Abstract
Chemotherapy has historically proven toxic and ineffective for the treatment of metastatic hormone-refractory prostate cancer (HRPC), a disease with substantial morbidity and mortality. Progress has been made in symptom relief, and the combination of mitoxantrone and prednisone is considered the palliative standard of care. The effects of a variety of chemotherapeutic agents, both alone and in combination, on prostate-specific antigen decline rates, measurable disease response, and survival have been examined in numerous phase I and II trials. Results suggest that combining vinblastine or paclitaxel with estramustine confers a survival advantage over either agent alone. In addition, docetaxel-based therapy has been found to be effective and well tolerated, and phase III trials will soon determine whether docetaxel-based therapy should replace mitoxantrone-based therapy as the standard of care for HRPC.
Key words: Hormone-refractory prostate cancer, Chemotherapy, Prostate-specific antigen, Docetaxel, Microtubule
The standard treatment for metastatic prostate cancer, androgen ablation, was developed by Charles Huggins more than 60 years ago. Although there have been reports of patients with metastatic prostate cancer surviving 5 years on androgen blockade, the majority of patients deteriorate and die from hormone-refractory metastases. Approximately 28,900 men will die from metastatic hormone-refractory prostate cancer (HRPC) in the year 2004.1
For patients with progressive metastatic HRPC, the median survival time is 9 to 12 months and varies with the extent of disease. Approximately 80% of patients have metastases to bone, resulting in significant morbidity, including pathologic fractures, anemia, cachexia, bone pain, and spinal cord compression. Although bisphosphonates, chemotherapy, external beam radiation therapy, and isotope administration therapy can help to prevent and relieve bone-related pain and complications, to date no treatment has been proven to improve survival. Although yet to be verified in a phase III study, the combination of docetaxel (Taxotere®, Aventis Pharmaceuticals, Bridgewater, NJ) and estramustine (Emcyt®, Pharmacia and Upjohn, Kalamazoo, MI) or paclitaxel (Taxol®, Bristol-Myers Squibb Company, New York) and estramustine has demonstrated survival times ranging from 17 to 23 months, and may represent the first step in improving the treatment of hormone-refractory disease. This article will review new developments in chemotherapy for HRPC.
Chemotherapy in Advanced Prostate Cancer: Historical Data
Both urologists and medical oncologists have viewed chemotherapy as toxic and ineffective for the treatment of metastatic HRPC. Because of the historically poor results with cytotoxic treatment, urologists have been reluctant to proceed with chemotherapy to treat men with advanced hormone-resistant disease. In 1993, Yagoda and Petrylak2 published a review of 26 chemotherapy trials performed between 1988 and 1991. The reported complete- and partial-remission rate of 8.7% (95% CI, 6.4–9.0) in 1001 assessable patients was similar to that of previous reviews. Survival appeared to be no different from that of historical controls. One problem with interpreting results from earlier chemotherapy trials is that patients often had advanced disease as well as concomitant medical conditions such as congestive heart failure or renal insufficiency, precluding administration of full drug doses. Clinical trial entry was based on progression on the bone scan, changes in symptoms, and progression in measurable disease. Rise in serum prostate-specific antigen (PSA), which may precede changes in bone scan by 7 to 12 months, was not used as a clinical trial entry criterion until the late 1980s. Thus, these trials may have treated patients with a higher tumor burden, which may cause difficulty in detecting low levels of drug activity.
Mitoxantrone-Based Chemotherapy
Two randomized trials have been instrumental in shifting the paradigm of the activity of chemotherapy in HRPC. Tannock and colleagues3 compared prednisone alone to the combination of mitoxantrone (Novantrone®, Serono, Inc., Geneva, Switzerland) and prednisone in 161 symptomatic men with HRPC. The Magill-Menzak pain scale and total narcotic analgesic consumption were used to assess changes in bone pain. The fact that this trial allowed patients to cross over from the prednisone arm to the mitoxantrone/ prednisone arm at progression precluded the detection of a survival advantage. The palliative response rate and duration of palliation were significantly improved with mitoxantrone/prednisone compared with single-agent prednisone.
