Abstract
Chemical sensing by cell-surface receptors to effect signal transduction is a ubiquitous biological event. Despite extensive structural biochemical study, detailed knowledge of how signal transduction occurs is largely lacking. We report herein a kinetic and structural study, obtained by stopped-flow IR spectroscopy, of the activation of the BlaR1 receptor of the Staphylococcus aureus bacterium by β-lactam antibiotics. The cell-surface BlaR1 receptor alerts the bacterium to the presence of β-lactam antibiotics, resulting in expression of the gene for a β-lactamase enzyme. This enzyme hydrolytically destroys the remaining β-lactam antibiotics. IR spectroscopic interrogation of the β-lactam–BlaR1 receptor reaction has allowed the simultaneous measurement of the chemical events of receptor recognition of the β-lactam and the characterization of the conformational changes in the BlaR1 receptor that result. The key chemical events in β-lactam recognition are serine acylation and subsequent irreversible decarboxylation of the BlaR1 active site lysine carbamate. Both events are observed by stopped-flow IR kinetics and 13C isotope-edited IR spectroscopy. The secondary structural changes in the BlaR1 receptor conformation that occur as a consequence of this acylation/decarboxylation are predicted to correlate to the signal transduction event accomplished by this receptor.
Keywords: receptor, signal transduction, infrared spectroscopy
The emergence of Gram-positive Staphylococcus aureus bacteria resistant to methicillin, a second-generation β-lactam antibiotic, was first reported in 1961. Four decades later, this same resistant strain (known as methicillin-resistant S. aureus, or MRSA) has emerged as a clinically significant pathogen (1–3) that is resistant to all classes of commercially available β-lactam antibiotics (4, 5). It is now known that the basis for β-lactam antibiotic resistance in MRSA is acquisition of an intriguing pair of signal sensing/transducing systems that unleash two separate and complementary antibiotic resistance mechanisms. One is the production of a β-lactamase, which hydrolytically destroys the β-lactam antibiotics. The second is the expression of a new penicillin-binding protein (PBP), referred to as PBP 2a, which resists the irreversible modification of its active site by the β-lactam (6–8). The expression of these antibiotic-resistance determinants is inducible (3). The biochemical commitment to antibiotic resistance is made only when the antibiotic is present in the milieu. The sensing/transducing systems used by MRSA are the BlaR1 and MecR1 receptors. The former has been implicated primarily in induction of β-lactamase expression and the latter in the induction of PBP 2a expression (1–3, 9–11).
The BlaR1 protein has three domains (Fig. 1): a C-terminal cell surface sensor domain, a transmembrane domain (that is believed to traverse the membrane four times; ref. 12), and a domain that is suggested to possess zinc-dependent protease activity. Reaction of the β-lactam with the surface domain of BlaR1 initiates signal transduction through the transmembrane domain, leading to activation of the cytoplasmic domain. The consequence of this activation is proteolytic degradation of a repressor protein that otherwise suppresses transcription of the blaZ gene encoding the β-lactamase. The mechanism of signal transduction by the MecR1 receptor is believed to have close similarity to that of the BlaR1 receptor.
Fig. 1.
Schematic of the BlaR1 protein and its overall action. The loss of interaction of the C-terminal domain with loop 2 is proposed in ref. 12, as is the tilting of the transmembrane helices in the signaling event.
The event following β-lactam binding to the BlaR1 surface domain is catalytic acylation of Ser-389 concomitant with opening of the β-lactam ring. The essential residue that promotes this serine for β-lactam acylation is an Nε-carboxylysine, obtained by the reaction of the sidechain amine of Lys-392 with carbon dioxide (1, 2). The use of an identical carbamate base for serine acylation also occurs in the class D β-lactamases (13). Indeed, the BlaR1 surface domain and the class D β-lactamases are believed to be evolutionarily related. Yet, the former is a receptor, and the latter is an enzyme. This divergence of function is suggested to relate to the chemical stability of the critical Nε-carboxylysine residue. In the example of the class D β-lactamases, the Nε-carboxylysine is stable, and acylation is followed by hydrolytic deacylation. In the example of the BlaR1 receptor, it is indicated that this residue is not stable. Rather, after its catalysis of sensor domain serine acylation, it undergoes N-decarboxylation. The resulting acyl-BlaR1 species is stable, fixing the receptor in the “on” state (1).
