Abstract
The overall structure of the DNase I hypersensitive sites (HSs) that comprise the β-globin locus control region (LCR) is highly conserved among mammals, implying that the HSs have conserved functions. However, it is not well understood how the LCR HSs, either individually or collectively, activate transcription. We analyzed the interactions of HS2, HS3 and HS4 with the human ε- and β-globin genes in chromatinized episomes in fetal/embryonic K562 cells. Only HS2 activates transcription of the ε-globin gene, while all three HSs activate the β-globin gene. HS3 stimulates the β-globin gene constitutively, but HS2 and HS4 transactivation requires expression of the transcription factor EKLF, which is not present in K562 cells but is required for β-globin expression in vivo. To begin addressing how the individual HSs may interact with one another in a complex, we linked the β-globin gene to both the HS2 and HS3. HS2 and HS3 together resulted in synergistic stimulation of β-globin transcription. Unexpectedly, mutated, inactive forms of HS2 impeded the activation of the β-globin gene by HS3. Thus, there appear to be distinct interactions among the HSs and between the HSs and the globin genes. These preferential, non-exclusive interactions may underlie an important structural and functional cooperativity among the regulatory sequences of the β-globin locus in vivo.
INTRODUCTION
The five members (5′-ε, Aγ, Gγ, δ, β-3′) of the human β-globin gene family are expressed sequentially during development (1), and depend for their high level expression on the locus control region (LCR), a complex, 15 kb regulatory element 10–60 kb upstream of the globin structural genes (2). The LCR encompasses four highly conserved (3) subdomains, HS1–HS4, which were originally identified as DNase I hypersensitive sites (HSs) (4,5). The LCR core sequences and the globin gene promoters share motifs for a small number of transcription factors (1). These include GATA-1 motifs, Maf recognition elements (MAREs, recognized by NF-E2 and other factors) and CACCC motifs [recognized by Kruppel-like proteins such as erythroid Kruppel-like factor (EKLF)] (6–8).
Despite considerable effort, it remains unclear how the LCR HSs, either individually or collectively, activate transcription (9). When individual HSs are linked to the globin genes in transgenic mice, they appear to have distinct globin gene targets (10). However, there has been less consistency in studies when individual HSs have been deleted and/or substituted for one another in the context of the complete human locus introduced into transgenic mice. In some cases, core HS deletion preferentially reduced the activity of particular globin genes (11,12). Other investigators found that deletion of HS2, HS3 or HS4 core sequences individually drastically lowered transcription of all the genes (13,14) and lessened the ability of the other non-mutated sites to form their characteristic DNase I-sensitive structures (14,15). The latter observations are consistent with the idea that the HSs act as a single synergistic entity, which has been termed the holocomplex (16). Further highlighting the complexity of LCR function, larger deletions encompassing individual HS cores with some flanking sequence were only modestly deleterious to transcription of the globin genes in either human transgenic loci in mice or in the mouse locus itself (17–20), and the remaining sites were able to form (21). These observations suggest an additive interaction, with considerable functional redundancy among the sites.
We have undertaken a reassembly approach to try to understand how the LCR functions. We have selected 400–600 bp LCR HSs sequences, which subsume the majority of LCR activity, encompass the characterized transcription activator sites shared by the LCR HSs and globin promoters, and are capable of forming their characteristic HS structure in chromatin (reviewed in 1). To avoid potential integration position effects while still analyzing LCR function in the context of chromatin in vivo, we have employed nuclear episomes. These minichromosomes are assembled into physiological chromatin and are maintained extrachromosomally at moderate copy number in human embryonic/fetal erythroid K562 cells. In earlier work, we studied interactions between a 4 kb human embryonic ε-globin gene and the human HS2 core sequence in minichromosomes. These and other studies provided evidence that a distant enhancer and gene promoter mutually affect each other’s chromatin conformation (22–24), suggesting they participate in a shared structure. Our aim in the present studies was to investigate whether this property is shared by other globin gene promoters and among the HSs themselves (e.g. in a holocomplex), as would be expected if they interact in chromatin, whether additively or synergistically.