The Cancer and Leukemia Group B (CALGB) randomized 242 patients with HRPC to hydrocortisone alone or mitoxantrone 14 mg/m2 combined with hydrocortisone.4 In contrast to the Tannock study, in which the primary endpoint was pain relief, the CALGB study’s primary endpoint was survival. Thus, symptomatic disease was not required for study entry, and the dose of mitoxantrone (14 mg/m2) was higher than in the Tannock study (12 mg/m2). An improved time to treatment failure, an improved PSA decline rate, and a trend towards improvement of bone pain were found in those patients treated with the mitoxantrone/ hydrocortisone combination; however, no difference in survival was demonstrated between the two treatment groups.
A recent randomized trial by Berry and associates demonstrated that there was no survival advantage to administering the combination of mitoxantrone and prednisone versus prednisone alone to asymptomatic patients with HRPC.5 Thus, based on these trials, the combination of mitoxantrone and prednisone is considered the palliative standard of care.
Targets for Improvement of the Treatment of Metastatic Prostate Cancer: Cytoplasmic Microtubules
Several pro- and anti-apoptotic pathways exist and have been identified in androgen-independent prostate cancer cell lines and tissues. The anti-apoptotic protein bcl-2 is expressed in approximately 65% of androgen-independent human prostate cancer specimens.6 Evidence suggesting that bcl-2 is part of the androgen-independent phenotype is derived from transfection experiments in which bcl-2 is introduced into human prostate cancer cell line LNCaP, imparting resistance to androgen deprivation. Bcl-2 transfection into the Dunning-G rat prostate cancer cell line will significantly decrease the cytotoxic effect of doxorubicin when compared with nontransfected lines. Thus, bcl-2 may impart both androgen-independent growth as well as chemoresistance. Chemotherapeutic agents, which inactivate bcl-2 by phosphorylation, include those that stabilize and destabilize tubulin, such as the taxanes and vinca alkaloids.
Estramustine-Based Chemotherapy
Estramustine (Emcyt®, Pharmacia and Upjohn, Kalamazoo, MI), a non-nitrogen mustard linked to an estrogen via a carbamate bone, administered orally at 10 mg/kg/day has a 14% response rate in metastatic HRPC. Estramustine binds to microtubule-associated proteins, thus disrupting cytoplasmic microtubules. This compound also inhibits assembly of the nuclear matrix and p-glycoprotein.7 Results of in vitro studies have demonstrated synergy between estramustine and each of the following agents: vinblastine (Velbe®, Eli Lilly Australia, West Ryde, New South Wales), paclitaxel, etoposide (VePesid®, Bristol-Myers Squibb, New York), and docetaxel.
Phase II studies demonstrated PSA decline rates of 54% to 61% and measurable disease response rates of 14% to 40% in men treated with the combination of estramustine and vinblastine. Similar response rates have been observed in patients treated with the combination of estramustine and vinorelbine (Navelbine®, GlaxoSmithKline, Research Triangle Park, NC). The concept of combination therapy directed at tubulin was tested in a phase III trial by Hudes and associates.8 Two hundred one patients were randomized to receive single-agent vinblastine 4 mg/m2 weekly for 6 weeks or the combination of estramustine 600 mg/m2 and vinblastine 4 mg/m2 weekly for 6 weeks. The trial was designed to detect a 50% increase in survival. The first analysis of this study demonstrated an improved time to disease progression (3.7 mo vs 2.2 mo, P < .0004) and PSA-level response (25% vs 3.2%, P < .0001), with a trend towards improved survival (11.9 mo vs 9.2 mo, P = .08). An update presented at the 2002 American Society of Clinical Oncology meeting demonstrated that the survival benefit was significant in favor of combination therapy (12.5 mo vs 9.4 mo, P = .051). However, this survival difference can also be explained by small differences in distribution of prognostic factors between arms of the study.9
Paclitaxel-Based Chemotherapy
Paclitaxel as a single agent as well as in combination with estramustine has been extensively evaluated for the treatment of patients with HRPC (Table 1). An early trial by the Eastern Cooperative Oncology Group administered a 24-hour infusion of paclitaxel at 135 to 170 mg/m2 every 3 weeks.10 A response rate of 4%, at the cost of a 26% rate of neutropenic sepsis and two toxic deaths, was observed. In a second study, higher PSA decline rate (39%) with a median survival of 14 months was found when patients were treated with weekly paclitaxel, 150 mg/m2 for 6 out of 8 weeks.11
Table 1.
