Abstract
Survival and biochemical outcome of patients with localized, high-risk prostate cancer treated with definitive three-dimensional conformal radiation therapy (3-D CRT) with or without hormonal therapy are poor. Other therapeutic strategies are needed to improve outcome in these poor-prognostic-group patients. One such strategy involves the use of chemotherapeutic agents to radiosensitize the effects of local 3-D CRT. Very few investigators have tested this novel concept of chemotherapeutic radiosensitization. Two studies evaluated the combination of estramustine phosphate and vinblastine (EV) with radiation therapy (RT). In both studies, the combination of EV and RT resulted in moderate to severe acute and late toxicity. A recently completed, phase I trial evaluated the maximally tolerated dose (MTD) of weekly docetaxel that could be concurrently delivered with 3-D CRT (70.2 Gy) in men with high-risk prostate cancer. The MTD of concurrent weekly docetaxel with 3-D CRT was determined to be 20 mg/m2, and this combination was shown to be safe and well tolerated. This was the first trial to evaluate taxane radiosensitization in prostate cancer. Other phase I/II studies are needed to further assess chemotherapeutic radiosensitization in localized, high-risk prostate cancer.
Key words: Prostate cancer, Three-dimensional conformal radiation therapy, Radiosensitization, Docetaxel
The American Cancer Society estimates that 220,900 new cases of prostate cancer will be diagnosed and that 28,900 men will die of this disease in the United States in 2003.1 Many of these men will present with intermediate or high-risk disease (ie, T3 or T4, prostate-specific antigen [PSA] ≥10 ng/mL, or Gleason score ≥7). Historically, radiation therapy (RT) has been the standard of treatment for these men. In a recent report, Hanks and colleagues updated the results of their three-dimensional conformal radiation therapy (3-D CRT) experience in 332 patients with 8–12 years follow-up.2 Biochemical failure was defined according to the American Society for Therapeutic Radiology and Oncology Consensus definition.3 The rates of no biochemical evidence of disease (bNED) at 8 years for patients treated with up to 70 Gy, according to pretreatment PSA levels, ranged from 10% to 45%. For PSA levels between 0 and 9.9 ng/mL and prostate cancer with unfavorable features (ie, T2B/T3 and/or Gleason score ≥ 7 and/or perineural invasion), the bNED rate was 45%; for PSA levels between 10 and 19.9 ng/mL, it was only 20%; and for PSA ≥ 20 ng/mL, only 10% of patients exhibited biochemical remission. Hence, anywhere from 55% to 90% of men experienced a biochemical relapse despite definitive 3-D CRT. Even at higher doses (75 Gy) of 3-D CRT, the 8-year actuarial bNED rates were not much better according to PSA levels: for PSA levels between 0 and 9.9 ng/mL with unfavorable features, only 60%; and for PSA ≥ 20 ng/mL, only 20% of patients achieved long-term biochemical remission.
Furthermore, attempts to enhance the therapeutic ratio by adding hormonal therapy to RT have not improved survival outcome,4–6 except in a single, randomized trial.7 Several randomized trials conducted by the Radiation Therapy Oncology Group (RTOG) and by the European Organisation for Research and Treatment of Cancer (EORTC) have explored adding hormonal therapy to RT over the past decade. Multiple randomized trials conducted by RTOG evaluating the role of neoadjuvant or adjuvant hormonal therapy have not shown a survival advantage. In a prospective, randomized trial conducted by RTOG, evaluating the role of neoadjuvant hormonal therapy (protocol 86-10), the updated 8-year survival outcome was not different in patients receiving RT alone compared with neoadjuvant hormonal therapy and RT (44% vs 54%, respectively, P = .10).4 More importantly, the PSA control rate was only 3% in the RT arm, compared with 16% in the hormonal-RT arm. Similarly, in another RTOG trial (protocol 85-31), the benefit of adjuvant hormonal therapy (goserelin acetate 3.6 mg/m2 [Zoladex®, AstraZeneca Pharmaceuticals, LP, Wilmington, DE]) to be continued indefinitely or until progression of disease) compared with RT alone was assessed.5 In this very large trial of 945 men, no difference in long-term overall survival at 8 years was noted between RT alone compared with RT and adjuvant androgen suppression (47% vs 49%, respectively, P = .36). The biochemical control rates were 21% in the RT-only arm, compared with 54% in the adjuvant hormonal therapy arm. Another RTOG trial (protocol 92-02) also failed to confirm a survival benefit for adjuvant total androgen suppression in locally advanced prostate cancer.6 In this trial, patients were treated with neoadjuvant (2 months) and concurrent (2 months) hormonal therapy followed by no further therapy or 2 years of additional adjuvant goserelin. The adjuvant hormonal therapy arm compared with the arm receiving no further adjuvant therapy showed no difference in five-year overall survival (78% vs 79%, respectively, P = ns). The biochemical control rates were only 21% in the RT arm, compared with 46% in the adjuvant hormonal therapy arm.