Because these events involve three distinct entities with unique IR signatures, the β-lactam, the CO2-derived Nε-carboxylysine, and the BlaR1 protein itself, IR spectroscopy has unique value to the temporal and structural elucidation of these events. Moreover, the change in the frequency of the IR absorbance often allows deduction of a change in bond strength upon protein binding. We have used IR spectroscopy previously to evaluate the acylation of the Streptococcus pneumoniae PBP 2x sensitive target transpeptidase by a range of β-lactam antibiotics and to evaluate β-lactam acylation of the class C β-lactamase from Citrobacter freundii. In both examples, one (or more) acyl-protein intermediates were detected, and the conformational changes that accompanied these reactions were observed (14–17). Herein we report the application of stopped-flow Fourier-transformed IR spectroscopy (18, 19) to the simultaneous observation of the rapid reaction kinetics, the evaluation of the structure of the reaction intermediates, and the interpretation of the induced conformational changes accompanying the reaction of the BlaR1 receptor with a β-lactam.
Results and Discussion
BlaRS Acylation by Ampicillin at 22°C.
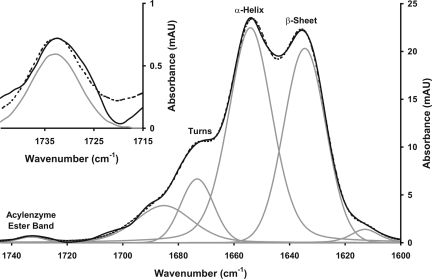
All experiments used the soluble recombinant C terminus sensor domain protein, termed BlaRS, of BlaR1. The functional competence of this domain for CO2-dependent lysine N-carboxylation and β-lactam acylation is proven (1, 2). The first experiment was acquisition of the IR spectrum after stopped-flow mixing and reaction of 0.3 mM BlaRS and 0.3 mM ampicillin in the reaction buffer (see Methods) at 22°C (Fig. 2). The difference spectrum obtained after reaction completion (see Methods) referenced against an equivalent spectrum of free protein shows inter alia the appearance of a new band (enlarged in Fig. 2 Inset) at 1,732 cm−1. This band is assigned as the ester carbonyl absorption of serine-acylated BlaRS. We assign this band with confidence, because its integrated absorption intensity (Table 2, which is published as supporting information on the PNAS web site) is close to that for a single ester carbonyl absorption at 0.3 mM (16). It is also apparent from Fig. 2 that significant change, involving numerous protein amide carbonyls, occurs concomitantly in the protein region of the spectrum. An approximate estimate of the magnitude of the conformational change may be made from the intensity of the difference spectrum (≈0.02 absorbance units). This value implies a conformational change involving ≈7% of the total amide 1 absorbance of the BlaRS protein (at this concentration, the absorbance of free BlaRS is 0.3). Notably, all of the change is positive in absorbance, resulting from increased transition dipole coupling (20) between the amide carbonyl groups resulting from reorientation into a more ordered, and likely less dynamic, state. An increase in hydrogen-bonding strength may also contribute. The conformational change is approximately equally expressed, in fractional terms, in all forms of the secondary structure. A similar effect has been observed by IR spectroscopy of the interaction of glutamate with its neuroreceptor (21) and interpreted in the same way.
Fig. 2.
The product (end of reaction) IR difference spectrum vs. free protein of the acylprotein formed at 22°C between ampicillin and BlaR. BlaRS (0.6 mM) was mixed with 0.6 mM ampicillin, both dissolved in reaction buffer, pH meter reading 7.5, in the stopped-flow apparatus. Sample spectra (8,320), four replicates, and 2,080 reference (one replicate) spectra were used to construct the difference spectrum. Solid line, experimental data; dashed line, overall fitted line; gray lines, individual fitted bands. (Inset) A magnified version of the acyl-protein ester band at 1,732 cm−1. Both sample and reference spectra were constructed from three replicate experiments by averaging 6,240 spectra at 4-cm−1 resolution. The band-fitting parameters are given in Table 2, together with values obtained from an equivalent stopped-flow experiment.