Paclitaxel-Based Treatment of Hormone-Refractory Prostate Cancer: Summary of Single-Agent and Combination Studies
| No. of | ≥ 50% PSA | Measurable Disease | Median Overall | ||
|---|---|---|---|---|---|
| Reference | Patients | Treatment | Decline, % | Response, % | Survival, mo |
| Roth et al.10 | 23 | P 135–150 mg/m2 IV | 0 | 4.3 | 9.0 |
| over 24 h q 3 wk | |||||
| Trivedi et al.11 | 17 | P 150 mg/m2 IV over | 39 | 50 | 13.5 |
| 1 h q wk × 6, rest 2 wk | |||||
| Paclitaxel Combination Treatments | |||||
| Hudes et al.12 | 34 | E 600 mg/m2/d po + | 53 | 44 | 15.9 |
| P 120 mg/m2 over | |||||
| 96 h q 3 wk | |||||
| Kelly et al.13 | 56 | E 10 mg/kg/d po + | 67 | 45 | 19.9 |
| P 60 100 mg/m2 over | |||||
| 1 hr q wk + | |||||
| C AUC = 6 q 4 wk | |||||
| Smith et al.14 | 37 | E 280 mg po tid d 1–7 + | 65 | 45 | 12.8 |
| VP-16 100 mg po qd d 1–7 + | |||||
| P 135 mg/m2 over 1 h | |||||
| d 2 q 3 wk | |||||
| Haas et al.15 | 24 | E 600 mg/m2/d po or 280 mg | 38 | 46 | 18.9 |
| bid + P 60–118 mg/m2 over | |||||
| 3 h q wk | |||||
| Athanasiadis | 41 | E 280 mg po bid + | 56 | 49 | 13 |
| et al.16 | P 60–90 mg/m2 q wk | ||||
| Hudes et al.17 | 63 | E 280 mg po bid for | 58 | 27 | NR |
| 3 qd wk × 6 + | |||||
| P 90 mg/m2/wk over 1 hr |
PSA, prostate-specific antigen; P, paclitaxel; E, estramustine; NR, not reported; VP-16, etoposide; C, carboplatin; AUC, area under curve.
Preclinical studies combining estramustine with paclitaxel found synergy against human prostate cancer cell lines. Several phase II trials have evaluated the estramustine/ paclitaxel doublet, with PSA response rates ranging from 38% to 67% and measurable disease responses ranging from 27% to 58% (Table 1). As with the combination of estramustine and vinblastine, there are data suggesting that the addition of estramustine to an antitubulin compound contributes to survival.12–17 A randomized phase II trial found a trend towards improved 3-month survival in patients treated with a course of estramustine 280 mg orally tid for 3 days combined with weekly paclitaxel 100 mg/m2 for 3 out of 4 weeks compared with weekly paclitaxel alone. Unfortunately, the size of the trial may be too small to detect a significant difference in 3-month survival.
Docetaxel-Based Chemotherapy
Docetaxel has been evaluated as a single agent and in combination regimens as treatment of HRPC in several phase II trials (Table 2). Single-agent docetaxel, when administered either weekly or every 3 weeks, results in PSA decline rates ranging from 38% to 58% and measurable disease response rates ranging from 17% to 33% (Table 2).18–21 Results from trials of docetaxel in combination regimens are discussed below.
Table 2.
