Abstract
In this review, gas-phase chemistry of interstellar media and some planetary atmospheres is extended to include molecular complexes. Although the composition, density, and temperature of the environments discussed are very different, molecular complexes have recently been considered as potential contributors to chemistry. The complexes reviewed include strongly bound aggregates of molecules with ions, intermediate-strength hydrogen bonded complexes (primarily hydrates), and weakly bonded van der Waals molecules. In low-density, low-temperature environments characteristic of giant molecular clouds, molecular synthesis, known to involve gas-phase ion-molecule reactions and chemistry at the surface of dust and ice grains is extended here to involve molecular ionic clusters. At the high density and high temperatures found on planetary atmospheres, molecular complexes contribute to both atmospheric chemistry and climate. Using the observational, laboratory, and theoretical database, the role of molecular complexes in close and far away is discussed.
Planetary atmospheres and interstellar media have traditionally been lumped together; they are complex dynamical entities whose understanding has been and continues to be a challenge. That this subject is new may be appreciated by the fact that the knowledge that hydrogen is the abundant element of the universe is less than 100 years old. Hydrogen and helium comprise more than 99% of the universe. Thus, interstellar chemistry must be quite different from that of the atmospheres of the minor planets Mars, Earth, and Venus. Conceding the dominance of hydrogen and helium on the cosmic scale and even in the major planets, more complex molecules than H2 attract our attention and dominate our thinking (1–4).
An understanding of the chemistry of the universe must involve a wide range of processes. A bit of history with respect to interstellar chemistry is relevant to our perspective. The observed interstellar molecules, CH, CH+, and CN, are quantitatively discussed by Herbig (5) in his classic study of z Oph. These species have optical transitions in spectral regions where Earth’s atmosphere is transparent. It was assumed that in interstellar clouds, which were translucent and thus bathed in the galactic radiation field, the major components were atomic. Because the average density of the galaxy is 1 atom cm−1 (6) three-body processes were readily dismissed. Kramers and ter Haar (7) in 1946 presented a calculation for the formation of CH by the radiative association of C and H, i.e., C + H = CH + photon. In 1951 Bates and Spitzer (8) showed that the CH calculated formation rate was seriously incorrect and with that ruled out synthesis by gas-phase association. Their concluding statement ushered in a lengthy period with emphasis on molecular synthesis on grain surfaces under a variety of conditions (3, 9–11).
With the advent of molecular radioastronomy (12) the molecular character of giant molecular clouds could be examined. These are the largest structures within the galaxy, with spatial extent of tens of light years. They are essentially impenetrable by visible radiation. These clouds are of high density, up to 106 molecules cm−3. The temperatures are low, in the range of 10–20 K. These are the objects in which stars are created. The chemistry therein appears to be dominated by cosmic ray-induced ion-molecule reactions (9). As may be obvious, the density of the interstellar medium within a galaxy is highly heterogeneous. In this sense there is a further contrast between planetary atmospheres and interstellar media.
The chemistries of different regions reflect the local conditions of the region. The contemporary interstellar medium has both dust and gas. Consequently, the very important process of H atom recombination occurs on grain surfaces, in contrast to the prestellar early universe in which the heavier elements carbon, oxygen, magnesium, and silicon had not yet been formed by nucleosynthesis. There, H2 is formed with the electron as catalyst in two steps, by the radiative association of H + e = H− followed by the fast ion-molecule reaction H + H− = H2 + e. The surface recombination of H atoms in interstellar clouds with low ionization appears much more efficient than electron-catalyzed formation. The difficulty in demonstrable surface reactions leading directly to gas-phase molecules at the interstellar condition of 20 K and H2 = 105 molecules cm−3 is severe. The essential problem is that for the dense molecular clouds with temperatures of 10–20 K, desorption from a grain surface is unlikely. It is, however, clear that in strongly heated regions many species appear in the gas phase. A good example of this is the abundance of interstellar water. The short-wavelength astronomical satellite (SWAS) (http://cfa-www.harvard.edu/swas) has receivers dedicated to the transitions from low-energy states of H2O. In cold molecular clouds the abundance of H2O is found to be 10−4 of CO (13). In heated regions such as the Orion hot core there is copious H2O emission (14) observed by the infrared satellite observatory (ISO) (http://isowww.estec.esa.nl/science). Clearly, H2O is of considerable interest in planetary atmospheres. Its low abundance in the gas phase of cold molecular clouds must be noted. This is particularly interesting given the cosmic abundance ratio O/C = 2.3. After H2, CO is the second most abundant gas-phase species in interstellar clouds unlike planetary atmospheres. Although N2 is relatively abundant, well traced by the ionic complex N2H+, O2 has not been observed. SWAS has set limits of O2/CO < 10−3, by means of its magnetic dipole-allowed transition. CO2 can be detected by its ionic complex CO2H+ in cold molecular clouds; however, its abundance is quite low. It is nevertheless seen in heated regions by ISO. The protonated species, H3+, HCO+, H2COH+, and HCNH+, as well as N2H+ and CO2H+, have been observed primarily by radioastronomy and are discussed later. Gas-phase synthesis schemes have been quite successful for elucidating the chemistry of giant molecular clouds (15–18). We examine extensions of this chemistry to involve molecular ionic clusters. This is quite natural because the importance of ion clusters in Earth’s atmosphere was a dominant theme in the work of Eldon Ferguson (19–22).