Only the EORTC (22863) trial has shown any benefit to the use of hormonal therapy.7 In this trial, men with locally advanced prostate cancer were randomized to either RT alone or RT with 3 years of adjuvant goserelin. At a median follow-up of 66 months, the 5-year survival rate was 78% in the RT and goserelin therapy arm, compared with 62% in the RT-only arm (P = .001).
Given the intermediate to poor results achieved thus far with either RT alone or in combination with hormonal therapy, other strategies are needed to improve local control and survival outcome for patients presenting with intermediate or high-risk prostate cancer. Because long-term cure rates cannot be achieved without first controlling local disease, a strategy that can enhance the local effects of RT is very appealing. One such strategy involves the use of concurrent chemotherapeutic agent(s) to sensitize the local effects of RT. This strategy has been successfully used in other malignant diseases, such as rectal and anal cancers, to improve local control and survival outcomes.8–10
Chemotherapeutic radiosensitizers for prostate cancer have not been widely investigated. Ideal properties of radiosensitizers include direct cytotoxic effects and the potentiation of the effects of ionizing radiation. Ionizing radiation causes direct and indirect DNA structural damage, especially during the G2-M phases of the cell cycle, which disrupts viable cell division, eventually leading to tumor cell death. Certain drugs, such as docetaxel (Taxotere®, Aventis Pharmaceuticals, Bridgewater, NJ) have similar mechanisms of cytotoxicity that are complementary to these lethal effects of radiation.
Docetaxel is an antineoplastic agent that targets the microtubular network in cells. Docetaxel binds to free tubulin and promotes the assembly of tubulin into stable microtubules while simultaneously inhibiting their disassembly.11,12 This leads to the production of microtubule bundles without normal function and to the stabilization of microtubules, which results in the inhibition of mitosis. By stabilizing the microtubular apparatus during the M phase of the cell cycle, docetaxel arrests and prevents tumor cell division. Moreover, by arresting tumor cells in the M phase of the cell cycle, docetaxel synergizes the lethal effects of RT, thereby serving as an ideal radiosensitizer.13–15 Both in vitro and in vivo studies have demonstrated the synergistic effects of docetaxel when combined with RT. Hennequin and colleagues13 and Milas and colleagues14 have shown that docetaxel significantly increases radioresponsiveness in vitro by a factor of 2.5 to 3.0. Mason and associates15 conducted in vivo experiments to assess the synergistic effects of docetaxel in murine MCa-K tumors. Murine MCa-K tumors were treated with radiation only or docetaxel plus radiation. Docetaxel increased tumor eradication rates by 3-fold.
Recognizing the laboratory and clinical synergy between radiation and docetaxel and that multiple phase I and II clinical studies have shown that single-agent docetaxel is safe and efficacious in metastatic prostate cancer,16–19 radiosensitization with docetaxel is an attractive therapeutic strategy. Hence, a phase I trial was conducted between January 2000 and August 2002 at Robert Wood Johnson Medical School/University of Medicine & Dentistry of New Jersey/The Cancer Institute of New Jersey to determine the maximally tolerated dose (MTD) of weekly docetaxel that could be concurrently delivered with 3-D CRT in the treatment of unfavorable localized adenocarcinoma of the prostate.20
Phase I Trial: Docetaxel and 3-D CRT
The primary eligibility criterion for men included in this phase I trial was biopsy-proven adenocarcinoma of the prostate with high-risk localized disease, defined as follows: American Joint Committee on Cancer (AJCC) stage T3N0M0 or T4N0M0; AJCC stage T1B/T1C/T2N0M0 and Gleason score ≥ 8; or AJCC stage T1C/T2N0M0 with Gleason score 5–7 and PSA ≥ 10 ng/mL. Other major eligibility criteria included Karnofsky performance status ≥ 70, no history of prior chemotherapy or pelvic irradiation, adequate bone marrow/liver function, and of course, informed consent. Neoadjuvant or adjuvant hormonal therapy could be given, but concurrent hormonal therapy was not allowed.
