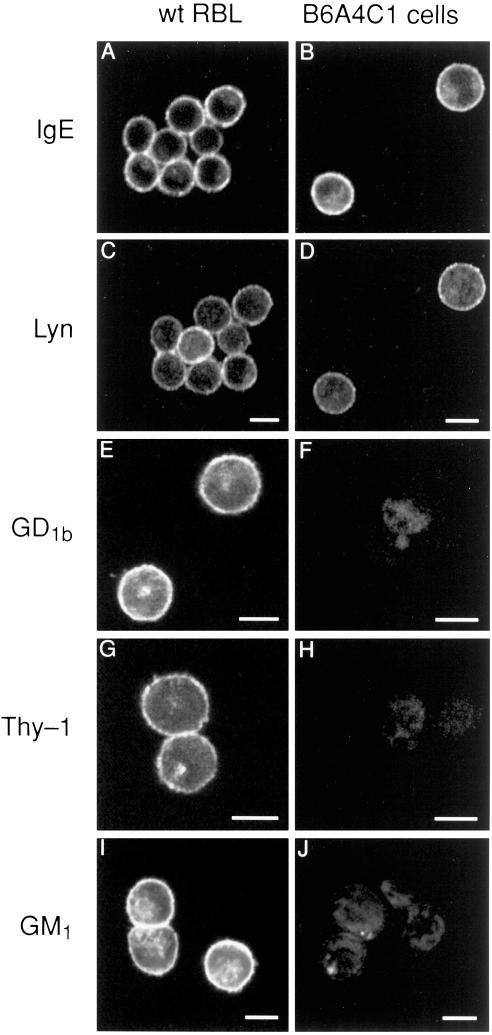
Figure 1.
Confocal fluorescence images of mutant B6A4C1 (right panels) and wild-type RBL-2H3 mast cells (left panels). Cells were sensitized with FITC-IgE (A-D) or unlabeled IgE (E-J), then fixed, permeabilized, and labeled as described in Methods. Cells with receptor-bound FITC-IgE (seen in A, B) were also labeled with rabbit anti-Lyn and Cy3-antirabbit secondary antibody (seen in C, D). Separate samples were labeled with Cy3-AA4 antiganglioside antibody (E, F), Cy3-anti-Thy-1 (G, H), or FITC-cholera toxin A specific for GM1 (I, J). Images shown are representative of at least 95% of the cells observed (>200 cells in two or more experiments for each marker). Scale bars = 10 μm.
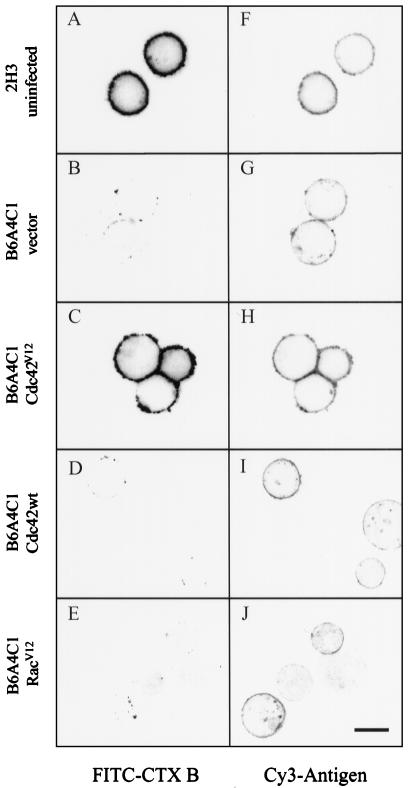
Figure 10.
Reconstitution of ganglioside biosynthesis and expression in B6A4C1 cells by activated Cdc42. Confocal fluorescence images (greyscale) show representative IgE-sensitized RBL-2H3 cells or B6A4C1 cells infected for 12 h with the indicated vaccinia virus constructs. Cells were labeled with FITC-cholera toxin B (A-E), then fixed and postlabeled with Cy3-DNP-BSA (F-J) as described in Methods. Some fluorescence bleedthrough of FITC into the Cy3 channel is noted. Scale bar = 10 μm for all images.
Degranulation and Ca2+ Measurements
Degranulation of RBL cells was measured by quantifying the release of β-hexosaminidase activity as described (Harris et al., 1997). For these experiments, cells were sensitized with biotinylated IgE (Field et al., 1995) and allowed to adhere for 4–24 h in 24-well culture plates. Cells were then triggered for 60 min in buffered salt solution (BSS, pH7.4; Harris et al., 1997) with 100 ng/ml DNP-BSA (Xu et al., 1998), 10 nM streptavidin, 200 nM thapsigargin, 700 nM A23187, or 80 nM phorbol myristoyl acetate (PMA) and 700 nM A23187 (Sigma Chemical Co).
Cytoplasmic Ca2+ responses were measured with indo-1 (Molecular Probes, Eugene, OR) as previously described (Pierini et al., 1997). Intracellular Ca2+ is represented as the ratio of the observed indo-1 fluorescence at each time point, minus background fluorescence, to the maximal fluorescence obtained after lysing the cells with TX-100, minus background fluorescence. Background fluorescence was determined following addition of 10 mM EGTA to samples in the presence of TX-100. Stimulants used were DNP-BSA, thapsigargin, or the adenosine agonist 5′-(N-ethylcarboxamido)-adenosine (NECA; Sigma Chemical Co.).
Anti-Phosphotyrosine Immunoblots
Cells sensitized with biotinylated IgE and suspended in BSS at 2 × 106 cells in 1 ml were stimulated with either 10 nM streptavidin, 100 ng/ml DNP-BSA or left unstimulated for 5 min at 37°C, then pelleted for 10 s at 5000 xg and resuspended in ice-cold lysis buffer (Field et al., 1995) with 0.5% TX-100. After 15 min on ice the lysates were cleared for 5 min at 5000 xg. For whole cell lysate immunoblots, 104 cell equivalents were analyzed, and the remainder of the samples were used for immunoprecipitation. Samples were immunoprecipitated by incubating for 4 h on ice with 5 μg rabbit anti-IgE (Menon et al., 1984), 2 μl rabbit anti-Syk antiserum (a gift from Dr. J.-P. Kinet, Harvard Medical School), or 5 μg rabbit anti-PLCγ2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by the addition of 50 μl of Protein A Sepharose beads (Pierce Chemical Co., Rockford, IL) and incubation at 4°C for 1 h. The immunoprecipitates were then washed twice in lysis buffer with 0.5% TX-100 and eluted by boiling in SDS sample buffer. The whole cell lysates and immunoprecipitates were then run on 12.5% polyacrylamide gels in the presence (anti-PLCγ2) or absence of reducing agent (anti-IgE, anti-Syk) and semidry transferred to Immobilon PVDF (Millipore Corp., Bedford, MA). The blots were blocked with BSA, probed with a 1:10,000 dilution of antiphosphotyrosine 4G10 conjugated to horseradish peroxidase (Upstate Biotechnology, Lake Placid, NY), and developed with Enhance chemiluminescent substrate (Pierce Chem. Co.).