Molecular Complexes in Planetary Atmospheres
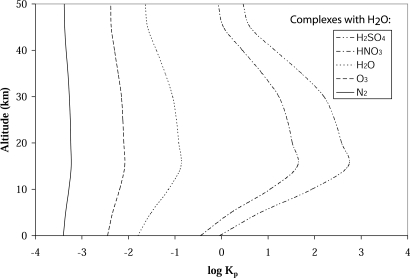
Planetary temperatures, climates, and chemistry are influenced by the absorption of solar radiation in the atmospheres of such cosmic bodies. At the high densities characteristic of planetary atmospheres, formation of weakly bound complexes is likely, yet relatively high temperatures characteristic of Earth’s and other dense planetary atmospheres such as Venus limit the lifetime and abundance of such complexes. In addition, spectroscopic signatures characteristic of weakly bound complexes are often masked by the absorption of the parent monomer. Using microwave and infrared spectroscopic information and theoretical chemistry, the thermodynamic properties of weakly bound molecular complexes have been estimated, and approximations to their atmospheric abundance have been attempted (23–29) as illustrated in Fig. 1. Based on such abundance calculations, close-in, on Earth, the role of weakly bound molecular complexes has been proposed (23, 25, 30, 31). A full description of thermodynamic properties is not possible because the appropriate theory of vibrational levels up to and above the dissociation limit for the complex is not yet available. These procedures seek to obtain the equilibrium constants for complexation, which are used to estimate their abundance. The estimates performed suggest that weakly bound van der Waals complexes (25, 32) (e.g., O2·N2), more strongly bound hydrogen-bonded clusters (23, 28, 33) [e.g., (H2O)2 and H2SO4·H2O], radical complexes (34–37) (e.g., OH·O2), and ionic clusters (38, 39) [e.g., H3O+·(H2O)n] could be important in atmospheric chemistry and climate.
Fig. 1.
Estimated atmospheric (Earth) vertical profile for molecular complexes with water.
Given theoretical estimates of relatively high abundances, it is somewhat surprising how few molecular complexes have been observed in planetary atmospheres. Only the hydrogen dimer has been identified in atmospheres of astronomical objects (Jupiter, Saturn, and Titan) through observations of the far-IR spectra of giant planets in the course of the Voyager space missions (40, 41). In the terrestrial atmosphere, only the oxygen dimer (O2)2 (42, 43) and the water dimer (H2O)2 (44) have been measured directly with long atmospheric absorption paths. The effect of these complexes and similar oxygen and water clusters with abundant atmospheric molecules (e.g., O2·N2, O2·H2O, and CO2·H2O) on radiative transfer and consequently on climate is receiving a great deal of attention (23, 28, 32, 42–44) (30). Model results rely mostly on laboratory model studies and chemical theory. The effects of complexes on climate have been evaluated in the case of O2·O2 and O2·N2 (43). The relevant spectroscopic features, missing from the high-resolution transmission molecular absorption (HITRAN) database (http://cfa-www.harvard.edu/hitran), are broad near-IR absorption bands assigned to transitions from the ground state to the electric dipole forbidden O2(1Δg) and O2(1Σg) states of O2 in the complex. Intensity for these transitions is acquired through vibronic spin-orbit coupling. The visible bands appear to involve simultaneous double electronic transitions of O2 in the O4 complex. Model results estimate ≈1 W m−2 contribution to the clear-sky absorption of solar radiation (43).