Docetaxel-Based Treatment of Hormone-Refractory Prostate Cancer: Summary of Single-Agent and Combination Studies
| No. of | Treatment | 50% PSA | Measurable Disease | Median Overall | |
|---|---|---|---|---|---|
| Reference | Patients | Treatment | Decline, % | Response, % | Survival, mo |
| Picus et al.18 | 35 | D 75 mg/m2 q 3 wk | 45 | 28 | 12 |
| Berry et al.19 | 61 | D 36 mg/m2 q wk × 6, | 41 | 33 | 9.4 |
| rest 2 wk | |||||
| Friedland et al.20 | 16 | D 75 mg/m2 q 3 wk | 38 | 17 | NR |
| Gravis et al.21 | 15 | D 35 mg/m2 q wk × 6, | 58 | NR | NR |
| rest 2 wk | |||||
| Beer et al.30 | 25 | D 36 mg/m2 q wk × 6, | 46 | 40 | 9 |
| rest 2 wk | |||||
| Docetaxel Combination Treatment | |||||
| Savarese et al.24 | 47 | E 10 mg/kg/d po d 1–5 + | 68 | 50 | 20 |
| D 70 mg/m2 d 2 q 3 wk + | |||||
| HC 40 mg/d | |||||
| Petrylak et al.22 | 35 | E 280 mg po tid d 1–5 + | |||
| D 70 mg/m2 d 2 q 3 wk | 74 | 57 | NR | ||
| Sinibaldi et al.26 | 32 | E 280 mg po q 6 h × 5 + | |||
| D 70 mg/m2 d 2 q 3 wk | 45 | 23 | NR | ||
| Kosty et al.25 | 21 | E 140 mg/d d 1–5 + | 71 | 11 | NR |
| D 43 mg/m2/wk d 2 × 3 | |||||
| out of 4 weeks |
D, docetaxel; E, estramustine; HC hydrocortisone; NR, not reported.
Phase I Trials: Docetaxel/Estramustine
Summaries of phase I trials of docetaxel plus estramustine are shown in Table 3. Two phase I trials combined docetaxel administered every 3 weeks with estramustine, and despite small variations in the treatment regimen, demonstrated similar maximum tolerated doses and response rates. Petrylak and colleagues22 combined estramustine (280 mg po tid days 1–5) with docetaxel (40–80 mg/m2 every 3 weeks) in men with minimally or extensively pretreated androgen-independent prostate cancer. Docetaxel administration was preceded by dexamethasone 20 mg for 3 doses. Dose-limiting myelosuppression was observed at 80 mg/m2; the recommended dose for phase II trials was 70 mg/m2 in minimally pretreated patients and 60 mg/m2 in extensively pretreated patients. At the time of study entry, 15 patients were receiving narcotic analgesics for the management of bone pain. After treatment, 8 patients discontinued use of narcotic analgesics for a median of 6 weeks, with 1 patient able to reduce his requirement by more than 50%.
Table 3.
Phase I Studies of Docetaxel Plus Estramustine in Hormone-Refractory Prostate Cancer
| Petrylak et al.22 | ||||
|---|---|---|---|---|
| MPT | EPT | Kreis et al.23 | Natale et al.29 | |
| No. of patients | 20 | 12 | 17 | 18 |
| PSA decline, %: | ||||
| >50% | 70 | 50 | 82 | 78 |
| >75% | 40 | 8 | 67 | 50 |
| Objective response | ||||
| > 50% tumor | ||||
| reduction, % | 28 | NR | 67 | |
| Symptomatic | ||||
| improvement, % | 53 | NR | 86 | |
| Median survival time, mo | ||||
| time, mo | NR | NR | NR | |
| Recommended | D 70 mg/m2 q | D 60 mg/m2 q | D 70 mg/m2 q 3 wk + | D 35 mg/m2 q wk + |
| Phase II regimen | 3wk | 3wk | E 12 mg/kg/d | E 280–420 mg |
| E 280 mg tid d 1–5 | tid d 1–3 | |||
D, docetaxel; E, estramustine; MPT, minimal prior therapy (≤ 2 prior chemotheraples, ≤ 2 prior radiation therapies, no history of radioisotope therapy, no evidence of superscan on bone scan, and no history of whole pelvic radiation therapy); EPT, extensive prior therapy (patients who did not fit the MPT category); NR, not reported.
