Abstract
All delivery forms of testosterone should be equally efficacious in treating the androgen-deficient aging male if adequate serum testosterone levels are obtained. The testosterone preparations available in North America include the oral undecanoate, injectable testosterone esters, the scrotal patch, the nonscrotal transdermal patch, and the transdermal gels. Selection of a specific testosterone preparation for replacement therapy depends on many factors, including the magnitude and pattern of serum testosterone levels produced, side effects of the particular formulation, reversibility if an adverse event should occur, convenience of use, cosmetic issues related to the preparation, and cost. In addition, potential adverse effects of testosterone therapy applicable to all forms of testosterone delivery, such as fluid retention, gynecomastia, polycythemia, worsening of sleep apnea, change in cardiovascular-disease risk, or alterations in prostate health, need to be considered both prior to therapy and during treatment monitoring.
Key words: Testosterone preparations, Dosing, Adverse effects
A number of factors should be considered when selecting a specific androgen modality for replacement therapy in the aging male. These factors include:
efficacy of the treatment, which relates to the sex-steroid levels obtained;
adverse effects of the therapy in general and of a particular preparation;
acceptability of the therapy to the individual patient.
Androgens used for therapy in the aging male encompass both those that can be aromatized to estrogens (such as testosterone) and those that cannot be aromatized (such as dihydrotestosterone [DHT]). This article will only discuss testosterone preparations, as they are the only preparations currently commercially available in North America. However, using an androgen such as testosterone, which can be metabolized to estrogens, may prove to be important in the treatment of some older men, because it appears that estradiol (or bioavailable estradiol) may be the major sex steroid affecting male bone.1 Table 1 lists those testosterone preparations currently commercially available in North America.
Table 1.
Testosterone Preparations Currently Commercially Available in North America
Efficacy of Testosterone Treatment
The efficacy of testosterone replacement depends on the goals of treatment, which should be defined on an individual basis by the desired androgen target organ effect(s). The goals of testosterone therapy might be improvement in symptoms involving mood, psychosexual function, physical stamina and performance, or general quality of life. Alternatively, they may involve improvement in, restoration of, or prevention of decline in areas that may have less readily recognized symptoms, such as bone density (eg, prevention of osteoporosis), body composition and strength, or cognition.
All forms of testosterone replacement are probably efficacious for any given androgen target response if adequate sex-steroid levels are obtained. Replacement of endogenous testosterone with equivalent levels of exogenous testosterone has no measurable effect; testosterone levels, whether total testosterone, free testosterone, or bioavailable testosterone, need to be increased over those levels found prior to diagnosis of androgen deficiency. Although theory in this area is easily understood, it is not easily put into practice. The magnitude of increase in testosterone level needed for an efficacious response has not been delineated for the older man; the androgen dose response for the various target organs, and interindividual variability in that response, are not known. In fact, based on the experience with testosterone replacement in hypogonadal, young adult men, a range of dose-response correlations between testosterone levels and target organs will likely be found. Improvement in mood and psychosexual functioning may require less testosterone than does improvement in bone density, which, in turn, may require less testosterone than that needed to significantly affect muscle. Data suggest that the dose effect of testosterone on cognition more closely approximates a bell-shaped curve.
In general, the effectiveness of testosterone delivery is assessed by measurement of serum sex-steroid levels. While serum levels of testosterone and the other sex steroids may not directly reflect levels obtained within a given target tissue, their measurement is the only practical method available for monitoring delivery and comparing preparations. Specific issues with regard to testosterone preparations and the serum levels obtained include (a) adequacy of testosterone levels obtained, (b) levels of estradiol and DHT obtained, (c) reliability of the preparation in obtaining adequate testosterone levels, and (d) flexibility of the preparation in allowing for dose adjustment. A discussion of these issues follows for each of the testosterone preparations listed in Table 1 that are recommended for therapy.
Testosterone undecanoate (Andriol® [NV Organon, Oss, The Netherlands]), which is not commercially available in the United States, provides serum levels of testosterone that are in the low end of the normal range for adult males and levels of DHT that tend to be mildly elevated.4 Delivery reliability is relatively poor; food consumption can have a significant effect on absorption and there can be large intraindividual and interindividual variability in obtainable testosterone levels. Testosterone undecanoate does offer some dosing flexibility because the preparation can be taken from two to four times a day.
