Short abstract
It can be hard to interpret information about potential harms from drugs, whether through adverse effects or drug interactions. A simple visual coding system could help
Prescribers and patients need information about the established harms of a drug as well as its benefits. This information helps prescribers to calculate the likely balance of benefit to harm before prescribing a drug. It also enables them to avoid using a drug in circumstances of particular risk (for example, renal insufficiency), to choose preventive strategies (for example, using a bisphosphonate to prevent glucocorticoid induced osteoporosis), to know when to monitor for harm when the risk is defined (for example, regular blood counts in patients taking clozapine), and to recognise an adverse reaction when it occurs. Patients need to know about harms for similar reasons. They need to be able to decide whether the likely benefit outweighs the potential harm. They may be more aware than the prescriber of circumstances that prevent their taking the drug. And it is in their interest to be alert to the possibility that an unwanted event that occurs while they are taking the drug is an adverse drug reaction. What is the best way to provide information about drug harms?
Necessary information
Ideally, prescribing information should list the potential harmful effects of every drug together with the following information about each effect: its relation to the dose, its time course, the factors that alter an individual's susceptibility to it, its seriousness, and the probability of it occurring, at least in the population and preferably in the individual.
In practice, however, this information is rarely available, for several reasons. Firstly, most drug studies focus on benefits and are relatively poor at detecting harms, for which larger studies are required. Harms can be hard to detect for reasons that relate to the predictability, clinical features, and frequency of the harm. A harm may be unpredictable because the full extent of a drug's pharmacological actions is initially unknown. For example, sildenafil was first intended for use in angina, was then found to reverse erectile impotence by inhibiting phosphodiesterase in the corpus cavernosum (a beneficial side effect), and was later found to cause visual adverse effects by inhibiting phosphodiesterase in the retina. Alternatively, the harm can be the result of effects that are divorced from the primary action of a drug, as with torsade de pointes caused by thioridazine, which results from inhibition of cardiac potassium channels. And sometimes the mechanism of harmful effects is unknown, as with the pulmonary fibrosis that can occur with pergolide.
The clinical nature of the harm can influence the chance of detecting it. Some harms are commonly associated with drugs. An unexpected increase in prothrombin time in a patient taking warfarin is commonly the result of an interaction with a drug the patient has recently started taking. Conditions such as Stevens-Johnson syndrome and aplastic anaemia are often caused by drugs. In such cases, suspicions of drug induced harm are readily raised. However, other harms are not obviously drug related. For example, it took several years for clinicians to realise that cough is common in women who take angiotensin converting enzyme inhibitors. Bizarre adverse effects, such as a change in perception of musical pitch with carbamazepine, can also evade detection.
The relative frequencies of a harm and related natural disorders can also influence the ease with which the reaction is detected. Rare harms can be hard to detect, even if they have distinct clinical features, and harder still when they resemble natural disorders and have to be distinguished from the background, as the recent cyclo-oxygenase 2 inhibitors saga has shown.1
If a harm is rare, a very large study may be needed to be reasonably sure that the harm is associated with a drug. Large randomised controlled trials are difficult to establish and run. They are also relatively poor at detecting harms, which are usually multiple and may be unknown before a trial, whereas benefits are single and carefully specified. Detection of a rare harm therefore usually requires an observational study, from which findings can be distorted by uncorrected biases and confounding, especially confounding by indication (was an effect due to the drug or the indication for which the drug was prescribed?).
Thus, the information used to establish harms often falls short of proof. When information on harmful effects exists, it is commonly of poor quality or hard to use. Often no clinical trials are available, and information comes only from observational studies or anecdotal reports,2 which makes it difficult to calculate frequencies. Furthermore, anecdotal reports are often not confirmed by subsequent systematic studies.3 Even when adverse events are logged in clinical trials, the causal relation to the drug is not always clear, especially when there is no comparison with placebo treatment. And even when estimates of frequencies of harms are available it is often difficult to translate those frequencies to the risk in the individual patient.
Information currently available
Standard sources of drug information, such as the summaries of product characteristics and British National Formulary (BNF), routinely present information on harms as contraindications, adverse reactions, and drug interactions. Specific types of adverse drug reactions, such as teratogenicity, are often listed separately.