The rate of 50% PSA decline was 63%, with 19% of patients attaining serum PSA levels of less than 4 ng/dL. PSA declines were observed at all dose levels; given the number of patients treated at each dose level, no clear pattern of dose/response could be ascertained. Of the 12 patients who received 40 mg/m2, 7 achieved at least 50% decline in PSA level. All 7 patients treated at the 70 mg/m2 dose level achieved a 50% or greater decline in PSA. An objective response was observed in 28% of the 18 patients with bidimensionally measurable disease. Two responses in measurable disease lasted 1 year, and soft tissue response was observed at all dose levels. The 1-year survival rate was 68%.22
Kreis and colleagues23 performed a similar phase I study combining docetaxel 40 to 80 mg/m2 every 3 weeks with continuous estramustine 14 mg/kg/day. As in the study by Petrylak and associates, neutropenia precluded dose escalation above 80 mg/m2, and 70 mg/m2 was selected as the recommended phase II dose. Overall, 82% of patients treated achieved a greater than 50% PSA decline.
Phase II Trials: Docetaxel/Estramustine
The docetaxel/estramustine doublet has been evaluated in several phase II studies (Table 2).18–22,24–26 PSA response rates ranged from 45% to 74% and response in measurable disease ranged from 11% to 57%. In the Columbia Presbyterian Phase II trial of docetaxel and estramustine, the occurrence of vascular events, including grade 4 and 5 cerebrovascular accidents (6%) and grade 3 deep vein thrombosis (5%), prompted the initiation of prophylactic anticoagulation. Since that time, no additional vascular events have been reported. The median survival in the Columbia Presbyterian study was 20 months. The concept of intermittent chemotherapy was also evaluated in this study. Patients who attained PSA levels lower than 4 ng/dL were offered the option of discontinuing therapy after 9 treatment cycles. Once the PSA level increased to 20 ng/dL, chemotherapy with estramustine/ docetaxel was resumed and continued until response, toxicity, or progression. Of the 5 patients treated, 4 demonstrated a PSA decline once their treatment resumed. These results imply that select patients may not require continuous chemotherapy, and thus need not be subjected to treatment-related toxicity.
In a trial reported by Savarese and associates,24 the CALGB found the combination of low-dose hydrocortisone, docetaxel, and estramustine to be active and well tolerated, with a 50% PSA decline rate of 68%. The reported median survival of 20 months was similar to that in the Columbia Presbyterian study. Similarly, Kosty and colleagues25 showed that a weekly regimen of dexamethasone, docetaxel, and estramustine in a low-dose weekly schedule produced a PSA decline rate of 71%, representing an alternative to the higher-dose (3 times a week) regimens.
Phase II Trial: Estramustine/Docetaxel/Prednisone vs Mitoxantrone/Prednisone
A randomized phase II study compared the following treatment arms: (A) docetaxel 70 mg/m2 every 3 weeks, estramustine 280 mg orally 3 times a day for 5 days, and prednisone 10 mg orally every day; (B) weekly docetaxel 35 mg/m2, estramustine 280 mg orally 3 times a day on days 1 to 3, and prednisone 10 mg orally every day; and (C) mitoxantrone27 2 mg/m2 combined with prednisone. A total of 120 patients were distributed over the 3 arms of the study. An improved PSA decline rate, measurable response rate, improved pain control rate, and improved performance status and survival were observed in patients treated with docetaxel/estramustine/prednisone either every 3 weeks or every week when compared with patients treated with mitoxantrone and prednisone. Nausea, diarrhea, and deep venous thrombosis were seen more commonly in the estramustine/docetaxel/prednisone arms than in the mitoxantrone/prednisone arms. Of note, the rates of deep venous thrombosis did not appear to be reduced when compared with those in previously reported phase II studies, despite the fact that the investigators administered warfarin 2 mg orally every day prophylactically. This study is too small to draw any significant conclusions regarding survival; the pattern of toxicity appears to be similar to that reported in previous studies of estramustine/docetaxel/prednisone and mitoxantrone/prednisone.