Patients received weekly docetaxel with concurrent daily 3-D CRT to a total dose of 70.2 Gy at 1.8 Gy/fraction (fx). Initially, the pelvis was treated with a dose of 45 Gy at 1.8 Gy/fx using a four-field box technique. The prostate, with or without the inclusion of the seminal vesicles, was then boosted to 70.2 Gy at 1.8 Gy/fx using a 3-D conformal technique. Every attempt was made to keep the bladder full during RT treatment to keep the bowel out of the field.
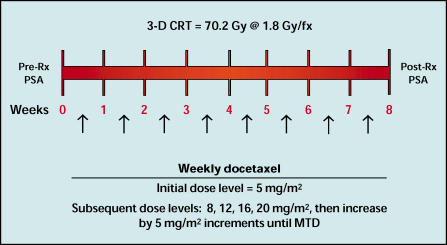
Patients received docetaxel once weekly during the course of the RT treatments. The initial docetaxel dose level (ie, level I) was 5 mg/m2 for the first cohort of patients, and the chemotherapy doses were escalated according to the modified Fibonacci method, as follows: level II, 8 mg/m2; level III, 12 mg/m2; level IV, 16 mg/m2; level V, 20 mg/m2. If the dose-limiting toxicity had not been achieved by level V, the docetaxel doses were then escalated by 5 mg/m2 dose increments until the MTD was reached (see Figure 1). Docetaxel was given as an intravenous infusion over 30 minutes. Patients were accrued in cohorts of three beginning at the 5 mg/m2 docetaxel dose level. Chemotherapy dose escalation was done with a classic phase I design. A dose-limiting toxicity was defined as any grade III or IV nonhematologic toxicity (including neurotoxicity), or grade IV hematologic toxicity lasting for more than 7 days.
Figure 1.
Schema for a phase I trial of docetaxel and three-dimensional conformal radiation therapy (3-D CRT) for the treatment of men with high-risk, localized prostate cancer. Rx, treatment; PSA, prostate-specific antigen; MTD, maximally tolerated dose.
All acute chemoradiation toxicities were graded with the Common Toxicity Criteria of the National Cancer Institute (version 2.0). Late toxicity was scored with the RTOG/EORTC scoring scheme.
Twenty-two men with unfavorable localized adenocarcinoma of the prostate completed the phase I trial as planned. These 22 patients were enrolled at weekly docetaxel dose levels of 5, 8, 12, 16, 20, and 25 mg/m2. No dose-limiting toxicity was observed at weekly docetaxel dose levels between 5 mg/m2 and 20 mg/m2. Dose-limiting toxicity occurred in the first two patients treated at the 25 mg/m2 docetaxel dose level, both of whom experienced grade III diarrhea. Subsequently, the docetaxel doses were reduced by 25%, and both patients went on to tolerate their chemoradiation therapy treatments without any further significant toxicity. Hence, 3 more patients were then enrolled to the 20 mg/m2 dose level, for a total of 6 patients, and no dose-limiting toxicity was observed. Overall, diarrhea occurred as follows: grade 0 in 7 patients, grade I in 5, grade II in 8, and grade III in 2. In all cases, the diarrhea was self-limiting or responsive to medication. Dysuria was observed as follows: grade 0 in 15 patients, grade I in 2, and grade II in the other 5. Dysuria was also either self-limiting or responsive to pyridium intervention in all cases. Of the 22 patients, 6 (27%) did not experience any diarrhea or dysuria.
No significant hematologic toxicity (grade II–IV) was encountered among these 22 patients. At a median follow-up interval of 8 months (range = 2–27 months), all patients are alive.