Expression of the adult β-globin gene in animals requires the erythroid transcription factor EKLF (25,26), and work from several laboratories has implicated EKLF in HS3 function and hypersensitivity (27–30). K562 cells, which have an embryonic/fetal phenotype, express neither EKLF nor the adult β-globin mRNA (31), and HS3 sequences in the natural K562 β-globin locus are very resistant to DNase I digestion (32; A.Dean, unpublished results). These observations suggest that EKLF might be required for adult β-globin transcription and HS3 function in K562 cells. Therefore, we created K562 cell lines in which EKLF was stably expressed and also identified conditions for highly efficient expression of EKLF in transiently transfected cells.
We found that HS2, HS3 and HS4 interact differently with the ε- and β-globin genes and differently from one another with the same gene. HS2 transactivated both the ε- and β-globin genes although activation of the β-globin gene required expression of the adult erythroid transcription factor EKLF (25,26). HS3 failed to activate the ε-globin gene but strongly activated the β-globin gene in an EKLF-independent manner. HS4 also failed to activate the ε-globin gene and stimulated β-globin transcription weakly even in the presence of EKLF. To begin studying the interactions among the various HSs, we inserted both HS2 and HS3 in their natural order upstream of the β-globin gene. HS2 and HS3 synergistically stimulated β-globin transcription in the presence of EKLF. Interestingly, linking HS3 to HS2 constructs altered its chromatin structure, and linking HS3 to HS2 mutants with impaired transactivation ability reduced transcription to levels lower than those observed with HS3 alone. These results suggest that the linked HS sites interact with one another in the context of the β-globin gene.
MATERIALS AND METHODS
Plasmids
Plasmids were constructed using conventional techniques (33). Minichromosomes were constructed in p220.2 (34) or a variant (see below). p220.2 contains the Epstein–Barr virus (EBV) origin of replication, a transcription unit for EBNA-1 which is required for replication, and a hygromycin resistance gene which permits selection and maintenance of the plasmid in human cells. To facilitate cloning of β-globin sequences into p220.2, NaeI and NotI restriction sites were introduced into the polylinker of p220.2 creating p220Nae/Not by site-directed mutagenesis (QuickChange kit; Stratagene). Stable expression vectors for the transcription factor EKLF were assembled in a variant of p220Nae/Not, p220neo, which carries a neomycin resistance marker from pMC1neopolyA (Stratagene) instead of the hygromycin resistance gene.
We studied the β-globin LCR core HS sequences that span the characterized transcription activator sites of each: HS4, SacI–SspI (GenBank coordinates 954–1338); HS3, PstI–AvaII (GenBank coordinates 4348–4942); HS2, HindIII–XbaI (GenBank coordinates 8486–8860) (3). A 3.7 kb EcoRI ε-globin genomic fragment (GenBank coordinates 17482– 21233) and a 4.5 kb β-globin genomic PstI fragment (GenBank coordinates 59855–64302) were used. GenBank coordinates refer to locus NG_000007 (25 April 2001 version). The –87 G→C thalassemia transversion (31) was introduced into the β-globin promoter in pBSβ using the QuickChange kit (Stratagene), verified by sequencing and used to create the mutant form of the gene in minichromosomes. Construction of wild-type and mutant HS2 ε-globin minichromosomes has been described (35). HS2 variants containing mutations in the NF-E2 and CACCC transcription factor binding sites (24) were linked to the β-globin gene.
Mouse EKLF expression vectors were created by transferring EcoRI–BamHI segments of pSG5 EKLF (wild-type) and pSG5 EKLFΔPro [DNA-binding domain (DBD) only deletion mutant] (8,36) into pCI (Promega). To create EKLF-expressing minichromosomes, the BglII–ClaI portion of pCI EKLF and of pCI EKLFΔPro were cloned into p220neo. Noteworthy differences between the mouse and human forms of EKLF are not apparent (37).