The Contribution of Dexamethasone to Response
Corticosteroid treatment can improve quality of life and induce PSA declines in men with prostate cancer. To determine the contribution of dexamethasone to the estramustine/docetaxel regimen, 12 patients were given single-agent dexamethasone 20 mg orally every 6 hours for 3 doses repeated every 3 weeks before starting cytotoxic treatment with estramustine and docetaxel.28 PSA levels were measured weekly, and if a patient had two consecutive rises, he was considered to have progressive disease on dexamethasone. None of the patients initially treated with single-agent dexamethasone had a 50% or greater decline in PSA level. With subsequent treatment with estramustine and docetaxel, 11 patients (92%) had a PSA decline of 50% or higher, and 7 patients (58%) had a PSA decline of 80% or higher. Three of four patients with bidimensionally measurable disease had a partial response. The conclusion from this trial was that the contribution of dexamethasone to the response rate of estramustine/docetaxel was no more than 20%.
Phase III Randomized Trials: Docetaxel-Based Therapy
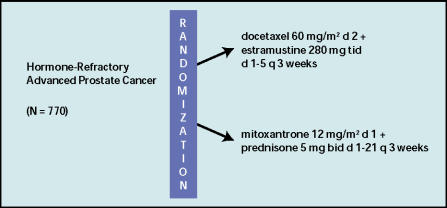
Two Phase III trials will determine whether docetaxel-based therapy should replace mitoxantrone-based therapy as standard treatment for metastatic HRPC. The results from both trials should be available in early 2004. The Southwest Oncology Group (SWOG) is leading study 99-16, comparing a 5-day course of estramustine 280 mg orally 3 times a day combined with docetaxel 60 mg/m2 to continuous prednisone and mitoxantrone 12 mg/m2 (Figure 1). Dose escalation is permitted to 70 mg/m2 and 14 mg/m2 for docetaxel and mitoxantrone, respectively. The trial was closed to accrual in January 2003, and the final analysis is due in February 2004. A total of 770 patients have been entered over a 3-year period; the trial is powered to detect a 33% difference in survival between the two treatment arms. Other outcomes to be measured include quality of life, response in measurable disease, and improvement in bone pain.
Figure 1.
Schema for Southwest Oncology Group study 99-16: a multicenter, randomized phase III study of docetaxel + estramustine versus mitoxantrone + prednisone in patients with hormone-refractory prostate cancer.
TAX 327, an international study, accrued 1004 patients. The three arms of this trial include the standard arm (mitoxantrone combined with prednisone) and two experimental arms (docetaxel combined with prednisone at varying doses). The dosing regimen for each arm is shown in Figure 2. The primary endpoint is similar to that of SWOG 99–16; the trial is designed to detect a 33% difference in survival. Unfortunately, neither SWOG 99-16 nor TAX 327 will answer the question of estramustine’s contribution to taxane-based therapy.
Figure 2.
Schema for international TAX 327 trial.
Conclusion
A trend towards improved survival has been observed in several phase I and II trials of estramustine combined with docetaxel in HRPC. However, the results of small phase II trials can be misleading, given the heterogeneity of disease in men with metastatic HRPC. The results of SWOG 99-16 and TAX 327 are crucial to the development of future treatment regimens and will define the standard of care. Newer-generation regimens, such as the combination of docetaxel with calcitriol, appear promising; however, the treatment regimens from these preliminary trials need to be compared in large randomized trials to the best regimen from either TAX 327 or SWOG 99-16.
Main Points.
For patients with hormone-refractory prostate cancer (HRPC), mitoxantrone combined with prednisone is considered the palliative standard of care; however, mitoxantrone combined with either prednisone or hydrocortisone has not demonstrated a survival advantage over single-agent prednisone or hydrocortisone.