Contraindications are pre-existing conditions that either preclude drug use (absolute contraindications) or make it less safe (relative contraindications, sometimes labelled cautions or precautions). For example, metoclopramide is absolutely contraindicated in patients with intestinal obstruction, which it exacerbates, and relatively contraindicated in children, in whom it can precipitate acute dystonic reactions.
Adverse drug reactions, which range from the trivial to the fatal, depend on the dose of the drug, the time from the start of prescribing, and the susceptibility of the subject.4 Dose relations can be characterised as toxic effects, collateral effects, and hypersusceptibility effects. The time course ranges from immediate reactions to effects that are greatly delayed. And there are various susceptibility factors, including genetic factors, age, sex, and comorbidities, all of which can alter the individual's risk of an adverse reaction. Classifying adverse drug reactions in this way gives insights into their detection, avoidance, and management.4
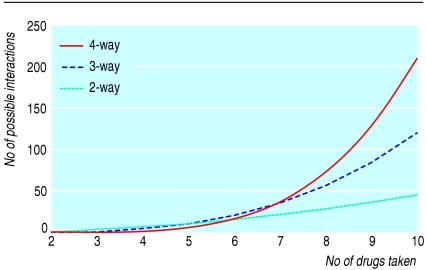
Drug interactions occur when one drug alters the concentration of another drug at the site of action or enhances or attenuates actions. Of the several thousand drugs used in modern practice, about 1200 are listed in the BNF; there are about 700 000 possible combinations of any two of these drugs, and the BNF lists about 3000 reported interactions of varying importance. Figure 1 shows the numbers of interactions that are theoretically possible from combinations of two, three, or four drugs when a patient takes up to 10 drugs. Small increases in the numbers of drugs make large differences to the numbers of potential interactions.
Fig 1.
Possible numbers of 2-way, 3-way, and 4-way interactions in patient taking multiple drugs
Communicating information
The three categories of potential harm share essential features that make communication difficult:
Lack of proof of the causality of an association
Evidence often confined to theory or unvalidated anecdotes
Poor estimates of the probability of harm and of its likely seriousness and intensity.
Furthermore, warning about every conceivable danger causes serious problems. Warnings may be so numerous that prescribers fail to heed them5,6; conversely, patients can be denied treatments because of unfounded concerns about dubious harms.
There is no clear system for deciding what information should be included, and different sources of data give inconsistent advice. This is illustrated by the widely varying information given about drug interactions in sources published in different countries. For example, information given about drug interactions with amiodarone shows wide disparities (see bmj.com). In all, 109 drugs or groups of drugs are mentioned in one or more of eight sources as interacting with amiodarone, but the range of drugs mentioned in each is 12-48 (median 38) and only five are in every source.
Proposed policy
Not all information should be included in practical guides to prescribing. The information that is included should enable the prescriber and patient to make rational decisions about drug treatment, based on the available evidence, with due regard to its quality and the risks involved. The threshold for including a warning will depend on the seriousness of the potential harm and the strength of the evidence. Clinical studies will generally carry more weight than in vitro or animal data, but such evidence (for example, teratogenicity in rabbits) often cannot be ignored. Some sources already use grading schemes for supposed frequencies of adverse reactions or seriousness of interactions. We advocate a consistent and transparent policy on how information should be incorporated into published sources such as the BNF.
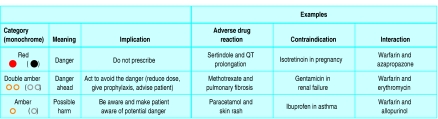
We suggest the inclusion of two types of information. Firstly, we propose including a warning about the degree of potential harm and the action to take. Users will want to have an estimate of the probability that harm will result from a given treatment, and this will vary from patient to patient. Complexity has to be balanced against clarity and accessibility of information, and we suggest three clearly signalled categories to guide the actions of the user, taking into account the combination of seriousness of harm and its probability (fig 2).
Fig 2.
Three categories of potential harm from drugs
This system should be intuitive and easy to understand; colour adds emphasis, but the signals have been chosen to be distinguishable in monochrome to allow for colour blindness or non-colour printing. A green signal could be added to this scheme to indicate that a particular course of action is known to be safe (for example, administration of a drug in pregnancy). However, drugs are usually not known to be harmful rather than known to be safe. We have therefore not included a green signal. In any case, when no harm is expected, no signal is required.