Phospholipase Assays
Phospholipase C activity was assayed by measuring total inositol phosphate production according to previously published methods (Apgar, 1997). Briefly, RBL-2H3 and B6A4C1 cells were incubated overnight in medium containing 3H-myo-inositol to label the polyphosphoinositides. The cells were activated with 50 ng/ml DNP-BSA for 45 min at 37°C in the presence of 10 mM lithium chloride. The cells were then extracted with chloroform/methanol (1:1), and the radiolabeled inositol phosphates were isolated using Dowex-1Cl− (Berridge et al., 1982; O'Rourke and Mescher, 1988) and measured in a liquid scintillation counter.
Phospholipase A2 activity was measured after culturing the cells overnight in medium containing 3H-arachidonic acid (Apgar, 1997). RBL-2H3 and B6A4C1 cells were activated with 50 ng/ml DNP-BSA or a combination of 500 nM A23187 and 50 nM PMA for 45 min at 37°C. Radiolabeled arachidonic acid and its metabolites released from the cells upon activation were quantified in the cell supernatants by liquid scintillation counting.
Production of radiolabeled phosphatidylethanol was used to measure phospholipase D activity (Lin et al., 1992; Apgar, 1997). After the cells were grown overnight in medium containing 3H-myristic acid to label the phospholipids, IgE-sensitized RBL-2H3 and B6A4C1 cells were stimulated either with buffer, 50 ng/ml DNP-BSA, or a combination of 500 nM A23187 and 50 nM PMA in the presence of 0.5% ethanol. The reaction was stopped after 45 min by extraction of the cells with chloroform/methanol. TLC, using a double one-dimensional system (Gruchalla et al., 1990), was used to isolate the 3H-phosphatidylethanol which was quantified by liquid scintillation counting.
Assay for Actin Polymerization
Total F-actin content in RBL-2H3 and B6A4C1 cells was measured using a modification (Frigeri and Apgar, 1999) of the method developed previously (Watts and Howard, 1992). IgE-sensitized RBL cells were incubated with either buffer, 50 ng/ml DNP-BSA, 10 nM PMA, or 10 μM NECA. The reaction was stopped by the addition of formaldehyde (3.7% final vol/vol). The fixed cells were permeabilized with buffer containing 1% TX-100, and F-actin was stained with NBD-phallacidin for 1 h at room temperature. The fixed cells were washed twice with PBS and bound NBD-phallacidin was extracted with methanol. The extracts were centrifuged to remove any insoluble material, and the relative fluorescence was measured using an AMINCO Bowman series 2 spectrofluorometer with excitation and emission wavelengths of 465 nm and 535 nm, respectively.
Vaccinia Virus Constructs and Infection
Construction of wild-type Cdc42, constitutively active Cdc42V12 and constitutively active RacV12 were previously described (Hong-Geller and Cerione, 2000). B6A4C1 cells were infected with recombinant vaccinia virus at 20 pfu/cell for 6 h for degranulation experiments and 12 h for biosynthesis experiments as previously described (Hong-Geller and Cerione, 2000).
RESULTS
The B6A4C1 cell line was derived from mutagenized RBL-2H3 mast cells and originally selected for the loss of expression of the mast cell-specific ganglioside, α-galactosyl GD1b, which is recognized by the monoclonal antibody AA4 (Stracke et al., 1987; Guo et al., 1989). This phenotype is confirmed in Figure 1, which also compares the labeling of permeabilized wild-type RBL-2H3 cells with the mutant B6A4C1 cells for several proteins and for ganglioside GM1. Cells colabeled with FITC-IgE (Figure 1, A and B) and with anti-Lyn and Cy3-modified secondary antibody (Figure 1, C and D) show similar, primarily plasma membrane staining in both cell lines. This demonstrates that the signaling defects characterized below are not due to the absence of one of these critical proteins. Labeling of α-galactosyl GD1b in permeabilized cells with Cy3-AA4 (Figure 1, E and F) results in bright surface staining of the wild-type cells, but no significant staining of the B6A4C1 cells, confirming that this ganglioside is not expressed in these mutant cells. The absence of significant intracellular staining indicates that the lack of surface expression is not due simply to a defect in post-Golgi trafficking of this antigen.
We previously showed that α-galactosyl GD1b gangliosides coisolate with detergent resistant membranes (i.e., lipid rafts) from RBL-2H3 cells (Field et al., 1995). Therefore, we examined the expression of two other raft components present on these cells: GPI-linked protein Thy-1 (Figure 1, G and H) and ganglioside GM1 (Figure 1, I and J). Similar to that observed for α-galactosyl GD1b, B6A4C1 cells express much less of these components than the RBL-2H3 cells, which exhibit abundant surface expression, as well as some intracellular label. These results suggest a general defect in the biosynthesis of outer leaflet raft components but normal expression of the inner leaflet raft component Lyn.