RNA protection assays
RNA was isolated from K562 cell clones using the PureScript kit (Gentra Systems). Probes were synthesized and radiolabeled using the MaxiScript system (Ambion) and RNA protection assays were performed using RPA II or RPA III kits (Ambion). The ε-globin probe protects a 149 nt band from endogenous K562 ε-globin transcripts and a novel 135 nt band from minichromosomal transcripts (35). Templates for the synthesis of radiolabeled probes that recognize either the 5′ or the 3′ sequences of mouse EKLF were constructed in pBS from pSG5 EKLF. The 5′ 225 nt probe protects a fragment of 160 nt. A 154 nt band is also observed presumably due to an A/T-rich region at the 3′ end of the EKLF sequence. The 445 nt 3′ probe protects a 401 nt portion of the EKLF message. Radiolabeled β-globin probes were synthesized from the genomic pBSβΔBam template created by deleting the 3′ portion of the β-globin gene from an internal BamHI site at +481 to the downstream BamHI site in the pBS polylinker. The 603 nt long probe protects a 114 nt fragment of the β-globin message corresponding to the first exon and a 204 nt fragment spanning most of the second exon. The 229 nt short probe protects a 189 nt segment of the second exon. Human poly(A)+ bone marrow RNA was obtained from Stratagene for use as a β-globin RNA positive control.
Transfections and cell culture
MEL cells were maintained in DMEM with 10% fetal bovine serum and 100 mM glutamine. K562 cells were propagated in RPMI 1640 with 10% fetal bovine serum, 100 mM glutamine and 200 µg/ml hygromycin (Roche) and/or 200 µg/ml G418 (Invitrogen/Life Technologies) as appropriate. K562 cells were collected by centrifugation, washed twice in a large volume of RPMI 1640 with 10% fetal bovine serum and suspended in this medium at 2–3 × 106 cells/300 µl on ice. The cells were electroporated (38) in the presence of 10 µg of plasmid DNA to create minichromosomal cell lines. EKLF expression minichromosomes were super-transfected into clonal cell lines already carrying globin minichromosomes without difficulty. The copy number, between 10 and 20 copies/cell, and integrity of minichromosomes in clonal cell lines were analyzed by Southern blotting. Within this moderate copy number range, copy number effects were not evident. Electroporation conditions for transient transfections were the same as above. Unless otherwise noted in the figure legends, clonal minichromosome-containing cells were transfected with 25 µg of EKLF expression plasmid or empty vector, with pBS added to 60 µg of total DNA. Approximately 80% of cells were transfected with pEGFP (Stratagene) under these conditions, and dose–response experiments demonstrated that β-globin transcription had reached a plateau.
Nuclear extract preparation and western blotting
Nuclear extracts were prepared (39) from 1–2 × 107 cells. Total protein yield was typically 2–3 µg/µl in a total volume of 100 µl of buffer D. EKLF expression was analyzed by SDS– PAGE and western blotting using a Novex Mini-Cell apparatus according to the manufacturer’s recommendations. Samples of 30 µg of protein were fractionated in 8% resolving gels. The quantity and quality of extracts was confirmed by examining a Coomassie Blue-stained gel run in parallel. EKLF in blotted nuclear extracts was detected using an anti-EKLF polyclonal antibody and the Enhanced Chemiluminescence system (ECL, Amersham-Pharmacia).