The kinetics at 22°C for the appearance of the 1,732 cm−1 acyl-serine ester were too rapid to follow experimentally by stopped-flow IR spectroscopy (>10 s−1). The kinetic change in protein secondary structure measured at 1,640 or 1,653 cm−1 was biphasic. The first phase showed a rapid increase in absorbance (>10 s−1, ΔA = +0.04). This rapid increase is followed by a decrease (relaxation) in absorption with ΔA = −0.02 to the final state shown in Fig. 2. The kinetics of this second phase were first-order with k = 3.1 ± 0.3 s−1. The assumption that the first kinetic phase of conformational change coincides with, or is slightly slower than, acylation by ampicillin, and that the second kinetic phase coincides with a protein relaxation that may be involved in the signaling process, is supported by experiments at lower temperatures (see below).
Properties of the Acyl-Serine Ester.
The presence of carbonyl-stabilizing hydrogen bonds, provided by two amides of the protein backbone (the so-called oxyanion hole), is seen in the crystal structures of both β-lactamase and PBP acyl-proteins. A representative value for the carbonyl frequency of these acyl-proteins is the 1,712-cm−1 absorption seen for the acyl-protein of S. pneumoniae PBP2x, a β-lactam-sensitive transpeptidase that is rapidly acylated by β-lactam antibiotics (14). The 1,732-cm−1 absorbance seen here indicates that the BlaRS acyl-protein is less strongly hydrogen bonded or is in a less polar environment (having a dielectric constant of ≈55, as compared with the value of 80 for water). All three crystal structures of BlaRS acyl-protein species (1, 11, 22) show two reasonably symmetric hydrogen bonds from the oxyanion hole to the carbonyl. Consistent with these structures, the IR spectrum of the BlaRS acyl-protein indicates a single and well ordered conformation, as indicated by its narrow bandwidth (13 cm−1). A representative value for the esters resulting from β-lactam solvolysis is an absorption frequency of 1,727 cm−1 and a bandwidth of 35 cm−1 in D2O solution (14).
The outcome of the identical experiment using a 5-fold excess, rather than a stoichiometric quantity, of ampicillin is shown as Fig. 6, which is published as supporting information on the PNAS web site. This experiment shows consumption of 20–25% of the initial ampicillin. Hence, within experimental error, in both experiments BlaRS acylation is stoichiometric.
BlaRS Acylation by Ampicillin at 10°C.
The BlaRS acylation reaction was examined at lower temperature to allow a more comprehensive kinetic analysis. The difference spectrum obtained upon β-lactam acylation at 10°C is very similar to that observed at 22°C (Fig. 7 and Table 3, which are published as supporting information on the PNAS web site). The notable difference is the presence of a major acyl-protein species at 1,732 cm−1 and a minor acyl-protein at 1,717 cm−1. Integration of these two peaks gives within error the correct absorbance for a single ester group (16). Both acyl-protein species also are seen in the experiment by using a 5-fold excess of ampicillin (Fig. 8, which is published as supporting information on the PNAS web site).
The kinetics for the appearance of the ester band at 1,732 cm−1 at 10°C are close to the experimental limit for stopped-flow Fourier-transformed IR. Nonetheless, the kinetic curve fits analysis for a first-order transformation having a rate constant of 3.8 ± 0.8 s−1 (Fig. 3A). The kinetics of this reaction previously were evaluated as having a rate constant of 1 s−1 (by a competition method with a reporter substrate) and a Ks = 23 μM (2) at ambient temperature.
Fig. 3.
The kinetic traces of the formation at 10°C (A) and 5°C (B) of the acyl-protein ester species at 1,732 cm−1. Four replicate experiments were averaged. The data were fitted to a first-order equation with k = 3.8 ± 0.8 s−1 at 10°C and 0.6 ± 0.1 s−1 at 5°C.