Excitation energies corresponding to the UV can induce photochemical reactions in oxygen (e.g., O2·O2) (45) and ozone (e.g., O3·H2O) (46) complexes. The UV absorption cross sections of oxygen complexes in the Herzberg O2 continuum (187–242 nm) corresponding to the A3Σu+ X3Σg− forbidden transition have been investigated from the point of view of ozone formation (45). The effect of temperature and pressure on the abundance, spectra, and dissociation dynamics of oxygen complexes revealed that the weakly bound metastable dimer of oxygen has an important effect on both the absorption cross section and photolysis rate of O2 in the Herzberg continuum. At these high excitation energies, perturbations of the reactive potential energy surfaces in clusters alter the monomer’s reaction dynamics. An important consequence of complex formation is the ability of the clustered reagents to use the excitation energy provided by the Sun in the UV with little loss through nonreactive processes. In the case of oxygen, the balance of odd (O + O3) and even (O2) oxygen is altered with consequences to the concentration of stratospheric and mesospheric O3 and OH. Laboratory quantum yields and model results have shown that this chemistry has only a small effect in Earth’s mesosphere (45).
The photochemistry of ozone in complexes with water has been proposed to lead to OH formation at lower excitation energies than the standard process that involves UV photolysis of ozone and subsequent reaction with water of the energetic photoproduct O(1D). The abundance of the complex is estimated to be greatest near the ocean surface, yet even in these environments only a small fraction, estimated at <0.001%, of ozone is complexed to water. The estimates also show that this seemingly insignificant fraction of hydrated ozone might be responsible for a few percent of atmospheric OH especially at high zenith angles (46). The reaction in the complex effectively uses all of the excitation energy that has a red-shifted spectrum while its bimolecular counterpart dissipates much of the excitation energy in relaxation of O(1D) in collisions with N2 and O2.
Water is the most significant greenhouse gas in Earth’s atmosphere. Water complexes have been suggested as potential contributors to climate by their absorption in the mid-IR and the near-IR (31). Water dimer has only recently been investigated and observed in the atmosphere (30, 44). The estimates suggest that the vibrational overtone transitions of (H2O)2 in the near-IR contribute as much as 3 W m−2 to absorption of solar radiation in the tropical atmosphere (31). Additional absorption is expected from complexes such as O2·H2O, N2·H2O, CO2·H2O, etc., but no experimental evidence exists to support such conjecture (32). In the atmosphere of Earth, open-shell radical species, although present in small concentrations, are important intermediates in chemical reactions. Recent laboratory and theoretical work highlighted the properties of radical complexes with water with potentially significant chemical effects in the atmosphere (34–37, 47). In the D region of Earth’s atmosphere, hydrated molecular clusters such as the protonated water cluster H3O+(H2O)n have been observed (17, 19–22). Laboratory studies suggest that series of reactions starting with NO+ and O2+, formed in the atmosphere by cosmic-ray ionization (48), are responsible for their formation. Although the atmospheric ion concentration varies with altitude, the integrated 50-km column concentration was estimated at 1014 m−2, about six order of magnitude less than the estimated neutral water dimer concentration. Based on the laboratory IR spectra of cluster ions, one expects effects on climate through radiative transfer; yet given their small concentration, the effects could be small (48). It is interesting to note that in a global warming scenario, a nonlinear increase in the concentration of water complexes (H2O)n and X+(H2O)n compared with water monomer is expected to provide positive feedbacks to anthropogenic global warming (31).
Molecular complexes of oxygen have been observed on the water-ice covered surfaces of Ganymede and Europa (49, 50). Odd oxygen production from oxygen dimer photolysis in the UV was used to explain ozone formation on these planetary moons. Observation of the Saturnian (Rhea and Dione) (51) and Galilean (Europa, Ganymede, and Callisto) (49, 50) moons with the Hubble space telescope’s Faint Object spectrograph and Galileo’s UV spectrograph identified condensed oxygen, oxygen dimers, and ozone. These observations have shown that dimer oxygen and ozone have opposite trends with latitude, implying a possible photochemical path between O2·O2 and O3 trapped in ice on Ganymede.