Discussion
Very few investigators have tested the novel concept of combining concurrent chemotherapy with radiation therapy in localized adenocarcinoma of the prostate.20 To our knowledge, this is the first trial that has investigated the use of concurrent docetaxel and radiation therapy for localized adenocarcinoma of the prostate. Zelefsky and associates21 recently reported their results in 27 patients treated with 3-D CRT with concurrent estramustine phosphate and vinblastine (EV). Patients were treated with neoadjuvant EV (estramustine phosphate [Emcyt®, Pharmacia and Upjohn, Kalamazoo, MI] at 10 mg/kg p.o. tid and vinblastine [Velbe®, Eli Lilly Australia, West Ryde, New South Wales] at 4 mg/m2 weekly for 6 of 8 weeks × 2 cycles) followed by EV (1 cycle) and concurrent 3-D CRT to 75.6 Gy to the prostate only; the pelvis was omitted from the radiation field. Of 27 patients, 23 (85%) completed the entire course of therapy. Two patients developed grade III hematologic toxicity, and 2 patients developed grade III hepatotoxicity necessitating discontinuation of the chemotherapy and withdrawal from the treatment program. Medications were required for relief of acute grade II rectal and urinary symptoms in 35% and 48% of patients, respectively. Three patients developed acute grade III genitourinary (GU) toxicities. The 2-year actuarial likelihood of late grade II GI toxicity was 20%. The 2-year actuarial likelihood of late grade II and III GU toxicities were 25% and 12%, respectively. Although these investigators concluded that neoadjuvant and concomitant EV with 3-D CRT is feasible for patients with high-risk prostate cancer, the incidence of late GI and GU toxicities appeared to be increased compared with 3-D CRT alone or in combination with hormonal therapy.
Khil and colleagues22 also reported their outcome with EV and RT for patients with locally advanced T2 to T4 prostate cancer. In this study, 65 patients were treated with concurrent EV (estramustine phosphate at 450 mg/m2 p.o. daily and vinblastine at 3 mg/m2 IV weekly) in combination with conventional external-beam RT (whole pelvis treatment to 45 Gy followed by a prostate boost to doses of 20–25 Gy). One grade III leukopenia and one grade III small bowel toxicity were observed, which required hospitalization in each case. In addition, 1 patient required a diverting colostomy for grade IV radiation proctitis. The incidence of grade II diarrhea and proctitis was 57% and 39%, respectively, and the incidence of grade II GU symptoms was 72%. The myelosuppression from the multiagent EV chemotherapy was so high that the last 19 patients were treated with only estramustine phosphate and concurrent 3-D CRT, because vinblastine was omitted from the treatment regimen. Hence, only 70% of the men completed the original chemotherapeutic regimen.
Weekly docetaxel with 3-D CRT was very well tolerated in our trial. The incidence of significant acute GI and GU toxicities was much lower in our series than that reported by Khil and colleagues22. The incidence of grade II diarrhea and grade II dysuria was 37% and 23%, respectively, in our trial compared with 57% and 72%, respectively, in the Khil trial (Table 1). Additionally, GI toxicity was also more common in the Khil series (ie, grade III small bowel toxicity in 1 patient, grade IV radiation proctitis requiring colostomy in another patient, and a grade II proctitis rate of 39%) compared with our series, in which proctitis and small bowel toxicity were not observed. This may be attributed to our use of 3D-CRT, the treatment of patients with a full bladder, and the use of low-residue diet before the initiation of therapy. Furthermore, the incidence of grade II diarrhea in our trial (36%) was similar to that in the Zelefsky study (35%), despite the use of whole-pelvic RT in our trial compared with the omission of the pelvis in the Zelefsky study and the use of multiagent chemotherapy.
Table 1.