Restriction enzyme accessibility
Nuclei of K562 cells carrying various minichromosomes were prepared (35) and aliquots (2–5 × 107 nuclei) were incubated with 100 U of restriction enzyme for 30 min at 37°C, conditions previously determined to obtain maximum digestion of chromatin. After purification, the DNA (20 µg/sample) was cut to completion with a second restriction enzyme, purified, separated on 1% agarose gels and transferred to nylon membranes by Southern blotting. The membranes were hybridized to the 32P-labeled DNA probes described in the text and figure legends, and the results quantitated using a PhosphorImager (Molecular Dynamics) and ImageQuant software. The percent digested was calculated by quantitating the intensity of the cut/(cut + uncut) signals.
RESULTS
We previously showed that the chromatin structures of the human ε-globin gene promoter and a linked HS2 core sequence were interdependent using minichromosomes stably maintained in K562 cells. To ask whether such a structural and functional interdependence is a general feature of HS–globin gene promoter interaction and/or whether there are preferential interactions between particular HSs and promoters, we constructed minichromosomes containing 4 kb genomic fragments, from ∼2 kb upstream to ∼2 kb downstream of the transcription start site, from the human ε- and β-globin genes with and without HS2, HS3 and HS4 core sequences. The inserted globin sequences are schematically depicted in Figure 1.
Figure 1.
Structures of the β-globin and ε-globin genes studied on minichromosomes. The various HS cores (HS2, 374 bp; HS3, 594 bp; HS4, 384 bp; see Materials and Methods) of the β-globin LCR were inserted 2–2.7 kb upstream of the globin transcription start sites in the natural genomic orientation. The black line and rectangles represent globin gene sequences and the gray line represents vector sequences.
Expression of EKLF at high levels in transfected minichromosome cell lines
Because K562 cells do not express EKLF, which is required for the expression of the adult β-globin gene from an intact β-globin locus in animals (25,26), we anticipated that forced expression of EKLF would be required for expression of minichromosomal β-globin genes, and possibly for the function of HS3 (27–30). We employed both stable and transient vectors to express EKLF in K562 cells. To construct the vector for stable EKLF expression, we replaced the hygromycin resistance cassette in the minichromosome backbone with a neomycin resistance gene and introduced an EKLF or control transcription unit into this neoR minichromosome. Hence, it was possible to select simultaneously for the globin- and EKLF-expressing minichromosomes.
We devised transient transfection conditions in which up to 80% of electroporated K562 cells express green fluorescent protein (Fig. 2A) and used these conditions to transfect K562 cells carrying globin minichromosomes with an expression vector containing an EKLF expression cassette. Figure 2B presents a western blot of nuclear extracts from similar numbers of transiently (lane 2) and stably (lanes 3 and 4) transfected K562 cells probed with anti-EKLF. As positive controls, we assayed recombinant six histidine tagged EKLF expressed in bacteria (lane 7) and nuclear extract prepared from MEL cells (lane 5), which normally express EKLF and the endogenous adult β-globin gene. Transfected K562 cells express abundant EKLF compared with MEL cells. The pattern and migration of EKLF bands from transfected K562 cells and from MEL cells was identical, suggesting that the protein was similarly post-translationally modified in both cell types (40). Recombinant EKLF containing a six histidine tag migrates more slowly as expected.
Figure 2.

EKLF is expressed at high levels in transfected cells and stable minichromosome cell lines. (A) Fluorescence microscopy of K562 cells transfected with pEGFP. At 36 h after transfection up to 80% of cells expressed GFP under the conditions employed. (B) Western blot of protein extracts (30 µg) from K562 cells transiently transfected with pSG5EKLF and from K562 cell lines stably expressing EKLF from minichromosomes. After PAGE and transfer to a nylon membrane, the blot was probed with anti-EKLF polyclonal antibodies and proteins detected by ECL. Lane 1, K562 cells transfected with pSG5 empty vector; lane 2, K562 cells transfected with pSG5EKLF; lane 3, high-expressing K562 cell clone carrying an EKLF minichromosome; lane 4, low-expressing K562 cell clone carrying an EKLF minichromosome; lane 5, MEL cell extract; lane 6, K562 cell extract; lane 7, recombinant 6-His tagged EKLF (D.A.Jackson and A.Dean, unpublished results). Mr, molecular weight markers.