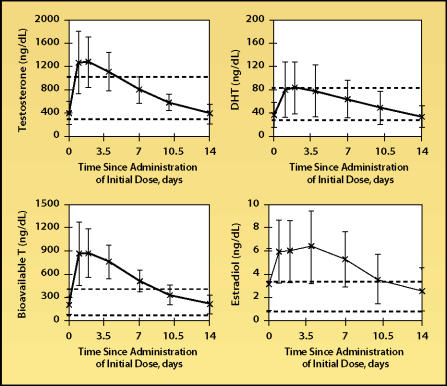
Of the injectable esters, which are usually given as intramuscular injections in the buttocks or thigh, the propionate ester generally is not used for replacement in men because of the need for frequent dosing (3 times/wk). The cypionate and enanthate esters have essentially equivalent pharmacokinetics5 and the usual dosing interval in an older man is every 1 to 2 weeks. High, often supraphysiologic, serum testosterone levels can be obtained with the injectable esters, and levels can fluctuate widely over the dosing period (Figure 1).6,7 At high testosterone levels, serum estradiol levels may be increased to above the normal range. The reliability of the injectable esters in delivery of testosterone is excellent and dosage adjustment is the most flexible of all the testosterone preparations. High serum testosterone levels also are achievable with the testosterone implants and delivery reliability is good.8 Some flexibility with dosage adjustment also exists with the implant.
Figure 1.
Steady-state mean serum levels of testosterone, bioavailable testosterone (T), dihydrotestosterone (DHT), and estradiol in thirty-three hypogonadal 22- to 65-year-old men given 200 mg of intramuscular testosterone enanthate every 2 weeks. Error bars indicate standard deviations. Dashed lines denote upper and lower limits of normal range. Adapted, with permission, from Dobs et al.7
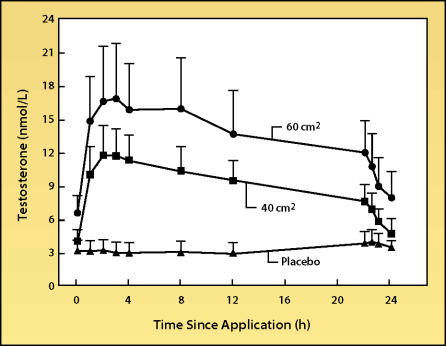
The scrotal testosterone patch (Testoderm® [ALZA Corp., Mountain View, CA]) generally produces serum testosterone levels in the low end of the normal range (Figure 2).9 Since the patch is applied daily, the testosterone levels produced can mimic the normal circadian rhythm in plasma testosterone levels10,11; however, the physiological importance of this daily variation in average plasma testosterone concentration has not yet been demonstrated. Due to the high level of the steroid 5-alpha-reductase enzyme in scrotal skin, supraphysiological plasma DHT levels are produced with the scrotal patch.10 Reliability of delivery of testosterone via the scrotal patch is fair to poor. Patch adherence, particularly in hot and humid climates, may be problematic, and good preparation of the scrotum by shaving is essential. Some dosage adjustment is possible, as the patch is available in two strengths (40 cm2 and 60 cm2), but the patches should not be divided.
Figure 2.
Steady-state mean serum levels of testosterone in hypogonadal men treated with either placebo patch or 2 different doses of the Testoderm scrotal patch system. Error bars indicate standard deviation. Adapted, with permission, from Place et al.9
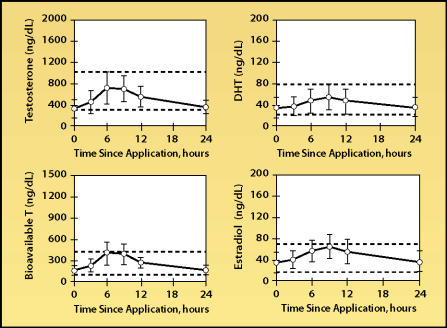
Currently, one nonscrotal testosterone patch is commercially available (Androderm®, Watson Pharma Inc., Corona, CA). Production of the Testoderm patch, which was marketed up until 2002, has been discontinued. Like the scrotal patch, the transdermal nonscrotal patch is applied daily, and therefore produces serum testosterone levels that can mimic normal circadian changes.7 The Androderm patch can deliver serum testosterone levels that are in the midrange of normal for young adult men (Figure 3), with fair to good delivery reliability. Transdermal absorption profiles can vary greatly among individuals, even those who apply the patch correctly. The Androderm patch affords some dosing flexibility because it is available in two sizes (2.5 mg and 5 mg), but the patches should not be divided.
Figure 3.