Secondly, it would be helpful to include some indication of the quality of the evidence for the association between drug and harm. We therefore propose that sources of information should indicate whether the evidence is based on:
A—Anecdotes: case reports or case series
D—Data from laboratory (animal or cellular) experiments or extrapolated from theory or
R—Randomised trials or observational studies.
Summary points
Prescribers and patients need information about the harms that drugs can cause
Potential harms come from adverse drug reactions, drug interactions, and contraindications
Evidence on all of these is patchy
Some potential harms are much more common, or more serious, than others
A grading system that signals the need for action and the evidence on which advice is based would help decision making
This is not a hierarchy of evidence. In some cases laboratory evidence may be sufficient to contraindicate a drug. Equally, a case or case series may be so convincing that randomised or observational studies are not necessary. In other cases a randomised study may not be the best way of showing an adverse effect or interaction and may give false reassurance about a true effect that has been shown in a case series or case-control study.7 In all cases the type of evidence that is most convincing needs to be considered by the prescriber.
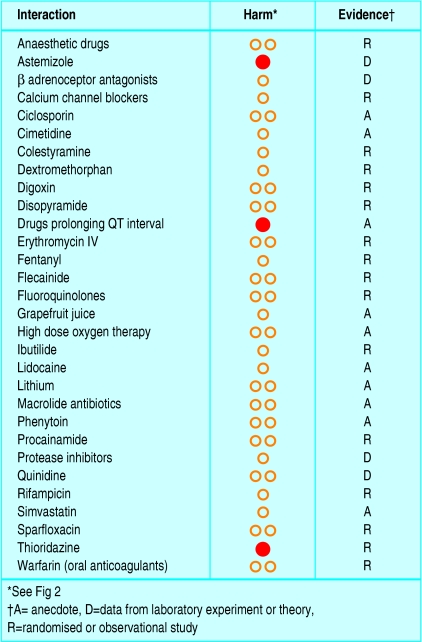
Figure 3 shows how drug interactions with amiodarone would be classified by this grading scheme. The principles should also be applicable to harms from interventions other than drugs—for example, the complications of surgical operations.
Fig 3.
Conclusions
The information currently provided in prescribing manuals is of uncertain validity, is often inconsistent between sources, and gives little or no indication of its importance. Assigning different pieces of information to different categories will be a challenge, but it is one that we need to face if prescribers and patients are to make informed therapeutic choices.
 List of harmful interactions between amiodarone and other drugs and references w1-w10 are on bmj.com
List of harmful interactions between amiodarone and other drugs and references w1-w10 are on bmj.com
We thank Sarah McDowell for help in checking table data and staff at the British National Formulary for helpful discussions.
Contributors and sources: JKA is a member of the joint formulary committee of the British National Formulary and of the paediatric formulary committee and REF is BMA representative on the formulary development committee of the BNF.
Competing interests: None declared.
References
- 1.Hawkey CJ, Fortun PJ. Cyclooxygenase-2 inhibitors. Curr Opin Gastroenterol 2005;21: 660-4. [DOI] [PubMed] [Google Scholar]
- 2.Aronson JK, Derry S, Loke YK. Adverse drug reactions: keeping up to date. Fundam Clin Pharmacol 2002;16: 49-56. [DOI] [PubMed] [Google Scholar]
- 3.Loke YK, Price D, Derry S, Aronson JK. Case reports of suspected adverse drug reactions—systematic literature survey of follow-up. BMJ 2006;332: 335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ 2003;327: 1222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferner RE. Computer aided prescribing leaves holes in the safety net. BMJ 2004;328: 1172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingart SN, Toth M, Sands DZ, Aronson M, Davis RB, Phillips RS. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med 2003;163: 2625-31. [DOI] [PubMed] [Google Scholar]
- 7.Aronson JK. Classifying drug interactions. Br J Clin Pharmacol 2004;58: 343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter K, ed. Stockley's drug interactions. 7th ed. London: Pharmaceutical Press, 2005.
- 9.Hantsen PD, Horn JR. Drug interactions—analysis and management. St Louis, MO: Wolters Kluwer Health, 2005.