Important climate effects result from the role of clusters (hydrates) in nucleation of aerosols and clouds. The role of ambient ionization in tropospheric aerosol formation has been discussed (52, 53). Hydrates of sulfuric acid are major contributors to climate through formation of aerosols, ubiquitous in the terrestrial and Venusian atmospheres (52, 54). Aerosols scatter solar radiation directly, and nucleate clouds, which scatter very efficiently radiation, back to space. The effect of aerosols on climate is potentially large, but as yet both the magnitude and the sign of the effect are poorly understood and under investigation (55).
Interstellar Complexes
Molecular complexes play a large role in the chemistry of the interstellar medium, in particular in giant molecular clouds. Perhaps the most familiar type of molecular complex is the proton adduct of a stable saturated molecule. Adducts that have been observed are H3+, HCO+ and its high-energy isomer COH+, HN2+, HCNH+, OCOH+, H3O+, H2COH+, and H-CCCNH+. In every instance, the parent unprotonated species exists. In interstellar media, H2 is the most abundant molecule, and CO appears to be second (56, 57). In cool dense clouds, both HCN and HNC are observed in essentially equal abundance. Of particular interest and importance are HN2+ and HCO2+. These species provide a means of determining the interstellar abundance of the nonpolar molecules N2 and CO2. These complexes are very strongly bound and thus quite stable.
In dense molecular clouds where volume ionization is achieved by high-energy cosmic rays, the dominant initial process is ionization of H2 (to H2+) and He, which account for >99% of the interstellar gas. The secondary reaction yielding complexes is H2+ + H2 = H3+ + H, which is exothermic by 165 kJ/mol.
The reactions of He+ with H2 are considerably more exoergic but do not occur at the low cloud temperatures. The difficulty of facile conversion of electronic energy into nuclear kinetic energy has been discussed for the He+, H2 system, in terms of the location of the potential energy curve crossings. We remark that the lack of reactivity of He+ with the highly abundant H2 lead to the facile production of C+ by the fast ion-molecule reaction of He= with CO, the second most abundant interstellar molecule. The proton affinity of CO is considerably greater than that of H2 leading to the conversion of H3+ to HCO+, the most abundant molecular ion in dense interstellar clouds.
Quite clearly, the discussion of protonated molecules as molecular complexes of a molecule and a proton place these species in the class of standard strongly bound complexes. They play an important role in the chemistry of the interstellar medium for several reasons. First, this chemistry is kinetically determined. That this is true is shown by several examples. In cool regions (≈20 K), the abundances of HNC and HCN are essentially equal. Because their energy difference is 65 kJ/mol, this points to a far-from-equilibrium condition. Perhaps even more dramatic is the ratio of CO to CH4. This ratio may be estimated to be [CO]/[CH4] ≈ 104. The equilibrium constant at 20 K for the reaction CO + 3H2 = CH4 + H2O is 10490. Thus, at [H2] = 106 molecules cm−3 the predicted equilibrium ratio is [CO]/[CH4] > 10500, showing that equilibrium arguments are seriously flawed because they lead to predictions of less than one molecule of CO in the observable universe, in conflict with observation.
The question of other complexes is an interesting one. Most interesting is that of weakly bound species, van der Waals molecules, bound in a complex by <1 kJ/mol. At first sight, their formation can be dismissed on the basis of the low molecular density of interstellar environments. The standard laboratory formation technique for such complexes is adiabatic expansion of a high-pressure gas mixture.
This scheme depends on three-body collisions. Because the density in a dense molecular interstellar cloud will be below 107 cm−3, the feasibility of three-body processes can be discarded. This has suggested to many of us that the formation (and therefore existence) of weakly bound complexes is unlikely. Although these arguments sound persuasive, they ignore one essential difference between laboratory and interstellar chemistry, namely the much larger time scale available in interstellar space. In particular, radiative association is usually a slow process that is unlikely to be of importance in laboratory conditions where gas densities of 1011 cm−3 or greater exist (58, 59). Thus, the laboratory measurement of slow radiative association rates requires considerable care in avoiding three-body association. Because the relevant temperature for dense cloud chemistry is 10–20 K, experimental measurements under these conditions are relatively rare (60).
