Abstract
Androgen ablation is effective therapy for metastatic prostate cancer, but the majority of men eventually become refractory to this intervention. Cytotoxic chemotherapy offers palliation to symptomatic patients with hormone-refractory prostate cancer (HRPC); however, no chemotherapy regimen has yet been shown to prolong survival. There is a clear need for new agents and drug targets for the treatment of HRPC. A number of innovative therapeutic approaches that are rationally based and target driven are under investigation. This article reviews the development of antisense oligonucleotides that inhibit the anti-apoptotic bcL-2 protein. Approaches that target the epidermal growth factor receptor, the platelet derived growth factor receptor, and nuclear factor kappa-B are also discussed. There is much expectation that these therapies alone or in combination with cytotoxic chemotherapy will impact the clinical outcome of patients with HRPC.
Key words: Hormone-refractory prostate cancer, Antisense oligonucleotides, Docetaxel, Androgen-independent prostate cancer
Androgen ablation has proved to be an effective therapy for metastatic prostate cancer. Although rapid and dramatic regression of metastatic lesions often follows the achievement of castrate testosterone levels, this response generally lasts only 18 to 24 months. The majority of men eventually become refractory to androgen blockade, with a median survival time of 9 to 12 months. Cytotoxic chemotherapy for the treatment of hormone refractory prostate cancer (HRPC) has carried the historic burden of ineffectiveness and toxicity. The seminal review by Yagoda and colleagues1 a decade ago provided little optimism that HRPC was a chemosensitive disease. This review demonstrated the need for new agents and drug targets. Despite this history, there has been progress over the past decade in the treatment of HRPC. Two randomized trials led to the approval of mitoxantrone combined with corticosteroids as palliative therapy for symptomatic HRPC.2,3 Recently published data from phase II studies suggest that docetaxel (Taxotere®, Aventis Pharmaceuticals, Bridgewater, NJ) as a single agent or in combination with estramustine may be efficacious in treating advanced prostate cancer.4,5 To date, however, no established chemotherapy regimen has been shown to increase survival. Two ongoing multicenter phase III trials will address whether docetaxel-based chemotherapy offers an improvement in survival compared with standard palliative therapy.
In recent years, a number of new therapeutic targets and treatment strategies have been identified. These rationally based, target-driven approaches may provide meaningful improvement in the treatment of advanced prostate cancer. This article reviews some of the innovative treatment approaches to HRPC (Figure 1).
Figure 1.
Novel approaches to the treatment of hormone refractory prostate cancer.
Targeting Anti-Apoptotic Genes Using Bcl-2 Antisense Oligonucleotides
Progression to androgen independence is a complex process involving variable combinations of clonal selection, adaptive upregulation of anti-apoptotic survival genes, ligand-independent activation of the androgen receptor from mutations or increased levels of co-activators, and alternate growth factor pathways.6,7 The processes involved in progression to an androgen-independent state have been studied in animal tumor models.8,9 One such model is the human LNCaP model, an androgen-responsive, prostate-specific antigen (PSA)- secreting cell line. As in clinical prostate cancer, serum levels in this model are initially regulated by androgen and are directly proportional to tumor volume. Apoptotic tumor regression does not consistently occur after castration. However, tumor growth stabilizes and serum tumor cell PSA levels decrease by 80% for several weeks post-castration, after which LNCaP tumor growth rates and PSA expression increase above pre-castrate levels.8 The Shionogi tumor model has also proved to be useful for studying the effects of androgen ablation on gene expression and apoptosis.9 The Shionogi tumor model is of mouse mammary origin but is androgen-dependent and contains a functional androgen receptor. The patterns of change in gene expression after castration in such tumor models as the LNCaP and the Shionogi cell lines are similar to that seen in human prostate cancer, validating their use as models of human disease. The investigation of the shift in gene expression after androgen ablation allows identification of molecular events that mediate tumor progression and may help to identify therapeutic targets.