Steady-state mean serum levels of total and bioavailable testosterone (T), dihydrotestosterone (DHT), and estradiol after application of 5 mg/day Androderm patch. Error bars indicate standard deviations. Adapted, with permission, from Dobs et al.7
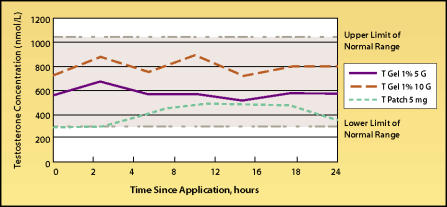
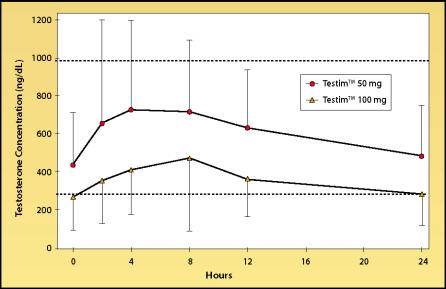
With the transdermal gel Andro-Gel, (Unimed Pharmaceuticals, Inc., Deerfield, Il), testosterone levels are usually within the midrange of normal (Figure 4), whereas plasma DHT levels may be mildly elevated.13 Delivery reliability is generally good, with the same caveat as with the transdermal patches that absorption profiles can vary greatly among individuals. Recently, another transdermal testosterone gel, Testim™ (Auxilium Pharmaceuticals, Inc., Norristown, PA), was approved for use in the United States. Serum levels of testosterone obtainable with Testim (Figure 5) appear to be similar to those obtained with AndroGel and, like AndroGel, serum DHT levels may become slightly above the normal range with the higher Testim dose of 100 mg/day.
Figure 4.
24-Hour serum testosterone (T) levels in hypogonadal men on day 90 of therapy with 5 grams or 10 grams of 1% AndroGel with the Androderm 5-mg patch. Adapted from: Proceedings of the Endocrine Society Andropause Consensus Conferences 2000–2001, The Endocrine Society Continuing Medical Education Services, January 2002.12
Figure 5.
24-Hour serum testosterone levels in older hypogonadal men on day 30 of therapy with 50 mg/day or 100 mg/day of the transdermal gel Testim. Data from personal communicaton.
Adverse Effects
The decision to use testosterone therapy and the selection of the specific method of testosterone delivery should involve consideration of the potential adverse effects that could occur. Another consideration in selecting the method of testosterone therapy may be the ease and speed of reversibility of the therapy should an adverse effect occur. Adverse effects of testosterone replacement in the older male can be divided into those effects that are related to testosterone itself (or to its metabolites, eg, estradiol or DHT) and those that are, instead, related to the method of testosterone delivery. Many of these latter effects relate to problems that occur at the site of application. Table 2 lists possible hormone-related and non-hormone-related adverse effects of testosterone therapy. The incidence of these effects is highly variable. The issue of testosterone treatment and prostate health is discussed in another article and will not be addressed here.
Table 2.
Potential Hormone-Related and Non-Hormone-Related Adverse Effects of Testosterone Therapy
| Hormone Related | |
| Promotion of fluid retention | |
| Increase in cardiovascular-disease risk | |
| Precipitation or worsening of sleep apnea | |
| Gynecomastia | |
| Polycythemia | |
| Fluctuations in mood | |
| Increased rate of development of benign or malignant prostate disease | |
| Not Hormone Related | |
| Preparation | Effect |
| T undecanoate | Gastrointestinal bloating and irritation |
| Injectable esters | Pain at sight of injection |
| Implantable pellets | Pain or infection at site; extravasation of pellet |
| Scrotal patch | Local site irritation |
| Nonscrotal patch | Local site skin irritation, sometimes significant |
| Transdermal Gels | Occasional mild skin irritation |
With testosterone therapy, a transient increase in body weight can occur, which is assumed to be due to fluid retention. To date, however, blood pressure has not been reported to increase in middle-aged or older men on testosterone therapy. Effects of testosterone therapy on other aspects of overall cardiovascular health are not clear. A higher incidence of cardiovascular disease in men than in age-matched women, the decrease in high-density lipoprotein (HDL) cholesterol levels at puberty in boys, and the increase in HDL cholesterol when young men are made hypogonadal have led to the speculation that testosterone therapy might have a negative impact on the cardiovascular system in older men. On the other hand, epidemiological studies support a positive correlation between testosterone levels and factors associated with improved cardiovascular-disease risk,14 and short-term testosterone-infusion studies have shown a direct arterial vasodilatory effect and prolongation of time until ischemia during exercise in men with known cardiovascular disease.15,16 In addition, studies in which middle-aged or older men were given testosterone have shown that cholesterol profiles were not adversely affected. In fact, testosterone therapy tends to promote a decrease in plasma total cholesterol, low-density lipoprotein (LDL) cholesterol, and lipoprotein (a) levels with little to no effect on HDL cholesterol.17 However, until specific cardiovascular endpoints such as myocardial infarction or stroke are evaluated in a large, multicenter trial of testosterone therapy in older men, the relative risks and benefits of testosterone therapy with regard to cardiovascular disease will not be known. At this time, known cardiovascular disease or elevated cholesterol levels are not contraindications to testosterone therapy.