The relation between radiative association and three-body association rates frequently has been discussed. The collision duration of the pair of interest is determined by the stabilization of the collision complex with helium encounters. The radiative association is then estimated by the emission rate during the lifetime of the collision complex (61). The species forming the collision complexes of interest here are in their ground electronic states. These states are nondegenerate orbitally; thus, the emission is the result of vibrational transitions, which may be estimated (62).
Below we consider possible candidates for both formation of weakly bound complexes and their observation. Because the abundance of a collision complex will depend on the abundances of the collision partners, their collision frequency, and the binding energy of the complex, it appears most likely that the attractive species are an ion and H2. The most abundant ion in dense molecular clouds is HCO+. Thus, the species of interest initially is H2-HCO+. This complex is well characterized spectroscopically (63). The radiative association
was thought initially to proceed efficiently to the stable protonated formaldehyde ion (64). The enthalpy change for this process is 122 kJ/mol. This was shown not to be the case, and instead the much higher energy form H2-HCO+, a weakly bound complex, is the product observed in helium stabilized collisions (61). The binding energy of H2-HCO+ is 1,370 cm−1. There are two forms, pH2-HCO+ and oH2-HCO+, which are quite different spectrally (63). The stability of these complexes is relatively slight, although they show no reactivity with H2 and are facilely converted to OC-HCO+. The abundance ratio [OC-HCO+]/[ H2-HCO+] ≈ 102 is estimated to be quite similar to the ratio of [HCO+]/[H3+]. The binding energy of OC-HCO+ is 4,900 cm−1.
The observation of these interstellar complexes of HCO+ presents interesting difficulties. The abundance ratio clearly favors OC-HCO+; however, this species is quite nonrigid. The complex has a linear structure but proton tunneling, i.e., OC-HCO+ ↔ +OCH-CO, is readily estimated to be extremely facile. The barrier to this proton motion is estimated (K. Higgins and Z. Yu, personal communication) to be relatively low, 1,100 cm−1. Thus the observable spectrum is not pure rotational but rotation tunneling. This species should be extremely interesting from a chemical viewpoint because it exhibits interconversion of a van der Waals (or hydrogen) bond and a covalent bond. In a sense, this is a gas-phase zwitterion.
The abundance of these species depends on the rate of radiative association. This has been estimated (61) to be 10−18 cm3 sec−1. Using this value and [H2] = 106, we obtain a steady state abundance of [OC-HCO+] = 10−7 cm−3. The abundance of H2-HCO+ is estimated to be two orders of magnitude smaller. Such estimates point to the likelihood that, in addition to strongly bound complexes of molecules with protons, weakly bound complexes may play a role in interstellar chemistry. A much more detailed theoretical analysis of the radiative association rate of H2-HCO+ is clearly of importance. It is likely that laboratory spectroscopic studies of these species, H2-HCO+ and OC-HCO+, will provide the precision frequencies to allow astronomical searches. The further question of whether these species are useful intermediates for the synthesis of interstellar species such as H2CO depends most likely on their reactivity with H atoms as well as its abundance in dense molecular clouds.
Concluding Remarks
In the context of this review, molecular complexes include strongly bound aggregates of molecules with ions, as well as intermediate strength hydrogen bonded complexes (H2O)n and H2SO4·(H2O)n and weakly bonded van der Waals molecules (O2·N2, O2·O2, Ar·HF, and H2HCO+). The stability of complexes varies with their bond dissociation energy, resulting in shallow potentials for weak van der Waals complexes stable only at low temperatures. The intermolecular interactions responsible for cluster formation modify the electronic structure, spectra, and reaction dynamics of the isolated precursors. Such complexes form readily in dense and low-temperature environments. In this review, we have discussed their formation and possible roles in planetary atmospheres (high density and high temperature) and in dense molecular clouds (low density and low temperature).
Understanding the radiation budget of a planet and the effects of atmospheric greenhouse gases is crucial to developing predictive climate models. It has recently been proposed that small contributions may have a cumulative impact. We reviewed the interesting possibilities that in the terrestrial atmosphere, nonlinear spectroscopic contributions due to complexes of oxygen and of water may occur. Water clusters (H2O)n and hydrates of water with O2, N2, Ar, and CO2 as well as complexes of oxygen (O2-O2, O2-N2, etc.) contribute through shifted and modified lineshapes caused by intermolecular interactions responsible for complex formation. Such contributions to the atmospheric “continuum absorption” have been estimated for the contemporary Earth and shown via general circulation models (GCM) to increase nonlinearly in a global-warming scenario. Laboratory and field data are now available to estimate the effects of water clusters in terrestrial atmospheric radiative transfer. An older literature exists proposing that molecular complexes are important in atmospheres of other cosmic bodies.