Preclinical studies in LNCaP and Shionogi tumor models have identified upregulation of genes associated with tumor cell apoptosis early after androgen ablation. The increased activity of such anti-apoptotic pathways may play an important role in hormonal resistance and chemoresistance in patients with prostate cancer. BcL-2 appears to be particularly important in the transition from androgen-dependent to androgen-independent growth. BcL-2 belongs to a family of related genes whose proteins regulate apoptosis in normal and abnormal cell populations. The ratio of death antagonists (bcL-2, bcL-xL) to death agonists (Bax, bcLxS, Bad) determines whether a cell will respond to an apoptotic signal. In both LNCaP and Shionogi tumor models, bcL-2 gene expression increases several-fold following castration and during androgen-independent progression.7 In patients with prostate cancer, increased bcL-2 expression has been observed in prostate cancer cells after androgen withdrawal. Tsuji and colleagues10 found that radical prostate specimens obtained following neoadjuvant hormone therapy had a higher incidence of bcL-2 expression as shown with immunohistochemical staining (57%) compared with radical prostatectomy specimens obtained from the hormone-naive patients (22%). Studies have also demonstrated a disparity between the levels of bcL-2 expression in androgen-dependent versus androgen-independent tumors.11 Some investigators have reported bcL-2 expression in up to 100% of specimens from patients with HRPC.12 There is accumulating evidence that overexpression of bcL-2 protects prostate cancer cells from the apoptotic cell death induced by a variety of stimuli, including androgen ablation and cytotoxic chemotherapy, and thereby accelerates progression to androgen independence and confers resistance to chemotherapy. Preclinical studies have shown that transfection of bcL-2 into LNCaP human prostate cancer cell lines imparts resistance to androgen ablation; bcL-2 expression is higher in LNCaP cells that have metastasized in nude mice. Furthermore, increased expression of bcL-2 has been correlated with poor prognosis in patients with prostate cancer.13,14
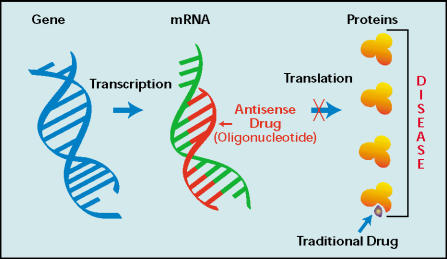
Antisense oligonucleotide therapy specifically targets bcL-2 gene expression. Antisense oligonucleotides are chemically modified stretches of single- stranded DNA that are complementary to mRNA regions of a target gene and inhibit translation by forming RNA/DNA duplexes, thereby reducing mRNA and protein levels of the target gene (Figure 2). Reducing the levels of these proteins alters the subsequent cascade of protein-protein signaling that controls cellular proliferation, differentiation, and apoptosis in the cancer cell. The specificity and efficacy of antisense oligonucleotides rely on the precise targeting afforded by strand hybridization, in which only a perfect match between the target sequence and the antisense oligonucleotides leads to hybridization and inhibition or translation. Phosphorothioate antisense oligonucleotides are water-soluble, stable, agents manufactured to resist nuclease digestion through the substitution of a non-bridging phosphoryl oxygen of DNA with sulfur. Antisense oligonucleotides become associated with high-capacity, low-affinity serum binding proteins after parenteral administration. One limitation of the phosphorothioate backbone is its short in vivo half-life, which necessitates administration as a continuous intravenous infusion.13,14
Figure 2.
Mechanism of action of antisense oligonucleotides.