Concurrent Chemotherapy and Radiation Therapy for Localized Adenocarcinoma of the Prostate: Comparison of Three Trials
| Trial | N | Therapy | Feasibility | GI Toxicity | GU Toxicity | Hematologic Toxicity |
|---|---|---|---|---|---|---|
| Kumar et al. 200320 | 22 | Docetaxel + 70.2 | 100% | Grade II: 36% | Grade II: 36% | Grade II: 0% |
| Gy (pelvis) | Grade III: 10% | Grade III: 0% | Grade III: 0 | |||
| Late grade II: 10% | ||||||
| Khil et al.199722 | 65 | EV + 65–70 Gy | 70% | Grade II: 57% | Grade II: 72% | Grade III: 1% |
| Grade III: 1% | ||||||
| Grade IV: 1% | ||||||
| Zelefsky et al.200021 | 27 | EV → 75.6 | 85% | Grade II: 35% | Grade II: 48% | Grade III: 10% |
| Gy (p.o.) + EV | Late grade II: 20% | Late grade III: 12% | Liver grade III: 10% |
EV, estramustine phoshate and vinblastine; GI, gastrointestinal; GU, genitourinary; p.o., prostate only
Traditionally, the recommended administration schedule of docetaxel has been once every 3 weeks. Data on the weekly administration schedule of docetaxel, compared with the every-3-week schedule, suggest it to be at least as efficacious, with potentially fewer toxicities.16–19 These findings have substantially enhanced investigational strategies for docetaxel in many disease sites, such as hormone-refractory prostate cancer and non-small cell lung cancer, malignancies in which patients are usually elderly and unable to tolerate excessively toxic regimens. For example, Picus and Schultz16 investigated an every-3-week docetaxel schedule at 75 mg/m2 in 35 chemotherapy-naïve patients with hormone-refractory prostate cancer and found that hematologic toxicity occurred in 43% of patients. A greater than 50% PSA decline was demonstrated in 46% of patients. Friedland and associates17 conducted a similar phase II study of single-agent docetaxel at 75 mg/m2 every 3 weeks in 21 men with hormone-refractory disease. Again, hematologic toxicities were predominant, with grade III/IV neutropenia occurring in 71% of patients.
In contrast, the use of weekly docetaxel with various tumor types, including metastatic prostate cancer, has demonstrated a more favorable toxicity profile than the every-3-week regimens, while maintaining comparable levels of antitumor activity. For example, Berry and associates18 conducted a multi-institution phase II study of weekly docetaxel in 60 heavily pretreated, hormone-refractory prostate cancer patients and found infrequent myelosuppression. Patients were scheduled to receive three cycles of therapy with docetaxel at 36 mg/m2 per week for 6 weeks, followed by 2 weeks of rest (one cycle). Therapy was well tolerated, and grade III/IV neutropenia occurred in only 3% of patients. Grade III/IV asthenia and diarrhea were each reported in 10% of patients. On an intent-to-treat basis, an objective tumor response was reported in 24 patients (41%). A nearly identical study was conducted by Beer and colleaguesB19 in 25 men who had not received prior chemotherapy. Patients received treatment with single-agent docetaxel at 36 mg/m2 weekly for 6 consecutive weeks of an 8-week cycle. Therapy was well tolerated, with 25% of patients experiencing grade III/IV hematologic toxicity and 36% of patients experiencing grade III nonhematologic toxicity. Grade III/IV neutropenia was reported in 16% of patients; however, no cases of neutropenic fever were reported. A PSA response was achieved in 11 of 24 evaluable patients (46%). The authors concluded that single-agent weekly docetaxel was well tolerated with acceptable toxicity and efficacy.
The lack of myelosuppression and the ease of tolerability of chemotherapy in our trial can also be explained readily by the weekly dosing schedule of docetaxel. No thrombocytopenia or neutropenia were observed in our trial. The only observed hematologic toxicity was grade I anemia, which occurred in 14 of 22 patients. However, in 5 of these patients, the anemia was present before the start of the chemoradiation therapy treatments. This ease of tolerability also translated into an overall higher dose intensity of weekly docetaxel with 3-D CRT than might be possible with other chemotherapy dosing schedules, such as every 3 weeks. Obviously, the radiosensitization effects of docetaxel are also better enhanced with a weekly schedule compared with a longer dosing schedule.
In summary, this phase I trial showed that the combination of concurrent weekly docetaxel and 3-D CRT is very well tolerated, with acceptable toxicity. The MTD of weekly docetaxel was determined to be 20 mg/m2 with concurrent 3-D CRT. A phase II trial will soon be initiated to test the feasibility and efficacy of weekly docetaxel at 20 mg/m2 and concurrent radiation therapy in men with unfavorable localized adenocarcinoma of the prostate.
Main Points.
For patients presenting with intermediate or high-risk prostate cancer, both radiation therapy (RT) alone or RT in combination with hormonal therapy have produced intermediate to poor results; other strategies are needed to improve local control and survival outcome for patients presenting with intermediate or high-risk prostate cancer.
One such strategy involves the use of concurrent chemotherapeutic agent(s) to sensitize the local effects of RT; this strategy has been successfully used in other malignant diseases, such as rectal and anal cancers, to improve local control and survival outcomes.
Docetaxel, when combined with RT, has been demonstrated to increase radioresponsiveness by a factor of 2.5- to 3.0-fold in vitro; and murine MCa-K tumors treated with docetaxel plus radiation had a 3-fold increase in tumor cure.