The conformational change of the protein at 10°C, following the first conformational change, is similar to that at 22°C in both magnitude (ΔA = 0.035) and the relative fractional changes in secondary structure (see Fig. 8 and Table 3). The amplitude change in the α-helix band seen at 1,653 cm−1 (Fig. 4) fits a biphasic curve with successive rate constants of 1.00 ± 0.05 s−1 and 0.030 ± 0.004 s−1. Because the value of the faster rate constant is determined from the relatively small absorbance change that is kinetically observable (that is, the final 15% rise in absorbance), the value of 1.00 ± 0.05 s−1 for the first phase may not be accurate (notwithstanding the small standard error). Thus the possibility that this transient (1.00 ± 0.05 s−1) and the acyl-protein transient (3.8 ± 0.8 s−1) are identical events cannot be excluded.
Fig. 4.
The kinetic trace of the change in absorbance at 1,653 cm−1 ascribed to α-helix at 10°C. Four replicate experiments were averaged. The data were fitted to a biexponential function to give first-order rate constants of 1.00 ± 0.05 s−1 and 0.030 ± 0.004 s−1. The kinetic trace for β-sheet at 1,632 cm−1 was very similar.
BlaRS Acylation by Ampicillin at 5°C.
The kinetic trace for acyl-protein formation at 5°C, measured by the appearance of the 1,732-cm−1 band, is shown in Fig. 3B. The reaction velocity is ≈6-fold slower than at 10°C (rate constant of 0.60 ± 0.05 s−1). We also followed the first-order loss of the β-lactam carbonyl absorbance of the ampicillin. The rate constant for this transformation is 0.78 ± 0.08 s−1 (not shown), similar to the rate constant for the acyl-protein ester formation. The changes in both the α-helix and β-sheet are monophasic and positive, and both changes occur with a rate constant (0.80 ± 0.07 s−1) that is essentially identical to the acylation rate constant. The second relaxation event is absent or is too slow to measure. These results show that, although acylation continues to occur at these lower temperatures, the fully manifested signaling process occurs at higher temperature, where the organism is more active.
Summary of the Kinetic Mechanism.
At each temperature, clearly identifiable protein conformational changes are observed. At the lower temperatures, the kinetics of the protein conformational change are very similar to the kinetics of acylation, as is reasonable given the sensor/transducer function of BlaR1. The kinetic mechanism and the kinetic constants are given in Table 1. Our interpretation of the relationship between these conformational changes and signaling will focus upon the events observed at 22°C, because this is the temperature most relevant to previous mechanistic study. The very sharp temperature dependence of the second conformational change implies a large enthalpy of activation, where relaxation requires the simultaneous weakening of many noncovalent interactions.
Table 1.
The kinetic model of BlaRs acylation and the ensuing protein conformational changes at 22°C, 10°C, and 5°C
| ||||||
|---|---|---|---|---|---|---|
| t, °C | kacyl, s−1 | First conformational change |
Second conformational change |
|||
| kc1, s−1 | ΔA‡ | kc2, s−1 | ΔA‡ | |||
| 22 | >10 | >10 | +0.04 | 3.1 ± 0.3 | −0.02 | |
| 10 | 3.8 ± 0.8 | 1.0 ± 0.05 | +0.005, +0.035§ | 0.030 ± 0.004 | −0.005 | |
| 5 | 0.6 ± 0.1 | 0.80 ± 0.07 | +0.05 | 0¶ | 0¶ | |
*The value for Ks at room temperature is 23 μM (2).
†The rate constants measured for changes in the α-helix and β-sheet content are identical.
‡The magnitude of the IR absorbance change under the experimental conditions.
§The first value is the kinetically observed part of the change. The second value is the total absorbance change.
¶Under the conditions of the experiment (length of observation at 5°C), this conformational change does not occur.
The Nature of the Kinetic Intermediates.
The likelihood that IR could be used to directly evaluate the proposal that Lys-392 is N-carboxylated in the native protein and experiences decarboxylation subsequent to acylation of BlaRS by β-lactam antibiotics is evident from the literature values for the IR absorbance frequencies of the carbamate group (23). Although the positions in the IR spectrum for both the bicarbonate anion and the carbamate anion are partly beneath the signal for the much more abundant protein amides, the use of 12C/13C isotope editing untangles this overlap. Bicarbonate anion in aqueous solution absorbs at 1,625 cm−1 in 12C form and at 1,575 cm−1 in 13C form. The carbamate anion absorbs at 1,565–1,575 cm−1 in 12C form (23) and at 1,525–1,535 cm−1 in 13C form, as estimated from an expected isotopic shift of 40 cm−1.