Hydrates of acids such as H2SO4-(H2O)n and HNO3-(H2O)n and ions like H3O+-(H2O)n are responsible for nucleation of aerosols, formation of cloud condensation nuclei, and ultimately clouds, which scatter solar radiation back to space and provide a nonlinear cooling effect. Despite significant interest and research, insufficient information is currently available to understand nucleation in planetary atmospheres. We point out that aerosols and clouds provide a feedback mechanism able to counter to some as-yet-unknown extent warming due to greenhouse gases. The role of complexes in nucleation of aerosols and formation of clouds is relevant to planetary atmospheres, notably that of Venus. We have not touched on the interesting role of circumstellar molecular chemistries. The question of complex formation occurring in stellar outflows is relatively unexamined.
The role of gas-phase ion-molecule reactions in planetary and interstellar media has been recognized, yet limitations of gas-phase processes have been noted in explaining observation of molecular species. In the low-density environments of molecular clouds, removal of excess energy via three-body collisions is unlikely because of the very low density. The question of whether ionic complexes with H2 will form by radiative association in sufficient number to provide new reaction pathways is posed as a question here. We note that for gas-phase reactions, the level of both theory and experiment is high. The detection and identification of gas-phase species by rotational emission spectroscopy (www.ph1.uni-koeln.de/vorhersagen) has an extremely high level of reliability if pursued in a serious manner. The recent experience (65) with the discredited detection of glycine (66) provides a useful example of the level of methodology required for reasonable certainty.
As thinking turned to gas-surface interactions and reactions, the lack of understanding of such processes, the low density of grains, and the incomplete knowledge of their composition and morphology, as well as the difficulty in proposing plausible mechanisms of removal of reaction products formed at low temperatures on the surface, remained challenging issues. We have discussed in this review the possibility that gas-phase molecular complexes provide an alternative chemical environment. It is plausible that such complexes can form in media relevant to interstellar chemistry and effectively carry their own third body for energy relaxation and molecular synthesis. Laboratory model compound studies in concert with direct astronomical observations are needed to elucidate the role of molecular complexes in “close in” planetary atmospheres and “far away” interstellar media.
Acknowledgments
V.V. thanks the Radcliffe Institute for Advanced Study of Harvard University and the John Simon Guggenheim Memorial Foundation for fellowships during 2004 and 2005, and Dr. V. Bierbaum for helpful discussions. Funding from the National Science Foundation is gratefully acknowledged in support of our work on molecular complexes.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kaiser R. I. Chem. Rev. 2002;102:1309–1358. doi: 10.1021/cr970004v. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenfreund P., Charnley S. B. Annu. Rev. Astron. Astrophys. 2000;38:427. [Google Scholar]
- 3.Williams D. A. Faraday Discuss. 1998;109:1–13. [Google Scholar]