Degranulation
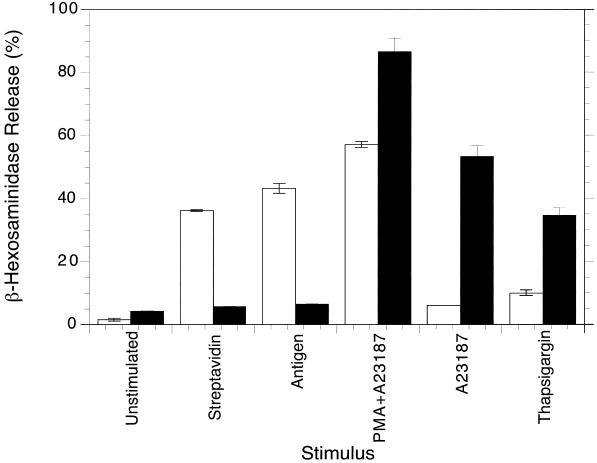
B6A4C1 cells were originally characterized as degranulating poorly in response to antigen (unpublished results). Figure 2 compares the release of β-hexosaminidase for RBL-2H3 (open bars) and B6A4C1 (filled bars) cells that were sensitized with biotinylated IgE and stimulated with various secretagogues. RBL-2H3 cells degranulated in response to streptavidin or antigen-mediated cross-linking of biotinylated IgE bound to FcεRI. In contrast, B6A4C1 cells showed only marginal responses to these stimuli. To determine if the defect in FcεRI signaling in the mutant cell line is before, or subsequent to Ca2+ mobilization, we triggered the cells with stimuli that bypass FcεRI. The Ca2+ ionophore A23187, together with the protein kinase C (PKC) activator PMA, stimulated strong degranulation in both RBL-2H3 and B6A4C1 cells (Figure 2). This demonstrates that the signaling defect in this mutant cell line does not prevent activation of downstream events. Also shown in Figure 2, B6A4C1 cells responded to Ca2+ ionophore alone (700 nM) to a larger extent than RBL-2H3 cells. Similarly, thapsigargin, an inhibitor of endoplasmic Ca2+ ATPase which activates Ca2+ influx by causing depletion of internal Ca2+ stores (Ali et al., 1994), stimulates some degranulation of RBL-2H3 cells, and B6A4C1 cells are stimulated significantly more (Figure 2). The results indicate that B6A4C1 cells are very sensitive to these downstream stimuli; thus, the defect in FcεRI signaling in these mutant cells appears to be upstream of Ca2+ influx.
Figure 2.
Degranulation of RBL-2H3 (open bars) and B6A4C1 (filled bars) mast cells. Adherent cells were sensitized overnight with biotinylated anti-DNP IgE and stimulated for 60 min at 37°C with no trigger (unstimulated), 10 nM streptavidin, 100 ng/ml DNP-BSA (antigen), 700 nM A23187 plus 80 nM PMA, 700 nM A23187, or 200 nM thapsigargin, as indicated. Cellular supernatants were assayed for β-hexosaminidase released from the cells as described in Methods. Degranulation is expressed as a percent of the total β-hexosaminidase activity released by TX-100 lysis, which was similar for both cell lines.
Ca2+ Mobilization
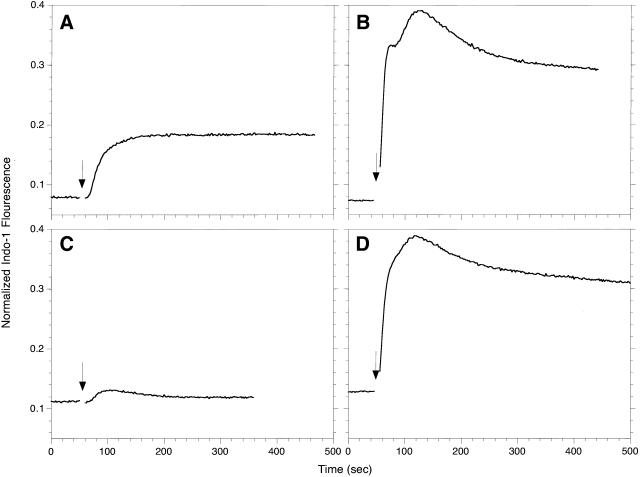
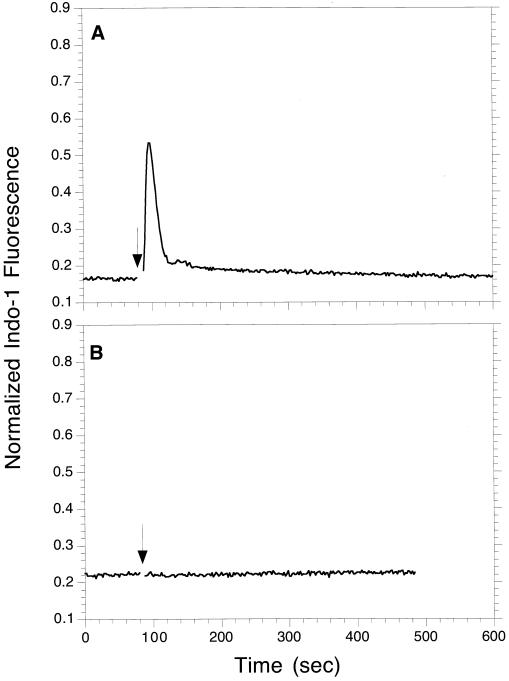
The signaling defect in the B6A4C1 cells was further investigated by measuring their antigen-stimulated Ca2+ response. RBL-2H3 and B6A4C1 cells loaded with the fluorescent Ca2+ indicator, indo-1, were triggered with multivalent antigen while monitoring indo-1 fluorescence. RBL-2H3 cells (Figure 3A) displayed a typical response characterized by a short delay, an initial rise contributed by the release of Ca2+ from internal stores, and a sustained plateau phase due to Ca2+ influx across the plasma membrane (Millard et al., 1988). In contrast, B6A4C1 cells showed only a small, transient response (Figure 3C), suggestive of some Ca2+ release from internal stores that does not trigger sustained Ca2+ influx. When thapsigargin is used to stimulate the B6A4C1 cells (Figure 3D), the Ca2+ response was qualitatively similar to that observed for RBL-2H3 cells (Figure 3B). This confirms that the B6A4C1 cells are able to undergo calcium influx across the plasma membrane when depletion of intracellular Ca2+ stores is sustained.
Figure 3.
Ca2+ responses of RBL-2H3 (A, B) or B6A4C1 (C, D) mast cells. IgE sensitized, indo-1 loaded cells were suspended at 106/ml, and indo-1 fluorescence was monitored at 400 nm. Where indicated by an arrow, the cells were stimulated with 100 ng/ml DNP-BSA (A, C) or 250 nM thapsigargin (B, D). The indo-1 fluorescence for each sample was normalized as described in Methods.