In previous mechanistic studies, 50 mM bicarbonate was used for complete activation of Lys-392 of BlaRS as the Nε-carboxylysine (1, 2). This bicarbonate concentration is too high for IR analysis. However, the use of 2.8 mM bicarbonate still gives full protein activation and enables identification of the carbamate absorption in 12C−13C difference spectra of BlaRS. The key IR experiment was performed by using a single sample of ε-amino Lys-392 of BlaRS (that is, purposefully N-decarboxylated) divided into three portions. One portion was kept N-decarboxylated (that is, was not activated with bicarbonate). The second was activated with 2.8 mM 12C-bicarbonate, and the third was activated with 2.8 mM 13C-bicarbonate, all at 10°C. IR spectra of the two protein samples, having, respectively, 12C- and 13C-labeled Nε-carboxylysine carbamate, were acquired. The two activated samples were then allowed to react with ampicillin (in 5-fold excess, 2.5-fold excess, or stoichiometric quantities), and the IR spectra were reacquired. The 12C minus 13C difference spectra before and after the reaction with ampicillin are shown in Fig. 5. The observations from these spectra are summarized. The only difference between the spectra is the carbon isotope of the carbamate functional group of the catalytic Nε-carboxylysine. The 12C-labeled protein will possess an IR signal for this carbamate centered approximately at 1,570 cm−1, whereas the 13C-labeled protein will possess an IR signal for this same functional group centered at 1,530 cm−1. Subtraction of these spectra before reaction with ampicillin should show a positive band at 1,570 cm−1 that falls to a downward-pointing 13C peak at 1,530 cm−1. This is seen in Fig. 5A, which shows the unadjusted difference spectrum. The lack of perfect balance between the spectra accounts for the absence of symmetry in the difference spectrum (the 12C peak is not mirrored below the wavenumber axis by the 13C peak, which remains positive, yet downward-going). Normalization of the spectra before subtraction was done by correction of the baseline contribution for the small quantity of precipitate that occurs during hydrogen exchange and by nulling the intensity of a narrow tyrosine absorption at 1,517 cm−1 (shown in Fig. 9, which is published as supporting information on the PNAS web site). This normalization has the effect of slightly attenuating the downward-bound 13C component of the spectra because of the proximity of the tyrosine band with the 13C lysine N-carboxylate component of the difference spectrum. However, this procedure allows a more accurate comparison with the spectra of the acylated BlaRS, where all spectra have been processed in exactly the same way. The 12C component is also slightly downshifted in frequency (1,560–1,565 cm−1) as a result of the normalization. The assignment of the positive band in the difference spectrum to the 12C-labeled Nε-carboxylysine is supported by its frequency (peak maximum near 1,570 cm−1), its intensity (3 milliabsorbence units), and its bandwidth of 40 cm−1, similar to carbamate in deuterium oxide. Should the Nε-carboxylysine undergo decarboxylation as a consequence of serine acylation by ampicillin, both carbamate peaks will disappear, and the resulting difference spectrum will be baseline. This is indeed what is observed. It is quite clear that lysine N-carboxylate absorption intensity has been lost between the acylated protein and the N-carboxylated BlaRS in the samples where 5- and 2.5-fold excesses of ampicillin have been used. The difference where only a single ampicillin equivalent is used is smaller, most likely because of incomplete acylation (1).
Fig. 5.
Isotope-edited spectra that show the loss of the Lys-392 N-carboxylate consequent upon acylation of BlaRS by ampicillin. Measurements shown were made at 10°C in 10 mM sodium phosphate (pH 7.5) containing 2.8 mM NaH12CO3 (sample) and the same buffer containing 2.8 mM NaH13CO3 (reference). (A) The uncorrected difference spectrum in which the 13C labeled sample was subtracted from the 12C-labeled sample. (B) The corrected difference spectra (see text) in the absence (black) and presence of a 5-fold excess of ampicillin over the 0.28 mM BlaRS (blue), a 2.5-fold excess (green), and at an equimolar concentration (red).