Some reports have shown that testosterone therapy may worsen pre-existing obstructive sleep apnea,18 whereas other reports have shown that some men with sleep apnea have low testosterone levels that normalize when their sleep apnea is treated.19 The relationship between testosterone and sleep apnea is poorly understood. A recent placebo-controlled study of about 100 older men given 3 years of treatment reported no increase in apneic episodes for those men on testosterone,20 suggesting that testosterone-induced sleep apnea may be relatively uncommon. Nevertheless, pre-existing sleep apnea should be considered a relative contraindication to testosterone therapy unless the sleep apnea is adequately treated prior to initiation of testosterone.
Occasionally, men who are given testosterone develop tender or enlarged breasts, and older men may be especially prone to this occurrence. Perhaps because of their increased adipose tissue, which contains the aromatase enzyme that converts testosterone to estradiol, older men on testosterone therapy can demonstrate with testosterone therapy a larger percentage increase in serum estradiol than in serum testosterone. The large increase in estradiol occurs more frequently when high serum levels of testosterone are obtained, such as when the injectable testosterone esters or the implantable pellets are used. This adverse effect can usually be controlled by a downward adjustment in the injected or implanted testosterone dose or by a change in the method of testosterone delivery to a patch or the gel, delivery methods that give lower, but more uniform, testosterone levels.
Most studies of testosterone therapy in middle-aged and older men have reported a significant increase in hemoglobin and hematocrit levels with therapy.17 These increases are usually much larger than those seen with testosterone treatment in young adult, hypogonadal men. The increase in oxygen-carrying capacity that results from the increased hemoglobin levels may contribute to the decrease in fatigue and improved energy levels reported by some men on testosterone therapy. In other instances, however, the increases in hemoglobin and hematocrit have been great enough to necessitate termination of therapy, phlebotomy, or a significant decrease in testosterone dose. Although coexisting sleep apnea or large body mass may contribute to the development of polycythemia, they have not done so in many cases. A greater increase in hemoglobin levels seems to occur with the injectable testosterone esters and implantable pellets, and may correlate with peak serum testosterone level.17 If a man has an elevated hematocrit prior to testosterone treatment, or if he develops a large increase in hematocrit with therapy, lowering the testosterone dose or selecting a delivery form that provides a more uniform testosterone level within the physiological range throughout the dosing period may be helpful.
Mood and libido have been reported to fluctuate in parallel with the fluctuations in serum testosterone levels in young adult, hypogonadal men given the injectable forms of testosterone. Experience suggests that such mood fluctuations also can occur in older men on testosterone, although much less frequently. In the event that these fluctuations do occur and are bothersome to the patient, it may be helpful to select a delivery form that produces less testosterone-level variability, such as the patches or gels.
Should a therapy-related adverse event occur, the patch forms of therapy are the quickest and easiest to terminate. Testosterone from the transdermal gel cannot be removed once it has entered the subcutaneous tissue, but delivery lasts for only about 24 hours. The implantable pellets would need to be surgically removed in order to terminate therapy, and there is no method to reverse the injectable esters once delivered, other than to wait out the course of metabolism.
Therapeutic Acceptability
The acceptability of one form of testosterone delivery over another depends primarily on the patient. In addition to some of the issues concerning potential adverse effects of the various preparations, convenience, cosmetic issues, and cost of the medication may play a role in patient preference. Whether the preparation is a pill, a gel, a patch, or an injection may be important; likewise, the frequency of dosing (2–4 times a day, daily, weekly, quarterly) and the patient’s ability, or inability, to selfadminister the preparation are important considerations. Some cosmetic issues such as the visibility of the agent (either during use or after removal), the need for preparation of the skin or scrotum by shaving prior to use, or the need to apply the agent to a large surface area can affect a patient’s selection and acceptance of a particular delivery method. Health care providers need to educate potential patients about all these issues and should never simply assume that the patient will prefer one form over another.