Preclinical studies in LNCaP and Shionogi models have demonstrated that species-specific bcL-2 antisense oligonucleotide therapy reduces bcL-2 mRNA and protein levels by over 90% in a sequence-specific, dose-dependent manner. The treatment of mice inoculated with the LNCaP prostate cancer cell line at the time of castration with bcL-2 antisense oligonucleotides has been shown to delay the emergence of androgen-independent recurrent tumors and prolong survival compared with controls treated with castration or a 1- or 2-base pair mismatch bcL-2 antisense oligonucleotide. BcL-2 antisense oligonucleotides also enhanced the cytotoxicity of chemotherapeutic agents (taxanes) in these tumor models.7,7–16
Oblimersen (G3139, Genasense,™ Genta Inc, Berkeley Heights, NJ) is a pharmacologic phosphorothioate antisense oligonucleotide. This 18-base synthetic oligodeoxyribonucleotide strand (sequence: 5′ tct ccc agc gtg cgc cat 3′) hybridizes to the first six codons of the bcL-2 open reading frame mRNA. The oligodeoxyribonucleotide mRNA hybrid recruits endogenous RNase H, which mediates scission of the bcL-2 mRNA and depletion of the bcL-2 protein. Clinical studies of oblimersen are ongoing. In a phase I study of patients with relapsed non- Hodgkin’s lymphoma, treatment with oblimersen was well tolerated and objective responses were achieved.17 Combination studies of oblimersen have been conducted in patients receiving mitoxantrone or docetaxel for metastatic prostate cancer.18,19 The downregulation of bcL-2 achieved with bcL-2 antisense oligonucleotides may improve the efficacy of docetaxel- based therapy.
A recently reported phase II study20 assessed the anti-tumor activity of oblimersen 7 mg/kg/d (continuous intravenous infusion days 1–8) and- docetaxel (75 mg/m2 on day 6) in patients with metastatic HRPC. Therapy was repeated every 21 days until progression or toxicity. Thirty-one men were enrolled and data for analysis was available on 29 of them. The median number of cycles was 4 (range 1–10), with 20 patients continuing protocol therapy. A partial response was achieved in 4 of 15 patients (27%) with measurable disease and a > 50% reduction in PSA levels was measured in 15 of 31 (48%) of patients. The most common grade 1–2 adverse events were fatigue (35%) and non-neutropenic fever (31%). Grade 3–4 neutropenia occurred in 13 of 31 (42%) patients. Five patients experienced grade 3–4 febrile neutropenia and grade 3 fatigue was reported in 3 patients (10%). The median decrement of normalized bcl-2 protein in peripheral mononuclear blood cells by day 6 was 50%. Overall the regimen appeared to be well-tolerated and associated with encouraging PSA and clinical responses.
Targeting Nuclear Factor Kappa-B
Nuclear factor-kappa B (NF-κB) is a transcription factor that regulates the expression of genes related to tumor invasion, metastases, cell proliferation, and apoptotic death. The activation of NF-κB is regulated by proteosome- mediated degradation of the inhibitor protein (I-κB). Inhibition of NF-κB activation through stabilization of the I-κB protein sensitizes cells to environmental stress and cytotoxic agents and ultimately leads to apoptosis. In the human prostate cancer cell lines PC-3 and DU 145, NF-κB is constitutively activated, whereas the hormone-sensitive LNCaP cell lines have significantly lower levels of NF-κB.21 A novel proteosome inhibitor, PS 341, is currently in clinical trials. This agent has demonstrated antitumor activity in colon cancer (HT-29), lung cancer (NCI-H23), and prostate cancer (PC-3) cell lines in human xenograft models.22 PS 341 (Velcade®, Millennium Pharmaceuticals, Inc., Cambridge, MA), has been shown to inhibit drug-induced NF-κB activation and, in doing so, sensitizes cells to drug-induced apoptosis. A recently completed phase I study established the tolerability of PS 341; dose-limiting toxicities included diarrhea and sensory neuropathy.22 Phase II testing of PS 341 is underway in multiple myeloma and a variety of solid tumors, including prostate cancer.