It is important to note that we detected a slow loss of the ampicillin β-lactam carbonyl absorbance as an indication that the protein was being acylated, despite the apparent absence of N-carboxylation of Lys-392. It is very difficult to exclude carbon dioxide from solutions. It is expected that traces of carbon dioxide in solutions of N-decarboxylated BlaRS will slowly proceed to carboxylate Lys-392. This now active BlaRS will undergo acylation and the requisite N-decarboxylation, whereby the process becomes catalytic with respect to the trace of carbon dioxide. These observations are consistent with the nanomolar Kd for carbon dioxide and with the earlier documentation of the absolute necessity of N-carboxylation at Lys-392 for acylation of BlaRS (2).
The amide 2 (primarily N-H bending) absorption occurs at 1,550 cm−1, in the middle of the IR region of interest. Although most of this absorption is removed by the prior hydrogen–deuterium exchange, some buried groups do not exchange. Because acylation causes a conformational change, this could expose buried amides, which could then exchange and cause a loss of absorbance at 1,550 cm−1. Such an effect could compromise experiments of this type, if the isotope-edited difference method is not used. Isotope editing specifically removes any effect on the protein that does not directly result from the N-carboxylation status, because the two “halves” of the experiment exactly compensate (19).
The structural interpretation of the kinetics enumerated in Table 1 is now evident. The initiating chemical event that follows β-lactam occupancy of the BlaRS active site is serine acylation promoted by the Nε-carboxylysine. The BlaRS protein responds to this acylation by a protein conformational change, which occurs at a rate equal to (or only very slightly slower than) serine acylation. The consequence of these events is destabilization of the Nε-carboxylysine functional group, resulting in N-decarboxylation. Thus serine acylation is made irreversible, and an altered sensor domain conformation signifying the presence of β-lactam antibiotics is attained.
Conclusion
Notwithstanding the challenge of the experimental design, we have demonstrated unequivocally the loss of CO2 from the Nε-carbamate of Lys-392 upon acylation of BlaRS. This observation provides a firm experimental basis for the proposal that N-decarboxylation of Lys-392 traps the acyl-protein species. Direct evidence for altered protein conformation, consistent with what is expected for the process of signal transduction, is provided by the IR spectrum of the acylated protein.
A major challenge in the investigation of multidomain proteins, and especially those that are membrane-bound, is to elucidate how in concert the entire protein functions. The investigations of function and of structure become more tractable when individual domains are studied. Our present investigations of BlaR1 focus on the surface sensor domain (BlaRS) of this protein. We have documented that BlaRS is a stable functional domain amenable to study, including crystallographic investigations. This reductionist approach to the study of the surface domain has revealed the details of how the protein experiences acylation by the antibiotic, activation by N-decarboxylation of Lys-392 to the “on” state, and attendant conformational changes that are intimately involved in transduction of the chemical message from the membrane surface to the cytoplasm. The challenge for the future is to make IR measurements on the entire receptor complex, in the membrane, to look for acylation-induced conformational change in the proposed α-helical transmembrane segments. Such experiments are feasible because IR radiation is remarkably immune to the scattering effects of inhomogeneous media. Finally, it might be possible to correlate these changes with proteolytic activation of the intracellular proteinase. In this way, the sequence of events in receptor activation might be charted in mechanistic and structural terms.
We add that BlaR1 and the aforementioned MecR1 are believed to be evolutionarily, structurally, and functionally related to each other. Therefore, knowledge of the processes of BlaR1 will shed light on MecR1 as well. It is important to note that sequence analyses of the two proteins reveal the conservation of the active site serine and the lysine, as well as the ensemble of the seven hydrophobic residues necessary for attenuation of the pKa of the lysine amine for the N-carboxylation reaction, a trait shared with the related class D β-lactamases (13). Whereas documentation of the biochemical properties of MecR1 awaits future investigations, this study implies that MecR1 should be a second protein that uses this lysine N-carboxylation, serine acylation, and N-decarboxylation to effect the receptor conformational change necessary to propagate signal transduction.