In a phase I trial conducted between January 2000 and August 2002, 22 patients with high-risk localized adenocarcinoma of the prostate received docetaxel once weekly during the course of RT treatments; this combination was very well tolerated, with acceptable toxicity.
The incidence of significant acute gastrointestinal and genitourinary toxicities was much lower with docetaxel than with a combination of estramustine phosphate and vinblastine used in another RT/chemotherapy trial.
The use of weekly docetaxel with various tumor types, including metastatic prostate cancer, has demonstrated a more favorable toxicity profile than the every-3-week regimens, while maintaining comparable levels of antitumor activity.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE, Hanlon AL, Epstein B, et al. Dose response in prostate cancer with 8–12 years follow-up. Int J Radiat Oncol Biol Phys. 2001;51(3) suppl 1:138–139. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 3.American Society for Therapeutic Radiology and Oncology Consensus Panel, authors. Consensus statement: Guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 4.Pilepich MV, Winter K, John MJ, et al. Phase II Radiation Therapy Oncology Group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 5.Lawton C, Winter K, Murray K, et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) Trial 85-31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;49:937–946. doi: 10.1016/s0360-3016(00)01516-9. [DOI] [PubMed] [Google Scholar]
- 6.Hanks GE, Lu JD, Machtay M, et al. RTOG Protocol 92-02: a phase III trial of the use of long term androgen suppression following neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2000;48(3) suppl 1:112. [Google Scholar]
- 7.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 8.Gastrointestinal Tumor Study Group, authors. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294–1295. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 9.Tveit KM, Guldvog I, Hagen S, et al. Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes b and C rectal cancer. Br J Surg. 1997;84:1130–1135. [PubMed] [Google Scholar]
- 10.Nigro ND. Multimodisciplinary management of cancer of the anus. World J Surg. 1987;11:446–451. doi: 10.1007/BF01655808. [DOI] [PubMed] [Google Scholar]
- 11.Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligrand stoichiometry, and competition. Biochemistry. 1993;32:2747–2755. doi: 10.1021/bi00062a003. [DOI] [PubMed] [Google Scholar]
- 12.Ringel I, Horwitz SB. Studies with RP 56976 (Taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- 13.Hennequin C, Giocanti N, Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B Cells. Cancer Res. 1996;56:1842–1850. [PubMed] [Google Scholar]
- 14.Milas L, Milas MM, Mason KA. Combination of taxanes with radiation: preclinical studies. Semin Radiat Oncol. 1999;9:12–26. [PubMed] [Google Scholar]
- 15.Mason K, Hunter N, Milas M, et al. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res. 1997;3:2431–2438. [PubMed] [Google Scholar]
- 16.Picus J, Shultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory cancer: preliminary results. Semin Oncol. 1999;29(suppl 17):14–18. [PubMed] [Google Scholar]
- 17.Friedland D, Cohen J, Miller R, et al. A phase II trial of docetaxel in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of bcl-2. Semin Oncol. 1999;26(5) suppl 7:19–23. [PubMed] [Google Scholar]
- 18.Berry W, Dakhil S, Regich M, et al. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28(suppl 15):8–15. doi: 10.1016/s0093-7754(01)90149-6. [DOI] [PubMed] [Google Scholar]
- 19.Beer T, Pierce WC, Lowe BA, et al. Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol. 2001;12:1273–1279. doi: 10.1023/a:1012258723075. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Perrotti M, Weiss R, et al. Radiosensitization with docetaxel and 3-D CRT. Results of a completed phase I trial. Proceeding of American Society of Clinical Oncology. 2003;22(1622):404. [Google Scholar]
- 21.Zelefsky MJ, Kelly WK, Scher H, et al. Results of a phase II study using estramustine phosphate and vinblastine in combination with high-dose three-dimensional conformal radiotherapy for patients with locally advanced prostate cancer. J Clin Oncol. 2000;18:1936–1941. doi: 10.1200/JCO.2000.18.9.1936. [DOI] [PubMed] [Google Scholar]
- 22.Khil MS, Kim JH, Bricker LJ, et al. Tumor control of locally advanced prostate cancer following combined estramustine, vinblastine, and radiation therapy. Cancer J Sci Am. 1997;3:289–296. [PubMed] [Google Scholar]