Cost of the testosterone preparation can be a major factor in selection, especially if the patient is responsible for the full cost of the medication. Unlike most oral or patch forms of estrogen delivery, whose full retail price is rather modest, the cost of many of the currently available testosterone preparations is much higher.
Main Points.
Selection of a specific type of androgen replacement therapy should include consideration of treatment efficacy, adverse-event profiles, reversibility of adverse events should they occur, and patient preferences.
Efficacy of treatment is generally assessed through measurement of serum sex-steroid levels; the available testosterone preparations vary in serum levels obtained, reliability in achieving adequate serum levels, and dosing flexibility.
Potential adverse events of testosterone replacement related to the testosterone or its metabolites include increase in cardiovascular-disease risk, worsening of sleep apnea, mood fluctuations, gynecomastia, and polycythemia.
Potential adverse events not related to hormones include pain at injection site and local skin irritation.
Additional factors that may play a role in patient preferences include convenience, cosmetic issues, and cost of treatment.
References
- 1.Khosla S, Melton J, Atkinson EJ, O’Malley WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 2.Westaby D, Paradinas FJ, Ogle SJ, et al. Liver damage from long-term methyltestosterone. Lancet. 1977;2:262–263. [PubMed] [Google Scholar]
- 3.Friedl KE, Hannan CJ, Jones RE, Plymate SR. High-density lipoprotein cholesterol is not decreased if an aromatizable androgen is administered. Metabolism. 1990;39:498–503. doi: 10.1016/0026-0495(90)90150-b. [DOI] [PubMed] [Google Scholar]
- 4.Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecenoate in hypogonadal men: a double-blind controlled study. Clin Endocrinol. 1989;14:49–61. doi: 10.1111/j.1365-2265.1981.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 5.Schulte-Beerbuhl M, Nieschlag E. Comparison of testosterone, dihydrotestosterone, luteinizing hormone, and follicle-stimulating hormone in serum after injection of testosterone enanthate or testosterone cypionate. Fertil Steril. 1980;33:201–203. doi: 10.1016/s0015-0282(16)44543-7. [DOI] [PubMed] [Google Scholar]
- 6.Snyder PJ. Clinical use of androgens. Annu Rev Med. 1984;35:207–217. doi: 10.1146/annurev.me.35.020184.001231. [DOI] [PubMed] [Google Scholar]
- 7.Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 8.Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71:216–222. doi: 10.1210/jcem-71-1-216. [DOI] [PubMed] [Google Scholar]
- 9.Place VA, Atkinson L, Prather DA, et al. Transdermal testosterone replacement through genital skin. In: Nieschlag E, Behre HM, et al., editors. Testosterone: Action, Deficiency, Substitution. Heidelberg: Springer-Verlag; 1990. pp. 165–181. [Google Scholar]
- 10.Findlay JC, Place V, Snyder PJ. Treatment of primary hypogonadism in men by the transdermal administration of testosterone. J Clin Endocrinol Metab. 1989;68:369–373. doi: 10.1210/jcem-68-2-369. [DOI] [PubMed] [Google Scholar]
- 11.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 12.The Endocrine Society Continuing Medical Education Services. Proceedings of the Endocrine Society Andropause Consensus Conferences 2000–2001; January 2002. [Google Scholar]
- 13.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 14.Barrett-Connor E, Khaw RT, Yen SS. Testosterone and risk factors for cardiovascular disease in men. Diabetes Metab. 1995;21:156–161. [PubMed] [Google Scholar]
- 15.Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary artery disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 16.Webb CM, Adamson DL, deZeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999;83:437–439. doi: 10.1016/s0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- 17.Tenover JL. Effects of androgen supplementation in the aging male. In: Oddens BJ, Vermeulen A, editors. Androgens and the Aging Male. New York: Parthenon Publishing Group; 1996. pp. 191–204. [Google Scholar]
- 18.Matsumoto AM, Sandblom RE, Schoene RB, et al. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Santamaria JD, Prior JC, Fleetham JA. Reversible reproductive dysfunction in men with obstructive sleep apnea. Clin Endocrinol (Oxf) 1988;28:461–470. doi: 10.1111/j.1365-2265.1988.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 20.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]