- 4.Kerridge J. F. Space Sci. Rev. 1999;90:275–288. doi: 10.1007/978-94-011-4211-3_25. [DOI] [PubMed] [Google Scholar]
- 5.Herbig G. H. Z. Astrophys. 1968;68:243. [Google Scholar]
- 6.Eddington A. S. Proc. R. Soc. London Ser. A; 1926. pp. 424–456. [Google Scholar]
- 7.Kramers h. A., ter Haar D. Bull. Astron. Inst. Neth. 1946;10:137–146. [Google Scholar]
- 8.Bates D. R., Spitzer L. Astrophys. J. 1951;113:441–463. [Google Scholar]
- 9.Herbst E. Adv. Gas Phase Ion Chem. 1998;3:1–47. [Google Scholar]
- 10.Tielens A. G. G. M., Charnley S. B. Origins Life Evol. Biosphere. 1997;27:23–51. [PubMed] [Google Scholar]
- 11.Sorel W. H. Astrophys. Space Sci. 1997;253:27–41. [Google Scholar]
- 12.Rank D. H., Townes C. H., Welch W. J. Science. 1971;174:1083. doi: 10.1126/science.174.4014.1083. [DOI] [PubMed] [Google Scholar]
- 13.Snell R. L. Astrophys. J. 2000;539:1101–1105. [Google Scholar]
- 14.Harwitt M., Newfeld D. A., Melnick G. J., Kaufman M. J. Astrophys. J. Lett. 1998;497:L105–L108. [Google Scholar]
- 15.Dalgarno A. Int. J. Mass Spectrom. 1995;149:429–437. [Google Scholar]
- 16.Huntress W. T., Mitchell G. E. Astrophys. J. 1979;231:456–467. [Google Scholar]
- 17.Heitmann H., Arnold F. Nature. 1983;306:747–751. [Google Scholar]
- 18.Smith D. M., Spanel P. Mass. Spectrom. Rev. 1995;14:255–278. [Google Scholar]
- 19.Ferguson E. E., Fehsenfeld F. C., Albritton D. L. Gas Phase Ion Chem. 1979;1:45–85. [Google Scholar]
- 20.Ferguson E. E., Arnold F. Acc. Chem. Res. 1981;14:327. [Google Scholar]
- 21.Fehsenfeld F. C., Ferguson E. E. J. Geophys. Res. 1969;74:2217–2222. [Google Scholar]
- 22.Ferguson E. E., Fehsenfeld F. C. J. Geophys. Res. 1969;74:5743–5751. [Google Scholar]
- 23.Vaida V., Headrick J. E. J. Phys. Chem. 2000;104:5401–5412. [Google Scholar]
- 24.Vigasin A. A. J. Struct. Chem. Engl. Transl. 1983;24:102–131. [Google Scholar]
- 25.Vigasin A. A., Slanina Z. Molecular Complexes in Earth’s Planetary, Cometary and Interstellar Atmospheres. Singapore: World Scientific; 1998. [Google Scholar]
- 26.Fellers R. S., Braly L. B., Brown M. G., Saykally R. J. J. Chem. Phys. 1999;110:6306–6318. [Google Scholar]
- 27.Schenter G. K., Kathmann S. M., Garrett B. C. J. Phys. Chem. A. 2002;106:1557–1566. [Google Scholar]
- 28.Vaida V., Kjaergaard H. G., Feierabend K. J. Int. Rev. Phys. Chem. 2003;22:203–219. [Google Scholar]
- 29.Evans G. T., Vaida V. J. Chem. Phys. 2000;113:6652–6659. [Google Scholar]
- 30.Ptashnik I. V., Smith K. M., Shine K. P., Newnham D. A. Q. J. R. Meteorol. Soc. 2004;130:2391–2408. [Google Scholar]
- 31.Vaida V., Daniel J. S., Kjaergaard H. G., Goss L. M., Tuck A. F. Q. J. R. Meteorol. Soc. 2001;127:1627–1643. [Google Scholar]
- 32.Kjaergaard H. G., Robinson T. W., Howard D. L., Daniel J. S., Headrick J. E., Vaida V. J. Phys. Chem. A. 2003;107:10680–10686. [Google Scholar]
- 33.Vaida V., Kjaergaard H. G., Hintze P. E., Donaldson D. J. Science. 2003;299:1566–1568. doi: 10.1126/science.1079297. [DOI] [PubMed] [Google Scholar]
- 34.Aloisio S., Francisco J. S. Acc. Chem. Res. 2000;33:825–830. doi: 10.1021/ar000097u. [DOI] [PubMed] [Google Scholar]
- 35.Brauer C. S., Sedo G., Grumstrup E. M., Leopold K. R., Marshall M. D., Leung H. O. Chem. Phys. Lett. 2005;401:420–425. [Google Scholar]
- 36.Ohshima Y., Sato K., Sumiyoshi Y., Endo Y. J. Am. Chem. Soc. 2005;127:1108–1109. doi: 10.1021/ja0442973. [DOI] [PubMed] [Google Scholar]