Tyrosine Phosphorylation
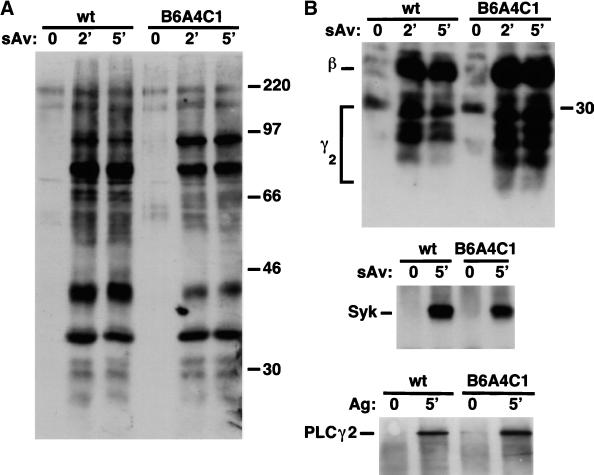
Because FcεRI-stimulated Ca2+ mobilization is defective in the B6A4C1 cells, we examined the upstream tyrosine phosphorylation events. Figure 4A compares streptavidin-stimulated tyrosine phosphorylation for B6A4C1 and wild-type RBL-2H3 cells sensitized with biotinylated IgE. Both cell lines exhibit robust stimulated tyrosine phosphorylation of a number of different proteins, and, although some differences appear in relative intensities of several bands, no consistent differences were noted in multiple experiments. To further compare and characterize activation of tyrosine kinases, we immunoprecipitated known substrates and examined their phosphorylation levels. The top panel of Figure 4B shows that streptavidin-induced tyrosine phosphorylation of FcεRI β and γ2 subunits by Lyn is similar for both RBL-2H3 and B6A4C1 cells. IgE-FcεRI cross-linking also caused similar amounts of phosphorylation of Syk tyrosine kinase in both cell lines (Figure 4B, center panel), indicating a similar amount of Syk activation (Rowley et al., 1995; Shiue et al., 1995). Furthermore, a known substrate of Syk, PLCγ2 (Zhang et al., 1996; Kurosaki, 1999) is tyrosine phosphorylated to the same extent in the two cell lines upon IgE-FcεRI aggregation by streptavidin (Figure 4B, bottom panel). In experiments analyzing PLCγ1 immunoprecipitates, a small amount of stimulated tyrosine phosphorylation was detectable in both cells lines (data not shown). These results indicate that the earliest signaling events stimulated by FcεRI, namely, tyrosine phosphorylation of this receptor and Syk-dependent substrates, occur equally well in the B6A4C1 and RBL-2H3 cells.
Figure 4.
Antiphosphotyrosine immunoblots of whole cell lysates and immunoprecipitates from RBL-2H3 (wt) and B6A4C1 cells sensitized with biotinylated IgE. (A) Time course of tyrosine phosphorylation stimulated by 10 nM streptavidin for whole cell lysates. (B) Stimulated tyrosine phosphorylation of FcεRI (top), Syk (middle), and PLCγ2 (bottom) immunoprecipitated from cell lysates. Numbers along right margins of immunoblots indicate positions of molecular mass markers in kDa.
Phospholipase Activation
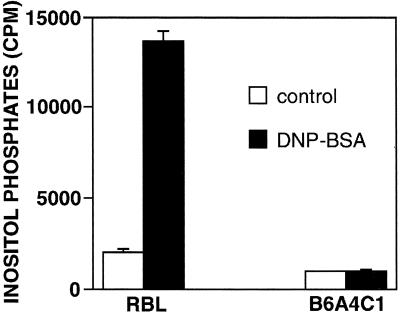
Our findings of stimulated tyrosine phosphorylation of PLC in the B6A4C1 cells, together with substantial reduction in Ca2+ mobilization, prompted us to investigate whether inositol phosphate production is stimulated in these cells. Figure 5 compares total inositol phosphates produced in the RBL-2H3 cells and B6A4C1 cells in response to antigen stimulation of IgE-FcεRI. RBL-2H3 cells exhibited an approximately sevenfold increase, whereas B6A4C1 cells show no significant increase following stimulation with DNP-BSA. In other experiments, B6A4C1 cells failed to stimulate IP3 as determined with a competitive binding assay that detected stimulated IP3 in RBL-2H3 cells (data not shown). Thus, the lack of stimulated inositol phosphate production by FcεRI cross-linking in B6A4C1 cells can account for the defect in stimulated Ca2+ mobilization.
Figure 5.
Total inositol phosphate production for RBL-2H3 and B6A4C1 cells. Radiolabeled cells were sensitized with IgE and activated with 50 ng/ml DNP-BSA (filled bars) or left unstimulated (open bars) for 45 min at 37°C in the presence of LiCl. Inositol phosphates were isolated and their radioactivity was quantified by liquid scintillation counting.
To determine whether the defect in receptor-stimulated inositol phosphate production in the B6A4C1 cells is specific for PLCγ isoforms, we compared activation of PLCβ in RBL-2H3 and B6A4C1 cells. In these cells, PLCβ can be activated by pertussis toxin-sensitive, G-protein-coupled receptors such as the A3 adenosine receptor (Ali et al., 1996). Stimulation by an agonist of this receptor, NECA, is not sufficient to cause degranulation, but it does stimulate a transient Ca2+ response and enhance the degranulation response to FcεRI (Ali et al., 1990). Figure 6 shows a representative experiment in which NECA stimulated a transient Ca2+ response in RBL-2H3 cells (Figure 6A) but did not stimulate a detectable response in B6A4C1 cells (Figure 6B). In the same experiment, antigen stimulated a transient Ca2+ response in B6A4C1 cells that was much smaller than the sustained response to antigen in RBL-2H3 cells, similar to Figure 3 (data not shown). These results indicate that B6A4C1 cells are defective in Ca2+ mobilization mediated by both PLCγ and PLCβ-activating receptors.
Figure 6.
Comparison of the stimulation of Ca2+ mobilization by NECA for RBL-2H3 cells (A) and B6A4C1 cells (B). Cytoplasmic Ca2+ was monitored with indo-1 fluorescence as described for Figure 3, and 10 μM NECA was added where indicated by the arrows.