There are data suggesting that combining estramustine with vinblastine or with an antitubulin compound, such as a taxane, contributes to survival.
In several phase II studies of docetaxel combined with estramustine, prostate-specific antigen (PSA) response rates ranged from 45% to 74% and response in measurable disease ranged from 11% to 57%.
A phase II trial demonstrated an improved PSA decline rate, measurable response rate, improved pain control rate, and improved performance status and survival in patients treated with a docetaxel/estramustine/prednisone combination either every 3 weeks or every week when compared with patients treated with mitoxantrone and prednisone.
Results are expected soon from two phase III trials that will determine whether docetaxel-based therapy should replace mitoxantrone-based therapy as the standard treatment for metastatic HRPC.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993;71:1098–1109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative endpoints. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 5.Berry W, Dakhil S, Modiano M, et al. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J Urol. 2002;168:2439–2443. doi: 10.1016/S0022-5347(05)64163-8. [DOI] [PubMed] [Google Scholar]
- 6.Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 7.Perry CM, McTavish D. Estramustine phosphate sodium. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer. Drugs Ageing. 1995;7:49–74. doi: 10.2165/00002512-199507010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Einhorn L, Ross E, et al. Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormone-refractory prostate cancer: a Hoosier Oncology Group and Fox Chase Network phase III trial. J Clin Oncol. 1999;17:3160–3166. doi: 10.1200/JCO.1999.17.10.3160. [DOI] [PubMed] [Google Scholar]
- 9.Hudes G, Ross E, Roth B, et al. Improved survival for patients with hormone-refractory prostate cancer receiving stramustine-based antimicrotubule therapy: final report of a Hoosier Oncology Group and Fox Chase Network phase III trial comparing vinblastine and vinblastine plus oral estramustine phosphate. Proc Am Soc Clin Oncol. 2002 doi: 10.1200/JCO.1999.17.10.3160. Abstract 704. [DOI] [PubMed] [Google Scholar]
- 10.Roth BJ, Yeap BY, Wilding G, et al. Taxol in advanced, hormone-refractory carcinoma of the prostate. Cancer. 1993;72:2457–2460. doi: 10.1002/1097-0142(19931015)72:8<2457::aid-cncr2820720825>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi C, Redman B, Flaherty LE, et al. Weekly 1-hour infusion of paclitaxel. Clinical feasibility and efficacy in patients with hormone-refractory prostate carcinoma. Cancer. 2000;89:431–436. doi: 10.1002/1097-0142(20000715)89:2<431::aid-cncr31>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Hudes GR, Nathan F, Khater C, et al. Phase II trial of 96-hour paclitaxel plus oral estramustine phosphate in metastatic hormone-refractory prostate cancer. J Clin Oncol. 1997;15:3156–3163. doi: 10.1200/JCO.1997.15.9.3156. [DOI] [PubMed] [Google Scholar]
- 13.Kelly WK, Curley T, Slovin S, et al. Paclitaxel, estramustine, phosphate, and carboplatin in patients with advanced prostate cancer. 2001;19(1):44–53. doi: 10.1200/JCO.2001.19.1.44. [DOI] [PubMed] [Google Scholar]
- 14.Smith DC, Esper P, Strawderman M, et al. Phase II trial of oral estramustine, oral etoposide, and intravenous paclitaxel in hormone-refractory prostate cancer. J Clin Oncol. 1999;17(6):1664–1671. doi: 10.1200/JCO.1999.17.6.1664. [DOI] [PubMed] [Google Scholar]