Methods
Ampicillin (Na+ salt), buffer materials, and deuterium oxide (99.9% enriched) were supplied by Sigma-Aldrich. The BlaRS C-terminal domain was expressed in Escherichia coli and purified as described (2). The lyophilized protein was dissolved to a concentration of 0.6 mM in 0.5 ml of 0.1 M phosphate buffer adjusted to a pH meter reading (pM) of 7.5, made up in D2O containing 0.3 M Na2SO4 and 2 mM sodium bicarbonate. This solution is sufficient for 10 replicate stopped-flow shots, three of which are used to fully wash the system before data are collected. A total of 0.1 ml of protein solution is required for a static spectrum. The protein solution was incubated at 4°C for 72 h in the D2O buffer to ensure a hydrogen–deuterium exchange as complete as possible. The buffer was adjusted to pM 7.5 at 22°C and used at 22°C, 10°C, and 5°C.
Ampicillin solutions, either 0.6 or 3 mM, were freshly prepared in the same buffer. Spectra of static (nonreacting) mixtures were acquired by using an in situ cuvette with a 100-μm pathlength (14). Stopped-flow time-resolved IR data were acquired by using a system derived, with the aim of reducing the dead volume and mixing dead time, from that described previously (18). Additional description is given in the Supporting Text, which is published as supporting information on the PNAS web site.
Spectra were collected by using a Bruker (Billerica, MS) IFS 66 spectrometer. For equilibrium spectra, the instrument was operated at 30 kHz (nine scans s−1) with a resolution of 4 cm−1. For stopped-flow measurements, the instrument was operated at 200 kHz (60 scans s−1) with a resolution of 4 cm−1. Spectra were accumulated as a geometric series of 10 × 1 scan, 10 × 2 scans, and 10 × 4 scans, up to a maximum of 10 × 512 spectra. This scheme was implemented not only to ensure that the complete reaction progress was captured but also to permit the averaging of many spectra at the end of the reaction so that a very low noise spectrum of the final products could be obtained.
Spectra of static (nonreacting or already-reacted) mixtures were acquired by using the 100-μm pathlength in situ cuvette. To make a detailed comparison of the 12C and 13C-carboxylated protein, it was necessary to ensure that all samples had an identical history before the IR experiment itself. Thus, 18 mg of purified BlaRS was N-decarboxylated by incubation at pH 4.5. The sample was divided into three equal portions. To the first portion was added 2.14 ml of 10 mM sodium phosphate (pH 7.5), containing 1 mM NaH12CO3. To the second portion was added 2.14 ml of 10 mM sodium phosphate (pH 7.5) containing 1 mM NaH13CO3. To the third portion was added 2.14 ml of 10 mM sodium phosphate (pH 7.5, without added bicarbonate). Each was freeze-dried, reconstituted with 0.76 ml of degassed D2O, and kept at 4°C for 72 h to allow hydrogen–deuterium exchange. The final BlaRS concentration of 0.25 mM is the minimum necessary for IR difference spectroscopy of a single functional group. The final concentration of bicarbonate in the first and second portions was 2.8 mM. Before loading into the in situ cuvette, 90 μl of the protein solution was mixed with 10 μl of 10 mM phosphate buffer (pH meter reading 7.5) made up in D2O containing 12.5 mM, 6.25 mM, or 2.5 mM ampicillin. Because the BlaRS concentration in the cuvette is 0.25 mM, the added ampicillin represents a 5-fold excess, a 2.5-fold excess, and an equal concentration to protein. Scheme 1, which is published as supporting information on the PNAS web site, shows the manipulations of the samples.
Spectra were collected at 10°C by collecting 256 scans every 30 s over 45 min, to give 90 sets of 256 scans. For most analyses, these 23,040 spectra were added together to give a low noise level.
Supplementary Material
Acknowledgments
We thank Tony Rothin (School of Biosciences mechanical workshop, University of Birmingham) for his engineering expertise in the design and construction of the stopped-flow apparatus and Baz Jackson for helpful advice. This work was supported by the Royal Thai Government (K.T.); the Biotechnology and Biological Sciences Research Council, United Kingdom (C.W.); and the National Institutes of Health (S.M.).