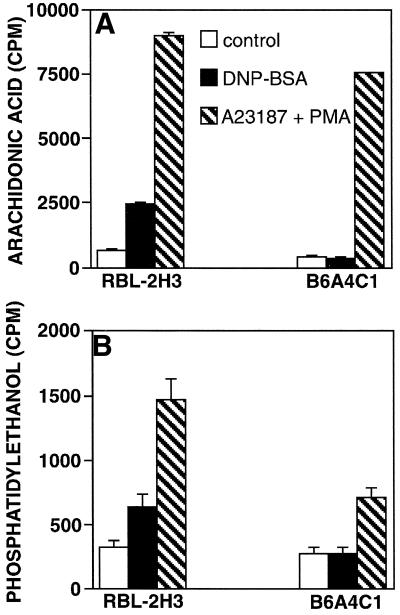
Because stimulated PLC activity is not detectable in the B6A4C1 cells, we tested antigen-mediated stimulation of two other lipases, phospholipase A2 (PLA2) and phospholipase D (PLD). Figure 7A shows that antigen-stimulated PLA2 activity, measured as production of 3H-arachidonic acid metabolites, was not detectable in B6A4C1 cells, whereas RBL-2H3 cells showed a 3.6-fold increase. For both cell lines, Ca2+ ionophore plus phorbol ester stimulated robust PLA2 responses, consistent with the observed degranulation responses (Figure 2). This indicates that PLA2 in B6A4C1 cells is functional but not activated by FcεRI cross-linking. Similar to these results, Figure 7B shows that DNP-BSA stimulated a significant PLD response in RBL-2H3 cells, but not in B6A4C1 cells. A23187 plus PMA stimulated a PLD response in both cell lines, but the magnitude of this response was smaller in the B6A4C1 cells. Because both PLA2 (Garcia-Gil and Siraganian, 1986) and PLD (Lin et al., 1991) require extracellular Ca2+ for antigen-stimulated membrane recruitment and cellular activity, the deficiencies in their activation by antigen in B6A4C1 cells may be a result of the loss of stimulated Ca2+ influx.
Figure 7.
Arachidonic acid production (A) and PLD activation (B) in stimulated RBL-2H3 and B6A4C1 cells. Cells were grown in the presence of 3H-arachidonic acid (A) or 3H-myristic acid (B) as described in Methods. IgE-sensitized cells were incubated with buffer (open bars), 50 ng/ml DNP-BSA (filled bars), or 500 nM A23187 plus 50 nM PMA (hatched bars) for 45 min at 37°C in the absence (A) or presence (B) of 0.5% (vol/vol) ethanol. Liquid scintillation counting was used to quantitate radiolabeled arachidonic acid metabolites released into the supernatant (A) or phosphatidylethanol isolated by TLC (B).
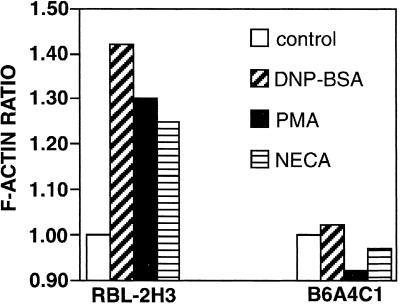
Stimulated production of PIP2 may be required for sustained activation of PLC, and it has also been implicated in stimulated actin polymerization as a sink for actin capping proteins such as gelsolin, thereby promoting microfilament growth (Apgar, 1995; Hartwig et al., 1995). We investigated the stimulation of actin polymerization in B6A4C1 cells by several different reagents previously shown to active this process in RBL-2H3 cells (Apgar, 1994). Figure 8 shows that DNP-BSA, PMA, and NECA all failed to stimulate significant increases in polymerized actin in B6A4C1 cells under conditions in which they stimulated strong responses in RBL-2H3 cells. These results indicate that B6A4C1 cells have a defect in stimulated actin polymerization which may involve decreased PIP2 production (see Discussion).
Figure 8.
Polymerization of actin in RBL-2H3 and B6A4C1 cells using different stimulants. Cells were sensitized with IgE and incubated with buffer (open bars), 50 ng/ml DNP-BSA, 5 min (diagonally hatched bars), 10 nM PMA, 5 min (solid bars), or 10 μM NECA, 1 min (horizontally hatched bars). Total F-actin content of the cells was assayed by fixing the cells with formaldehyde, permeabilizing with detergent, and measuring the amount of bound NBD-phallacidin. The data are expressed as the F-actin ratio which is calculated as (F-actin in activated cells - background)/(F-actin in unstimulated cells - background). The experimental error in all cases is less than 8%.
Reconstitution of the Signaling and Biosynthesis Defects in B6A4C1 Cells
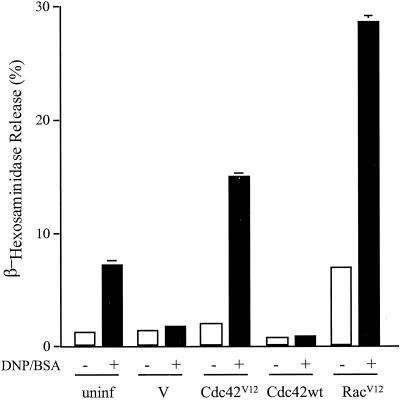
Our characterization of the signaling defects in B6A4C1 cells indicated that most of these could be accounted for by the failure to activate PLCβ and γ in these cells. A previous study indicated that GTP-bound Cdc42 and Rac could activate PLCβ2 in an in vitro assay with purified components (Illenberger et al., 1998). Furthermore, mutant forms of Cdc42 and Rac, Cdc42V12 and RacV12, which bind GTP in stable complexes that constitutively activate effector proteins were recently shown to enhance antigen-stimulated IP3 production and Ca2+ mobilization in RBL-2H3 cells (Hong-Geller and Cerione, 2000). We therefore tested the capacity of Cdc42V12 and RacV12 to reconstitute antigen-stimulated degranulation in B6A4C1 cells.
For these experiments, B6A4C1 cells were infected for 6 h with vaccinia virus constructs expressing Cdc42V12, RacV12, or wild-type Cdc42. Figure 9 shows the results from a representative experiment of this design. Infection with the empty vaccinia vector causes a decrease in the small response to antigen in B6A4C1 cells, similar to a previously observed reduction with RBL-2H3 cells (Hong-Geller and Cerione, 2000). Expression of Cdc42V12 restores antigen-stimulated degranulation, whereas wild-type Cdc42 does not. RacV12 causes a small increase in degranulation in the absence of antigen, and a more substantial increase in the antigen-stimulated response, similar to the FcεRI response in RBL-2H3 cells (Figure 2). Expression levels for these vaccinia constructs were similar to each other in the B6A4C1 cells and significantly greater than the endogenous levels of Cdc42 and Rac expression in these cells, as previously observed in RBL-2H3 cells (Hong-Geller and Cerione, 2000; data not shown). A more extensive characterization of the effects of these and related constructs reveals that Cdc42V12 and RacV12 also restore sustained antigen-stimulated Ca2+ responses in B6A4C1 cells (E. Hong-Geller, D. Holowka, R. Siraganian, B. Baird, and R.A. Cerione, submitted for publication). These results, taken together, support the hypothesis that activation of Cdc42 and/or Rac is a critical early event in antigen-stimulated degranulation. Furthermore, they indicate that the primary signaling defect in B6A4C1 cells is at or upstream of this activation process.
Figure 9.