- 15.Haas N, Roth B, Garay C, et al. Phase I trial of weekly paclitaxel plus oral estramustine phosphate in patients with hormone-refractory prostate cancer. Urology. 2001;58:59–64. doi: 10.1016/s0090-4295(01)01011-1. [DOI] [PubMed] [Google Scholar]
- 16.Thanasiadis A, Tsavdaridis D, Athanassiades I, et al. Paclitaxel (P) and estramustine phosphate (EP) in patients with hormone refractory prostate cancer (HRPC) — a phase II study [Abstract 755] Proc Am Soc Clin Oncol. 2001;20:189a. [Google Scholar]
- 17.Hudes GR, Manola J, Conroy J, et al. Phase II study of weekly paclitaxel (P) by 1-hour infusion plus reduced-dose oral estramustine (EMP) in metastatic hormone-refractory prostate carcinoma (HRPC): a trial of the Eastern cooperative Oncology Group [Abstract 697] Proc Am Soc Clin Oncol. 2001;20:175a. [Google Scholar]
- 18.Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol. 1999;26(5) Suppl 17:14–18. [PubMed] [Google Scholar]
- 19.Berry W, Dakhil S, Gregurich MA, Asmar L. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;4(suppl 15):8–15. doi: 10.1016/s0093-7754(01)90149-6. [DOI] [PubMed] [Google Scholar]
- 20.Friedland D, Cohen J, Miller R, et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of Bcl-2. Semin Oncol. 1999;26(suppl):19–23. [PubMed] [Google Scholar]
- 21.Gravis G, Bladou F, Salem N, et al. Efficacy, quality of life (QOL) and tolerance with weekly docetaxel (D) in metastatic hormone refractory prostate cancer (HRPC) patients [Abstract 2433] Proc Am Soc Clin Oncol. 2001;20:171b. [Google Scholar]
- 22.Petrylak DP, Macarthur RB, O’Connor J, et al. Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol. 1999;17:958–967. doi: 10.1200/JCO.1999.17.3.958. [DOI] [PubMed] [Google Scholar]
- 23.Kreis W, Budman DR, Fetten J, et al. Phase I trial of the combination of daily estramustine phosphate and intermittent docetaxel in patients with metastatic hormone refractory prostate carcinoma. Ann Oncol. 1999;10:33–38. doi: 10.1023/a:1008354600497. [DOI] [PubMed] [Google Scholar]
- 24.Savarese DM, Halabi S, Hars V, et al. Phase II study of docetaxel, estramustine, and low dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. J Clin Oncol. 2001;19:2509–2516. doi: 10.1200/JCO.2001.19.9.2509. [DOI] [PubMed] [Google Scholar]
- 25.Kosty MP, Ferreira A, Bryntesen T. Weekly docetaxel and low-dose estramustine phosphate in hormone refractory prostate cancer: a phase II study [Abstract 2360] Proc Am Soc Clin Oncol. 2001;20:152b. [Google Scholar]
- 26.Sinibaldi VJ, Carducci MA, Moore-Cooper S, et al. Phase II evaluation of docetaxel plus oneday oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer. 2002;94(5):1457–1465. doi: 10.1002/cncr.10350. [DOI] [PubMed] [Google Scholar]
- 27.Oudard S, Banu E, Voog E, et al. Results of a phase II randomized trial of docetaxel (D), estramustine (E) and prednisone (P)—two schedules—versus mitoxantrone (M) and prednisone (P) in patients (pts) with hormone-refractory prostate cancer (HRPC). Poster presented at Congress of the European Association of Urology; March 13–15, 2003; Madrid. Abstract #746. [Google Scholar]
- 28.Weitzman AL, Shelton G, Zuech N, et al. Dexamethasone does not significantly contribute to the response rate of docetaxel and estramustine in androgen independent prostate cancer. J Urol. 2000;163:834–837. [PubMed] [Google Scholar]
- 29.Natale RB, Zaretsky SL. Phase I/II trial of estramustine (E) and Taxotere (T) in patients with metastatic hormone-refractory prostate cancer (HRPC) [Abstract 1343] Proc Am Soc Clin Oncol. 1999;18:348a. [Google Scholar]
- 30.Beer TM, Pierce WC, Lowe BA, Henner WD. Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol. 2001;12:1273–1279. doi: 10.1023/a:1012258723075. [DOI] [PubMed] [Google Scholar]