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Birck C. J., Cross J., Schulze-Briese C., Meroueh S. O., Schlegel H. B., Mobashery S., Samama J. P. J. Am. Chem. Soc. 2004;126:13945–13947. doi: 10.1021/ja044742u. [DOI] [PubMed] [Google Scholar]
- 2.Golemi-Kotra D., Cha J. Y., Meroueh S. O., Vakulenko S. B., Mobashery S. J. Biol. Chem. 2003;278:18419–18425. doi: 10.1074/jbc.M300611200. [DOI] [PubMed] [Google Scholar]
- 3.Fuda C. C. S., Fisher J. F., Mobashery S. Cell. Mol. Life Sci. 2005;62:2617–2633. doi: 10.1007/s00018-005-5148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevons M. P., Rolinson G. N., Know R. Br. Med. J. 1961;1:124–128. [Google Scholar]
- 5.Bartley J. Infect. Control. Hosp. Epidemiol. 2002;23:480. doi: 10.1017/s0195941700082333. [DOI] [PubMed] [Google Scholar]
- 6.Fuda C., Hesek D., Lee M., Morio K., Nowak T., Mobashery S. J. Am. Chem. Soc. 2005;127:2056–2057. doi: 10.1021/ja0434376. [DOI] [PubMed] [Google Scholar]
- 7.Chambers H. F. Clin. Microbiol. Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers H. F. Trends Microbiol. 2003;11:145–148. doi: 10.1016/s0966-842x(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H. Z., Hackbarth C. J., Chansky K. M., Chambers H. F. Science. 2001;291:1962–1965. doi: 10.1126/science.1055144. [DOI] [PubMed] [Google Scholar]
- 10.Archer G. L., Niemeyer D. M. Trends Microbiol. 1994;2:343–347. doi: 10.1016/0966-842x(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 11.Kerff F., Charlier P., Colombo M.-L., Sauvage E., Brans A., Frère J.-M., Joris B., Fonze E. Biochemistry. 2003;42:12835–12843. doi: 10.1021/bi034976a. [DOI] [PubMed] [Google Scholar]
- 12.Haniquet S., Colombo M.-L., Goormahtigh E., Soumillon, Frère J.-M., Joris B. J. Mol. Biol. 2004;279:14264–14272. doi: 10.1074/jbc.M313488200. [DOI] [PubMed] [Google Scholar]
- 13.Golemi D., Maveyraud L., Vakulenko S., Samama J.-P., Mobashery S. Proc. Natl. Acad. Sci. USA. 2001;98:14280–14285. doi: 10.1073/pnas.241442898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittock R. S., Ward S., Wilkinson A. S., Caspers P., Mensch B., Page M. G. P., Wharton C. W. Biochem. J. 1999;338:153–159. [PMC free article] [PubMed] [Google Scholar]
- 15.Wharton C. W., Page M. G. P., Chittock R. S., Regan T. E., Ward S. Nat. Prod. Rep. 2000;1:447–453. doi: 10.1039/b002066o. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson A. S., Bryant P., Meroueh S. O., Page M. G. P., Mobashery S., Wharton C. W. Biochemistry. 2003;42:1950–1957. doi: 10.1021/bi0266941. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson A. S., Ward S., Kania M., Page M. G. P., Wharton C. W. Biochemistry. 1999;38:3851–3856. doi: 10.1021/bi990030i. [DOI] [PubMed] [Google Scholar]
- 18.White A. J., Drabble K., Wharton C. W. Biochem. J. 1995;306:843–849. doi: 10.1042/bj3060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johal S. S., White A. J., Wharton C. W. Biochem. J. 1994;297:281–287. doi: 10.1042/bj2970281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang R., Kubelka J., Barber-Armstrong W., Silva R. A. G. D., Decatur S., Keiderling T. A. J. Am. Chem. Soc. 2004;126:2346–2354. doi: 10.1021/ja037998t. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Q., Jayaraman V. J. Biol. Chem. 2004;279:26346–26350. doi: 10.1074/jbc.M403111200. [DOI] [PubMed] [Google Scholar]
- 22.Wilke M. S., Hills T. Y., Zhang H. Z., Chambers H. F., Strynadka N. C. J. J. Biol. Chem. 2004;279:47278–47287. doi: 10.1074/jbc.M407054200. [DOI] [PubMed] [Google Scholar]
- 23.Masuda K., Ito Y., Horiguchi M., Fujita H. Tetrahedron. 2005;61:213–229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.