Reconstitution of antigen-stimulated degranulation in B6A4C1 cells by activated Cdc42 and Rac. Release of β-hexosaminidase was measured in IgE-sensitized, uninfected B6A4C1 cells or B6A4C1 cells infected for 6 h with 20 pfu/cell of vaccinia virus vector only (V) or vaccinia virus containing Cdc42V12, Cdc42 wild-type, or RacV12, all in the presence or absence of 100 ng/ml DNP-BSA as in Figure 1. Error bars indicate the range of duplicate samples in a single experiment that is representative of results from three independent experiments for each construct.
We next characterized the effects of Rho family GTPases on the defects in ganglioside and GPI protein biosynthesis in B6A4C1 cells. Vaccinia infection of the mutant cells for 12 h with the Cdc42V12 construct resulted in abundant cell surface expression of GM1 detected with FITC-cholera toxin B subunit (Figure 10C), but infection with empty vector did not induce GM1 expression (Figure 10B). (The right-hand side of Figure 10, F, G, H, I, and J shows Cy3-antigen bound postfixation to the same cells, identifying those that are FITC-cholera toxin B-negative.) The appearance of newly-biosynthesized surface label in the Cdc42V12 -expressing cells is typically more punctate than the uniform plasma membrane distribution in RBL-2H3 cells shown in Figure 10A. Qualitatively similar expression of the α-galactosyl GD1b gangliosides and Thy-1 are also detected in B6A4C1 cells infected for 12 h with vaccinia virus expressing Cdc42V12 (unpublished observations). The more punctate distribution of these newly synthesized lipid raft components is consistent with results of Hannan et al. (1993), who found that newly synthetized GPI proteins are clustered and immobile when they first arrive at the plasma membrane.
In contrast to the results with activated Cdc42, wild-type Cdc42 expression does not cause the restoration of ganglioside expression (Figure 10D), consistent with the failure of this form to restore antigen-stimulated degranulation (Figure 9). Somewhat surprisingly, RacV12 fails to reconstitute biosynthesis of the outer leaflet lipid raft components (Figure 10E), contrary to its effective restoration of signaling (Figure 9). These results were quantified as summarized in Table 1. The difference between the large percentage of GM1-expressing cells with Cdc42V12 (67%) and the low percentage of GM1-expressing cells with RacV12 (5%) indicates that the function of Cdc42 in lipid raft biosynthesis probably involves interactions with different effector proteins than those involved in Cdc42/Rac-dependent signaling (see Discussion).
Table 1.
Quantification of GM1 expression on RBL mast cells
| Cells | GM1-positivea (%) |
|---|---|
| 2H3 | 100 |
| B6A4C1 + vector | 11 ± 6 |
| B6A4C1 + Cdc42V12 | 67 ± 18 |
| B6A4C1 + Cdc42wt | 13 ± 1 |
| B6A4C1 + RacV12 | 5 ± 2 |
Percentage of cells labeled with FITC–cholera toxin B based on scoring > 200 cells in two or more experiments ± SD.
DISCUSSION
The extensively studied RBL-2H3 mast cell line has permitted detailed characterization of FcεRI-mediated signaling, and thereby has provided a useful system for understanding the mechanisms of MIRR function in hematopoietic cells. In the present study, we describe a mutagenized RBL-2H3 cell line, B6A4C1, which fails to activate signaling pathways downstream of stimulated tyrosine phosphorylation, despite the apparently normal activation of Syk and tyrosine phosphorylation of its substrates, including PLCγ isoforms. Previous studies demonstrated that Syk activation is necessary for downstream signaling and degranulation mediated by FcεRI (Hirasawa et al., 1995; Zhang et al., 1996), and the present results indicate that there is a biochemical event downstream of stimulated tyrosine phosphorylation that is also essential for FcεRI-stimulated Ca2+ mobilization, degranulation, PLA2 activation, and actin polymerization.
The capacity of constitutively active Cdc42 and Rac to restore antigen-stimulated degranulation (Figure 9) and Ca2+ responses (Hong-Geller et al., submitted) suggest that activation of endogenous Rho family GTPases is the critical signaling event that is defective in the B6A4C1 cells. Consistent with this is the failure of wild-type Cdc42 or Rac expression to restore the signaling deficiencies in the mutant cells (Figure 9 and Hong-Geller et al., submitted). Based on these results, we hypothesize that activation of Cdc42 and/or Rac is a pivotal event in FcεRI-mediated stimulation of Ca2+ mobilization, degranulation, and other downstream signaling by FcεRI. However, it seems unlikely that activation of Cdc42/Rac is sufficient for Ca2+ mobilization and other downstream signaling leading to exocytosis, as there is little or no activation of these events by Cdc42V12 or RacV12 in the absence of antigen stimulation. Recent evidence indicates that activated Cdc42 and/or Rac participate in PLCγ activation in RBL-2H3 cells (Hong-Geller et al., submitted), but the mechanism of this effect is not yet clear. Stimulated tyrosine phosphorylation of PLCγ is necessary for its activation by growth factors (Kim et al., 1991) or antigen (Zhang et al., 1996) and appears to be independent of the Cdc42/Rac-dependent step. It is possible that a combination of tyrosine phosphorylation with a Cdc42/Rac-dependent event is required for PLCγ activation, and this may account for all of the subsequent downstream events that lead to exocytosis. In an analogous mechanism, activation of PLCβ by NECA may depend on Cdc42/Rac in addition to heterotrimeric G- protein βγ interactions (Illenberger et al., 1998).
Consistent with this hypothesis are recent descriptions of T cells and B cells from Vav-/- mice, in which several different signaling events downstream of tyrosine phosphorylation are diminished or absent (Fischer et al., 1998; Holsinger et al., 1998; O'Rourke et al., 1998; Costello et al., 1999). Vav is known to be a guanine nucleotide exchange factor with some preference for the Rho-family member Rac1 (Crespo et al., 1997), but the mechanism by which this protein participates in T and B cell receptor signaling is not established. For Vav-/- T cells, T cell receptor-mediated IP3 production (Costello et al., 1999), Ca2+ mobilization (Turner et al., 1997; Holsinger et al., 1998) and F-actin polymerization (Fischer et al., 1998) are substantially reduced or absent, as is stimulated IL-2 production and proliferation (Fischer et al., 1998; Holsinger et al., 1998; Costello et al., 1999). Similar to the signaling defects in B6A4C1 cells, all of the responses that are downstream of IP3 production are observed in Vav-/- T cells when Ca2+ ionophore and/or phorbol ester are used as the stimulatory agent(s).