Effects of EKLF and HS2, HS3 and HS4 on ε-globin gene transcription
HS2 sequences situated 2 kb upstream of the ε-globin gene transcription start site in a minichromosome stimulated transcription of the gene more than 100-fold (35). To ask whether HS3 and HS4 could also stimulate ε-globin transcription, we inserted the HS3 and HS4 core sequences (Fig. 1) into the same position in the minichromosome and created clonal cell lines carrying HS3ε or HS4ε along with either a control or EKLF expression minichromosome.
RNA protection was used to determine the abundance of ε-globin and EKLF RNA. ε-Globin transcripts from the minichromosomes are marked by a mutation in the 5′ UTR such that RNase digestion produces shorter protected fragments from them than from transcripts of the endogenous genes. The results for several clones carrying HS3ε (Fig. 3A) or HS4ε (Fig. 3B) and either an EKLF or a control expression minichromosome (vector) indicate that neither HS3 nor HS4 stimulates transcription of the ε-globin gene even when EKLF RNA is expressed at a high level compared with MEL cells (see Fig. 2B). ε-Globin transcription from the endogenous locus exhibits clone to clone variability (24,35) but is inde pendent of EKLF expression (Fig. 3A and B, endogenous). Others (28,31,41) have observed that EKLF can transactivate transiently transfected reporter genes in K562 cells as well as transfected minichromosomes containing the β-globin gene (see below). Therefore, we conclude that HS3 and HS4 are not able to interact functionally with the ε-globin gene in the context of K562 cells and that overexpression of EKLF alone in the K562 cellular milieu is not sufficient to mediate interaction. Overall, the lack of an EKLF effect on ε-globin transcription is consistent with observations made in EKLF knockout mice (25,26).
Figure 3.
Effects of EKLF and HS2, HS3 and HS4 on ε-globin gene transcription. Independent K562 cell clones were established which carried two minichromosomes, either hygR HS3ε or HS4ε, plus neoR EKLF or empty vector minichromosomes. (A) RNA was isolated from HS3ε clones and assayed by RNA protection separately for ε-globin and EKLF RNA. Representative RNA protection results are shown. Actin RNA served as the load control. M, marker DNA ladder in nucleotides. (B) RNA protection results for representative HS4ε clones as described in (A). (C) Column graph of ε-globin expression by the HS3ε and HS4ε clones in the presence (black bars) and absence (gray bars) of EKLF. Means of clones ± SEM are shown (n = 4–8 clones with data determined 3–5 separate times for each clone). The results are compared to transcription by HS2ε clones and clones containing the ε-globin gene (ε) unlinked to a LCR HS site (24).
Effects of EKLF and HS2, HS3 and HS4 on β-globin gene transcription
Endogenous β-globin RNA is undetectable by RNA protection analysis in K562 cells, and overexpression of EKLF does not stimulate transcription from the chromosomal β-globin gene (Fig. 4A, lanes 20–21) (31). Similarly, there was only trace signal from the β-globin gene on minichromosomes in K562 cells, which was not altered by transiently transfected EKLF (β, Fig. 4A, lanes 1–4, and B). To ask whether LCR core sequences affected transcription from the β-globin minichromosomes, we analyzed clonal cell lines carrying the β-globin gene linked to HS2, HS3 or HS4. We also tested the effect of EKLF expression on β-globin transcription. RNA isolated from the transfected cells was analyzed by RNA protection for the abundance of β-globin and EKLF RNAs. We studied three or four clones for each construct, and Figure 4A depicts the results for two different clones per construct. Multiple experiments are graphically summarized in Figure 4B.
Figure 4.