Targeting Signal Transduction Pathways
Epidermal Growth Factor Receptor Blockade
The progression of prostate cancer is associated with the upregulation of growth factors and growth factor receptor products and the downregulation of tumor suppressor gene products. Growth factors implicated in disease progression include transforming growth factor α (TGF-α), transforming growth factor β, and fibroblast growth factor (FGF).6 Specific growth factor receptors include all members of the erbB family (EGFR/erbB1, HER-2/neu/erbB2, HER-3/erbB3, and HER-4/erbB4). Receptors in the erbB family contain an extracellular ligand-binding domain, a hydrophobic transmembrane segment, and an intracellular domain that has catalytic tyrosine kinase activity. With the exception of HER-2, for which there is no ligand, each receptor binds to at least one growth factor. Ligands for the epidermal growth factor receptors (EGFRs) include epidermal growth factor (EGF), TGF-α, and amphiregulin. Ligand binding triggers receptor dimerization and phosphorylation. The activated receptors phosphorylate other proteins and provide docking sites for signaling molecules to initiate a cascade of signals to the cell nucleus. This signaling enhances cellular proliferation, promotes angiogenesis, and prevents apoptosis. EGFR is expressed in 40%–80% of malignant prostate cancer cells, and increased expression has been correlated with high Gleason score and tumor progression from an androgen-dependent state to an androgen-independent state.6,23,24 Preclinical research in the androgen-responsive prostate cancer cell lines MDA PCa 2a and MDA PCa 2b has shown that both dihydrotestosterone and the EGF can stimulate proliferation of these cell lines. Dual blockade of androgen receptor function and EGFR (with either C225 or trastuzumab) resulted in significant growth inhibition.25 Thus, the combination of EGFR and androgen blockade may provide a promising new therapeutic approach to the treatment of prostate cancer.
Several inhibitors of EGFR are being studied in clinical trials. These agents include monoclonal antibodies directed against the external ligand-binding domain of EGFR, such as cetuximab (Erbitax®, ImClone Systems, Inc., New York) and ABXEGF (Abgenix, Inc., Fremont, CA), and small molecules that inhibit the intracellular tyrosine kinase region of EGFR, such as ZD1839 (Iressa™, AstraZeneca PLC, London, UK) and OSI-774 (Tarceva™, OSI Pharmaceuticals, Melville, NY). ZD1839, an orally active selective inhibitor of EGFR tyrosine kinase, inhibits the growth of a wide range of human tumors, including prostate cancer grown as xenografts in nude mice. Combinations of cytotoxic agents and ZD1839 have also been studied in these models, and results suggest that the combination of ZD1839 and chemotherapy (carboplatin or paclitaxel) is synergistic in lung tumors and in androgen-independent TSU and PC-3 human prostate cancer xenografts.26 Phase I studies have shown treatment with ZD1839 to be generally well tolerated; acneiform skin rash and diarrhea were the most common toxicities. In one study, there was a confirmed partial response for longer than 6 months in a patient with HRPC.27 These observations have led to the initiation of phase II studies of ZD1839 for the treatment of HRPC.
Platelet-Derived Growth Factor Receptor Blockade
The various platelet-derived growth factor (PDGF) and platelet-derived growth factor receptor (PDGF-r) isoforms comprise a family of ligands and receptors. PDGF is a 30-kd protein consisting of disulfide-bonded homodimers or heterodimers of A and B chains. PDGF-r occurs as an α/β heterodimer and belongs to the protein tyrosine kinase family of receptors. The α receptor chain can bind to all dimeric isoforms of PDGF (AA, BB, AB), whereas the β receptor chain preferentially binds to the B isoform. After binding of the dimeric ligand to the extracellular portions of the two PDGF-r chains, autophosphorylation of receptor tyrosine residues initiates various signaling pathways that ultimately lead to changes in cell growth and prevention of apoptosis. The effect of PDGF and expression of PDGF-r on HRPC were recently examined. Studies using immunohistochemical analysis demonstrated that the PDGF A chain and PDGF-r-α are expressed in adenocarcinomas but not in benign prostate hypertrophy or normal prostatic epithelium. These two proteins are also expressed in the precursor lesion prostatic intraepithelial neoplasia, suggesting that de novo expression occurs early in the transformation process.28,29 These findings led to a multicenter, phase II clinical trial of a PDGF-r inhibitor, SU101, in patients with advanced prostate cancer.30 Patients received a 4-day intravenous loading dose followed by 10 weekly infusions. The primary endpoints were decline in PSA level and decrease in measurable tumor. Three of 39 patients who were evaluable for PSA level demonstrated a decline from baseline of greater than 50%. One of 19 patients with measurable disease exhibited a partial response. The modest clinical benefit observed can be explained by the short half-life of SU101 (SUGEN, South San Francisco,CA), which resulted in transient blockade of PDGF-mediated signaling. More recently, imatinib mesylate (STI571, Gleevec™, Novartis Pharmaceuticals Corporation, East Hanover, NJ), a potent inhibitor of PDGF-r tyrosine kinase, has been investigated for the treatment of HRPC.31 This agent inhibits several tyrosine kinases, including Abl and Bcr-Abl tyrosine kinase, the tyrosine kinase receptor for stem cell factor, c-kit, and PDGF-r. In preclinical studies of human prostate cancer cells grown in the bones of nude mice, systemic treatment with imatinib mesylate and paclitaxel was associated with significant induction of apoptosis in tumor cells and tumor-associated endothelial cells, resulting in inhibition of tumor growth and preservation of bone structure.32 These findings provide the rationale for combining imatinib mesylate with cytotoxic chemotherapy. At the Institute for Drug Development, we are currently studying the combination of imatinib mesylate, estramustine, and docetaxel for patients with advanced solid tumors, including HRPC.