Activation of Cdc42 and Rac during FcεRI-stimulated signaling in RBL-2H3 cells may be mediated by a guanine nucleotide exchange protein such as Vav, or by some alternate mechanism. In some cells, phosphoinositide 3-kinase has been shown to participate in Vav-dependent Rac activation (Rodriquez-Viciana et al., 1997; Han et al., 1998), and it is possible that this step or some other step upstream of Cdc42/Rac activation could be defective in B6A4C1 cells. However, although phosphoinositide 3-kinase is involved in antigen-stimulated IP3 production in RBL-2H3 cells, it does not appear to play a role in stimulated actin polymerization in these cells (Barker et al., 1995; 1998). This latter process is independent of extracellular Ca2+ (Pfeiffer et al., 1985) and can be activated by phorbol esters or diacyl glycerol in the absence of Ca2+ ionophores (Figure 8 and Apgar, 1995).
Our results indicate that the mutant phenotype of B6A4C1 cells can be accounted for by a single defect in Rho-family activation. As described above, PLCγ activation by antigen may depend on Cdc42/Rac activation in addition to stimulated tyrosine phosphorylation. In this model, the absence of antigen-stimulated PLA2 and PLD activation in the mutant cells could then be attributed to the absence of PLCγ activation leading to sustained Ca2+ mobilization. A Cdc42/Rac1-based defect in antigen-stimulated PIP2 synthesis may additionally play a role in the signaling deficiences of the B6A4C1 cells. In Vav-/- B cells, coreceptor CD19-dependent enhancement of B cell receptor activation is defective, and this defect correlates with dependence on Vav for stimulated PIP2 synthesis (O'Rourke et al., 1998). Consistent with this model, we observed that antigen-stimulated PIP2 synthesis is substantially less in the B6A4C1 cells than in RBL-2H3 cells (unpublished observations). Rho family members and PIP2 have been implicated in both actin polymerization (Hartwig et al., 1995) and PLD activation (Brown et al., 1993), so it is possible that the absence of stimulated actin polymerization (Figure 8) as well as stimulated PLD (Figure 7B) in the mutant cells could be explained by defective Cdc42/Rac activation separate from its effect on PLC activation. Further studies will be necessary to determine the relative contributions of Cdc42/Rac-dependent PIP2 synthesis and PLCγ activation on these downstream signaling pathways.
The lack of expression of gangliosides GM1 and α-galactosyl GD1b, and the GPI-linked protein Thy-1 in B6A4C1 cells suggests a defect in lipid raft-associated biosynthesis. These are all components of the plasma membrane outer leaflet, and their expression at the cell surface depends on trafficking from the Golgi complex via sphingolipid/cholesterol-rich lipid rafts (Simons & Ikonen, 1997). The capacity of active Cdc42 to restore this expression in the mutant cells suggests that Cdc42 may play an important role in this biosynthetic pathway. The incapacity of activated Rac to restore this pathway indicates that expression of gangliosides and GPI-linked proteins may depend on effector interactions that are specific to Cdc42. Previous studies indicated that Cdc42 is highly localized to Golgi in a brefeldin A-sensitive manner (Erickson et al., 1996), and other studies have suggested participation of this Rho-family member in membrane protein trafficking in polarized epithelial cells (Kroschewski et al., 1999).
Restoration of lipid raft-mediated biosynthesis by active Cdc42 but not wild-type Cdc42 further supports the hypothesis that activation of Rho-family proteins is the primary biochemical defect in B6A4C1 cells. Also consistent with this hypothesis are the findings that expression of o-Dbl, a guanine nucleotide exchange factor for the Rho family, partially restores both signaling (Hong-Geller et al., submitted for publication) and GM1 biosynthesis (unpublished results) in B6A4C1 cells. The differential capacity of active Rac to restore signaling deficiencies but not the biosynthetic defect indicates that this latter defect is not critical for FcεRI signaling in these cells. In this regard, although certain lipid raft components are not expressed in B6A4C1 cells, we find that cross-link-dependent IgE-FcεRI association with detergent-resistant membrane domains is preserved in these cells (data not shown), consistent with lipid raft participation in antigen-stimulated tyrosine phosphorylation.
In summary, analysis of mutant RBL mast cells provides evidence for a critical event in FcεRI signaling downstream of tyrosine phosphorylation that is necessary for stimulated actin polymerization, sustained Ca2+ mobilization, and activation of phospholipases important for mediator release. Restoration of antigen-stimulated signaling leading to exocytosis by expression of constitutively active Cdc42 or Rac implicates the activation of these Rho-family GTPases as the critical signaling defect in these cells. Restoration of ganglioside and GPI protein expression in these cells by active Cdc42 suggests that this Rho-family member also participates in the biosynthesis of outer leaflet lipid raft components. Future studies will explore the mechanisms by which these multifunctional GTPases carry out these diverse roles.
ACKNOWLEDGMENTS
This work was supported by Grants AI22449 (D.H.), GM42388 (J.A.) and AI42244 (J.A.) from the National Institutes of Health. Elizabeth Hong-Geller is an American Cancer Society postdoctoral fellow in the laboratory of Prof. R.A. Cerione, whom we thank for key insights and support.