Effects of EKLF and HS2, HS3 and HS4 on β-globin gene transcription. K562 cells and K562 clones carrying a β-globin gene on a minichromosome were transiently transfected with pCI EKLF using conditions (see Fig. 2) which resulted in greater than 80% of cells expressing a test reporter gene. (A) RNA was isolated and RNA protection experiments were carried out separately to measure β-globin (upper panel) and EKLF RNA (lower panel). Actin RNA served as the load control. Representative results for two clones (A and B) for each construct are presented. –, transfection with empty vector; +, transfection with pCI EKLF. Lanes 1–4, β minichromosome cell lines (no HS site); lanes 5–8, HS2β minichromosome cell lines; lanes 9–12, HS2β minichromosome cell lines with the promoter –87 thalassemia mutation; lanes 13 and 14, untransfected HS3β minichromosome cell lines (transfected HS3β cell lines are not shown on this gel); lanes 15–18, HS4β minichromosome cell line; lane 19, MEL cell RNA control; lanes 20 and 21, parent K562 cells. Controls fo β-globin RNA include HS3β (lane 22) and human bone marrow RNA (lane 23). M, DNA markers in nucleotides. (B) Column graph depicting the abundance of β-globin RNA (± SEM) measured by RNA protection in K562 cells carrying various minichromosomes, either transiently transfected with empty vector (gray bars) or with pCI EKLF (black bars). β-Globin RNA is normalized for loading and minichromosome copy number.
Linking the β-globin gene to HS2 renders the gene responsive to EKLF, with a 4.4-fold increase in β-globin RNA (Fig. 4A, lanes 5–8, and B). To confirm that EKLF was acting through its binding site at the proximal CACCC box in the β-globin promoter (42), we introduced a C→G transversion at position –87 to recreate a naturally occurring mutation known to result in β-thalassemia (31). The HS2β clones with the –87 mutation HS2β(CACm) failed to respond to EKLF and displayed a level of transcriptional activity similar to that of the wild-type gene with no enhancer (Fig. 4A, lanes 9–12, and B).
HS3 increased β-globin expression about 10-fold over the level of the β-globin gene alone, in striking contrast to the results seen with HS3 and the ε-globin gene (Fig. 4A, compare lanes 13–14 with lanes 1–4, and B). HS3 activates the β-globin gene in an EKLF-independent manner (Fig. 4B) although other workers have found that HS3 function requires EKLF (see Discussion). Unlike HS2 and HS3, HS4 exerts a marginal effect at most on β-globin gene expression, even in the presence of EKLF (HS4β, Fig. 4A, lanes 15–18, and B).
Transcriptional activation by EKLF requires the EKLF transactivation domain
A number of in vivo and in vitro approaches have demonstrated that the EKLF transactivation domain is required for stimulation of the β-globin promoter through the recruitment of chromatin remodeling complexes and probably other accessory factors (8,37,41,43,44). To explore the role of EKLF in HS2β transactivation, we transfected a HS2β cell line with increasing amounts of expression plasmids for full-length EKLF, the EKLF DBD alone, or an empty vector. Prior work indicates that the stability of wild-type and deleted forms of EKLF is similar (8,41). In contrast to the intact EKLF polypeptide, the EKLF DBD fails to stimulate transcription from HS2β even at high levels of expression (Fig. 5A and B). These observations indicate that the EKLF transactivation domain is required for transcriptional activation of the β-globin gene on minichromosomes, and suggest that EKLF also functions in this system by recruiting accessory factors to the β-globin promoter (8,44).
Figure 5.
EKLF stimulation of β-globin expression requires the EKLF transactivation domain. An HS2β cell clone was transfected with pCI EKLF, an expression plasmid for full-length EKLF, with pCI EKLFΔPro, which expresses the EKLF DBD, or pCI empty vector. (A) RNA was isolated and β-globin RNA and EKLF RNA were measured by RNA protection. Actin RNA served as the loading control. Transfection mixtures contained 3, 5 or 9 µg of EKLF or EKLF DBD expression plasmid plus pCI to a total of 10 µg. Empty, 10 µg of empty vector; control, human bone marrow RNA. (B) The column graph depicts fold induction of β-globin RNA normalized to actin RNA (black bars) and abundance of EKLF or EKLF DBD RNA normalized to actin (gray bars). M, marker DNA in nucleotides.