Conclusion
Over the past decade, our understanding of the molecular basis of resistance to androgen blockade and knowledge of growth factor-regulated pathways and their role in the pathogenesis of prostate cancer have advanced. This had led to rationally designed drug therapy for the treatment of HRPC. New combinations of cytotoxic chemotherapy and novel targeted agents are being explored as therapies for this disease. There is much expectation that these innovative therapies will impact the outcome of patients with HRPC. Further studies that integrate novel agents with available cytotoxic and bone-targeted therapy are necessary, and the introduction of novel agents earlier in the disease course needs to be considered
Main Points.
Although androgen ablation is effective for the treatment of advanced prostate cancer, the majority of men become refractory to this therapy. In addition, no chemotherapy regimen has yet been shown to increase survival in these patients.
New target-based approaches for the treatment of hormone-refractory prostate cancer are under investigation; these include antisense oligonucleotides that inhibit the bcL-2 protein, agents targeting nuclear factor-kappa B (NF-κB), inhibitors of the epidermal growth factor receptor (EGFR), and inhibitors of the platelet-derived growth factor receptor (PDGF-r).
The downregulation of bcL-2 achieved with bcL-2 antisense oligonucleotides, such as oblimersen, may improve the efficacy of docetaxel-based therapy. Studies to characterize the anti-tumor activity of this combination are ongoing.
References
- 1.Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993;71(3 suppl):1098–1109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative endpoints. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Shelton GB, England-Owen C, et al. Response and preliminary survival results of a phase II study of docetaxel (D) + estramustine (E) in patients (Pts) with androgen-independent prostate cancer (AIPC) Proc Am Soc Clin Oncol. 2000;19:334a. [Google Scholar]
- 5.Savarese DM, Halabi S, Hars V. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. J Clin Oncol. 2001;19:2509–2516. doi: 10.1200/JCO.2001.19.9.2509. [DOI] [PubMed] [Google Scholar]
- 6.Barton J, Blackledge G, Wakeling A. Growth factors and their receptors: new targets for prostate cancer therapy. Urology. 2001;58(2) suppl 1:114–122. doi: 10.1016/s0090-4295(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 7.Gleave ME, Zellweger T, Chi K, et al. Targeting anti-apoptotic genes upregulated by androgen withdrawal using antisense oligonucleotides to enhance androgen- and chemo-sensitivity in prostate cancer. Invest New Drugs. 2002;20:145–158. doi: 10.1023/a:1015694802521. [DOI] [PubMed] [Google Scholar]
- 8.Gleave ME, Hsieh JT, Wu HC, et al. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 1992;52:1598–1605. [PubMed] [Google Scholar]
- 9.Bruchovsky N, Rennie PS, Coldman AJ, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–2282. [PubMed] [Google Scholar]
- 10.Tsuji M, Murakami Y, Kanayama H, et al. Immunohistochemical analysis of Ki-67 antigen and Bcl-2 protein expression in prostate cancer: effect of neoadjuvant hormonal therapy. Br J Urol. 1998;81:116–121. doi: 10.1046/j.1464-410x.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 12.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 13.Miayake H, Tolcher A, Gleave ME. Chemosensitization and delayed androgen-independent recurrence of prostate cancer with the use of antisense Bcl-2 oligodeoxynucleotides. J Natl Cancer Inst. 2000;92:34–41. doi: 10.1093/jnci/92.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H, Tolcher A, Gleave ME. Antisense Bcl-2 oligodeoxynucleotides inhibit progression to androgen-independence after castration in the Shionogi tumor model. Cancer Res. 1999;59:4030–4034. [PubMed] [Google Scholar]