REFERENCES
- Ali H, Maeyama K, Sagi-Eisenberg R, Beaven MA. Antigen and thapsigargin promote influx of Ca2+ in rat basophilic RBL-2H3 cells by ostensibly similar mechanisms that allow filling of inositol 1,4,5-trisphosphate-sensitive and mitochondrial Ca2+ stores. Biochem J. 1994;304:431–440. doi: 10.1042/bj3040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, Choi OH, Fraundorfer PF, Yamada K, Gonzaga HMS, Beaven MA. Sustained activation of phospholipase D via adenosine A3 receptors is associated with enhancement of antigen- and Ca2+-ionophore-induced secretion in a rat mast cell line. J Pharmacol Exp Ther. 1996;276:837–845. [PubMed] [Google Scholar]
- Ali H, Cunha-Melo JR, Saul WF, Beaven MA. The activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells: evidence for a novel adenosine receptor. J Biol Chem. 1990;265:745–753. [PubMed] [Google Scholar]
- Apgar JR. Polymerization of actin in RBL-2H3 cells can be triggered through either the IgE receptor or the adenosine receptor but different signaling pathways are used. Mol Biol Cell. 1994;5:313–322. doi: 10.1091/mbc.5.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apgar JR. Activation of protein kinase C in rat basophilic leukemia cells stimulates increased production of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: correlation with actin polymerization. Mol Biol Cell. 1995;6:97–108. doi: 10.1091/mbc.6.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apgar JR. Increased degranulation and phospholipase A2, C, and D activity in RBL cells stimulated through FcεRI is due to spreading and not simply adhesion. J Cell Sci. 1997;110:771–780. doi: 10.1242/jcs.110.6.771. [DOI] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Hall A, Martinez AM, Pfeiffer JR, Oliver JM, Wilson BS. Wortmannin Blocks Lipid and Protein Kinase Activities Associated with PI 3-Kinase and Inhibits a Subset of Responses Induced by FcεRI Cross-linking. Mol Biol Cell. 1995;6:1145–1158. doi: 10.1091/mbc.6.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Pfeiffer JR, Wilson BS. Wortmannin-sensitive phosphorylation, translocation, and activation of PLCγ1, but not PLCγ2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: Isolation of releasing and nonreleasing clones. Eur J Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidyinolitol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase C activity. Cell. 1993;75:1137–44. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Costello PS, Walters AE, Mee PJ, Turner M, Reynolds LR, Prisco A, Sarner N, Zamoyska R, Tybulewicz VLJ. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc Natl Acad Sci U S A. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Schuebel LE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the Vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y-h. Ligand recognition by alpha-beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Zhang C, Kahn RA, Evans T, Cerione RA. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi Apparatus. J Biol Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. FcεRI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci, U S A. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- Frigeri L, Apgar JR. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J Immunol. 1999;162:2243–2250. [PubMed] [Google Scholar]
- Garcia-Gil M, Siraganian RP. Phospholipase A2 stimulation during cell secretion in rat basophilic leukemia cells. J Immunol. 1986;136:259–263. [PubMed] [Google Scholar]
- Gruchalla RS, Dinh TT, Kennerly DA. An indirect pathway of receptor-mediated 1,2-diacylglycerol formation in mast cell. I. IgE receptor-mediated activation of phospholipase D. J Immunol. 1990;144:2334–2342. [PubMed] [Google Scholar]
- Guo N, Her GR, Reinhold VN, Brennan MJ, Siraganian RP. Monoclonal antibody AA4, which inhibits binding of IgE to high affinity receptors on rat basophilic leukemia cells, binds to novel α-galactosyl derivatives of ganglioside GD1b. J Biol Chem. 1989;264:13267–13272. [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hannan LA, Lisanti E, Rodriquez-Boulan, Edidin M. Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cells. J Cell Biol. 1993;120:353–358. doi: 10.1083/jcb.120.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NT, Goldstein B, Holowka D, Baird B. Altered patterns of tyrosine phosphorylation and Syk activation for sterically restricted cyclic dimers of IgE–FcεRI. Biochemistry. 1997;36:2237–2242. doi: 10.1021/bi9619839. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hirasawa N, Scharenberg A, Yamamura H, Beaven MA, Kinet JP. A requirement for Syk in the activation of the microtubule-associated protein kinase/phospholipase A2 pathway by FcεRI is not shared by a G protein-coupled receptor. J Biol Chem. 1995;270:10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Cerione RA. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J Cell Biol. 2000;148:481–493. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-β2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 1998;17:6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- Kinet J-P. The high affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- Lin P, Wiggan GA, Gilfillan AM. Activation of phospholipase D in a rat mast (RBL 2H3) cell line. J Immunol. 1991;146:1609–1616. [PubMed] [Google Scholar]
- Lin P, Fung W-JC, Gilfillan AM. Phosphatidylcholine-specific phospholipase D-derived 1,2-diacylglycerol does not initiate protein kinase C activation in the RBL-2H3 mast cell line. Biochem J. 1992;287:325–331. doi: 10.1042/bj2870325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon AK, Holowka D, Baird B. Small oligomers of immunoglobulin E (IgE) cause large-scale clustering of IgE receptors on the surface of rat basophilic leukemia cells. J Cell Biol. 1984;98:577–583. doi: 10.1083/jcb.98.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard PJ, Gross D, Webb WW, Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci, U S A. 1988;85:1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke AM, Mescher MF. T cell receptor-mediated signaling occurs in the absence of inositol phosphate production. J Biol Chem. 1988;263:18594–97. [PubMed] [Google Scholar]
- O'Rourke LM, Tooze R, Turner M, Sandoval DM, Carter RH, Tybulewicz VLJ, Fearon DT. CD19 as a membrane-anchored adaptor protein of B. lymphpocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 1998;8:635–645. doi: 10.1016/s1074-7613(00)80568-3. [DOI] [PubMed] [Google Scholar]
- Oliver C, Sahara N, Kitani S, Robbins AR, Mertz LM, Siraganian RP. Binding of monoclonal antibody AA4 to gangliosides on rat basophilic leukemia cells produces changes similar to those seen with Fcε receptor activation. J Cell Biol. 1992;116:635–646. doi: 10.1083/jcb.116.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985;101:2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L, Harris NT, Holowka D, Baird B. Evidence supporting a role for microfilaments in regulating the coupling between poorly dissociable IgE–FcεRI aggregates and downstream signaling pathways. Biochemistry. 1997;36:7447–7456. doi: 10.1021/bi9629642. [DOI] [PubMed] [Google Scholar]
- Pierini L, Holowka D, Baird B. FcεRI-mediated association of 6-μm beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J Cell Biol. 1996;134:1427–1439. doi: 10.1083/jcb.134.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- Rodriquez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rowley RB, Burkhardt AL, Chao HG, Matsueda GR, Bolen JB. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophsophorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue L, Zoller MJ, Brugge JS. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Stracke ML, Basciano LK, Siraganian RP. Binding properties and histamine release in variants of rat basophilic leukemia cells with changes in the IgE receptor. Immunol Lett. 1987;14:287–292. doi: 10.1016/0165-2478(87)90006-x. [DOI] [PubMed] [Google Scholar]
- Turner M, Mee J, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VLJ. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- Watts R, Howard TH. Evidence for a gelsolin-rich, labile F-actin pool in human polymorphonuclear leukocytes. Cell Motil Cytoskel. 1992;21:25–37. doi: 10.1002/cm.970210104. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol. 1999;11:265–269. doi: 10.1016/s0952-7915(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Xu K, Goldstein B, Holowka D, Baird B. Kinetics of multivalent antigen DNP-BSA binding to IgE–FcεRI in relationship to the stimulated tyrosine phosphorylation of FcεRI. J Immunol. 1998;160:3225–3235. [PubMed] [Google Scholar]
- Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor–mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]