- 37.Suma K., Sumiyoshi Y., Endo Y. Science. 2005;308:1885–1886. doi: 10.1126/science.1112233. [DOI] [PubMed] [Google Scholar]
- 38.Dopfer O., Roth D., Maier J. P. Int. J. Mass Spectrom. 2002;218:281–297. [Google Scholar]
- 39.Ziemann P. J., Castleman A. W. J. Phys. Chem. 1991;94:718–728. [Google Scholar]
- 40.Frommhold L. Collision Induced Absorption in Gases. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]
- 41.McKellar A. R. W. Astrophys. J. 1988;326:3261. [Google Scholar]
- 42.Pfeilsticker K., Erle F., Platt U. J. Atmos. Sci. 1997;54:933–939. [Google Scholar]
- 43.Solomon S., Portmann R. W., Sanders R. W., Daniel J. S. J. Geophys. Res. 1998;103:3847–3858. [Google Scholar]
- 44.Pfeilsticker K., Loter A., Peters C., Bosch H. Science. 2003;300:2078–2080. doi: 10.1126/science.1082282. [DOI] [PubMed] [Google Scholar]
- 45.Brown L., Vaida V. J. Phys. Chem. 1996;100:7849–7853. [Google Scholar]
- 46.Frost G., Vaida V. J. Geophys. Res. Atmos. 1995;100:18803–18809. [Google Scholar]
- 47.Nelander B., Engdahl A., Svensson T. Chem. Phys. Lett. 2000;332:403–408. [Google Scholar]
- 48.Aplin K. L., McPheat R. A. J. Atmos. Solar Terr. Phys. 2005;67:775–783. [Google Scholar]
- 49.Calvin W. M., Spencer J. R. Icarus. 1997;130:505–516. [Google Scholar]
- 50.Noll K. S., Johnson R. E., Lane A. L., Domingue D. L., Weaver H. A. Science. 1996;273:341–343. doi: 10.1126/science.273.5273.341. [DOI] [PubMed] [Google Scholar]
- 51.Noll K. S., Roush T. L., Cruikshank D. P., Johnson R. E., Pendleton Y. J. Nature. 1997;388:45–47. doi: 10.1038/40348. [DOI] [PubMed] [Google Scholar]
- 52.Seinfeld J. H., Pandis S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: Wiley; 1998. [Google Scholar]
- 53.Finlayson-Pitts B. J., Pitts J. N. Chemistry of the Upper and Lower Atmosphere. San Diego: Academic; 1999. [Google Scholar]
- 54.Scappini F., Cecchi-Pestellini C., Smith H., Klemperer W., Dalgarno A. Mon. Not. R. Astron. Soc. 2003;341:657–661. [Google Scholar]
- 55.Intergovernmental Panel on Climate Change. Climate Change 2001: The Scientific Basis. New York: Cambridge Univ. Press; 2001. pp. 289–348. [Google Scholar]
- 56.Yan M., Dalgarno A., Klemperer W., Miller A. E. S. Mon. Not. R. Astron. Soc. 2000;313:L17–L18. [Google Scholar]
- 57.Bertoldi F., Cox P., Neri R., Carilli C. L., Walter F., Omont A., Beelen A., Henkel C., Fan X., Strauss M. A., Menten K. M. Astron. Astrophys. 2003;409:L47–L50. [Google Scholar]
- 58.Gerlich D., Horning S. Chem. Rev. 1992;92:1509–1539. [Google Scholar]
- 59.Herbst E., Smith D. M., Adams N. G., McIntosh B. J. J. Chem. Soc. Faraday Trans. 2. 1989;85:1655–1664. [Google Scholar]
- 60.Barlow S. E., Dunn G. H., Schauer M. Phys. Rev. Lett. 1984;52:902–905. [Google Scholar]
- 61.Fehsenfeld F. C., Dunkin D. B., Ferguson E. E. Astrophys. J. 1974;188:43–44. [Google Scholar]
- 62.Herbst E. Astrophys. J. 1985;291:226–229. [Google Scholar]
- 63.Bieske E. J., Nizgorodov S. A., Bennett F. R., Maier J. P. J. Chem. Phys. 1995;102:5153–5164. [Google Scholar]
- 64.Herbst E., Klemperer W. Astrophys. J. 1973;185:505–533. [Google Scholar]
- 65.Snyder L. E., Lovas F. J., Hollis M., Friedel D. N., Jewell P. R., Remijan A., Ilyushin V. V., Alekseev E. A., Dyubko S. F. Astrophys. J. 2005;619:914–930. [Google Scholar]
- 66.Kuan Y.-J., Charnley S. B., Huang H.-C., Tseng W.-L., Kisiel Z. Astrophys. J. 2003;593:848–867. [Google Scholar]