- 15.Gleave M, Tolcher A, Miyake H, et al. Progression to androgen independence is delayed by antisense Bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate tumor model. Clin Cancer Res. 1999;5:2891–2898. [PubMed] [Google Scholar]
- 16.Leung S, Miyake H, Zellweger T, et al. Synergistic chemosensitization and inhibition of progression to androgen independence by antisense BcL-2 oligodeoxynucleotide and paclitaxel in the LNCaP prostate tumor model. Int J Cancer. 2001;91:846–850. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1131>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Webb A, Cunningham D, Cotter F, et al. Bcl-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet. 1997;349:1137–1141. doi: 10.1016/s0140-6736(96)11103-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen HX, Marshall J, Trocky N, et al. A phase I study of Bcl-2 antisense G3139 (Genta) and weekly docetaxel in patients with advanced breast cancer and other solid tumors. Pro Am Soc Clin Oncol. 2000;19:178a. doi: 10.1093/annonc/mdh317. [DOI] [PubMed] [Google Scholar]
- 19.Chi KN, Gleave ME, Klasa R, et al. A phase I trial of an antisense oligonucleotide to Bcl-2 (G3139, Genta) and mitoxantrone in patients with metastatic hormone refractory prostate cancer. Proc Am Soc Clin Oncol. 2000;19:330a. [Google Scholar]
- 20.Chi KN, Murray RN, Gleave ME, et al. A phase II study of oblimersen sodium (G3139) and docetaxel in patients with metastatic hormone refractory prostate cancer. Amer Soc Clin Oncol. 2003;22 abstr # 1580. [Google Scholar]
- 21.Palayoor ST, Yournell MY, Calderwood SK, et al. Constitutive activation of Iκ B kinase α and NF-κ B in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;l18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 23.Ciadiello F, Di Lorenzo G, Tortora G, et al. Expression of epidermal growth factor receptor (EGFR) correlates with disease relapse and progression to androgen-independence in human prostate cancer. Proc Am Soc Clin Oncol. 2002;21:176a. [PubMed] [Google Scholar]
- 24.Scher HI, Sarkis A, Reuter V, et al. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor _ in the progression of prostatic neoplasms. Clin Cancer Res. 1995;1:545–550. [PubMed] [Google Scholar]
- 25.Ye D, Mendelsohn J, Fan Z. Androgen and epidermal growth factor down-regulate cyclindependent kinase inhibitor p27Kip1 and costimulate proliferation of MDA PCa 2a and MDA PCa 2b prostate cancer cells. Clin Cancer Res. 1999;5:2171–2177. [PubMed] [Google Scholar]
- 26.Sirotnak FM, Zakowski MF, Miller VA, et al. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
- 27.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 28.Fudge K, Bostwick DG, Stearns ME. Plateletderived growth factor A and B chains and the α and β receptors in prostatic intraepithelial neoplasia. Prostate. 1996;29:282–286. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Fudge K, Wang CY, Stearns ME. Immunohistochemistry analysis of platelet-derived growth factor A and B chains and platelet-derived growth factor α and β-receptor expression in benign prostatic hyperplasia and Gleason-graded human prostate adenocarcinomas. Mod Pathol. 1994;7:549–554. [PubMed] [Google Scholar]
- 30.Ko YJ, Small EJ, Kabbinavar F, et al. A multiinstitutional phase II study of SU101, a platelet derived growth factor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res. 2001;7:800–805. [PubMed] [Google Scholar]
- 31.George D. Platelet-derived growth factor receptors: a therapeutic target in solid tumors. Semin Oncol. 2001;28(5) suppl 17:27–33. [PubMed] [Google Scholar]
- 32.Uehara H, Kim SJ, Karashima T, et al. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]