The ε- and β-globin genes confer different structures on HS3
HS2 linked to the ε-globin gene was highly accessible to nucleases, and was required to remodel the chromatin structure of the ε-globin gene promoter to the transcriptionally active state (24). Since active genes and regulatory elements are typically found in open, nuclease-sensitive regions of chromatin, we wished to investigate the structures of ε- and β-globin genes linked to the various HSs and the structures of the HSs themselves. We also investigated whether the expression of EKLF in K562 cells could influence the structure of HS2 or HS3 because there is evidence implicating ELKF in HS3 formation (27,28) and chromatin remodeling (37,44).
Nuclei were isolated from cell lines carrying HS3ε or HS4ε minichromosomes and, as controls, ε-globin unlinked to a HS, linked to HS2, or linked to a mutated form of HS2 (NFm) which does not form a HS and fails to activate the ε-globin gene because the essential NF-E2 sites in HS2 have been destroyed by clustered point mutations (24). The nuclei were digested with AvaII or MscI and accessibility at the promoters and the HSs was analyzed by Southern blotting and indirect end labeling. Representative experiments are depicted in Figure 6A and the results of multiple experiments presented graphically in Figure 6C. Commensurate with the transcription results of Figure 3, restriction enzyme accessibility of the ε-globin promoter was not altered by HS3. Further, HS3 was inaccessible when linked to the ε-globin gene, with MscI cutting of 2%, comparable to the HS2 NF-E2 mutant (9%), and in contrast to over 60% accessibility for wild-type actively transcribing εHS2. Expression of EKLF from a second minichromosome did not affect this result (not shown). Interestingly, HS4 and the linked ε-globin gene promoter were accessible to restriction enzyme cutting, even though HS4 did not activate transcription from the gene (see Fig. 3B).
Figure 6.
The chromatin structure of HS3 depends on the linked globin gene. Nuclei of cells carrying various minichromosomes were prepared, digested with the appropriate restriction enzyme cleaving within either the promoter or enhancer, and processed as described in Materials and Methods. (A) Representative results obtained with ε-globin clones with no enhancer, or linked to HS2, HS3 or HS4, or to HS2 with mutated NF-E2 sites (NFm). To analyze the promoters, nuclei were incubated with AvaII and DNA cleaved secondarily with BglII. To analyze enhancers, nuclei were incubated with MscI and DNA cleaved secondarily with EcoRV. MscI cleaves within both HS2 and HS3 (see Fig. 7A). Blots were hybridized to an XbaI–EcoRV probe. (B) Representative results obtained with β-globin clones with no enhancer or linked to HS2 or HS3, and carrying a second EKLF-expressing minichromosome, or an empty vector. To analyze the promoters, nuclei were incubated with MscI and cleaved secondarily with EcoRI. To analyze enhancers, nuclei were incubated with MscI and cleaved secondarily with XcmI plus SphI. Blots were hybridized to a PmeI–SphI probe. (C) The mean percent accessibility of the promoters and enhancers (determined three times on 2–5 clones) is presented graphically ± SEM for the ε-globin (black bars) and β-globin (gray bars) clones studied.
In parallel experiments, we tested accessibility of the β-globin gene unlinked to a HS, or linked to HS2 or HS3 in cell lines in which a second minichromosome carried either a control or an EKLF-expressing cassette. Representative experiments are depicted in Figure 6B and the results of multiple experiments presented graphically in Figure 6C. HS2 and HS3 displayed similar levels of MscI accessibility of ∼40% when linked to the β-globin gene and accessibility was not affected by the expression of EKLF. HS4β does not contain a suitable restriction site with which to measure accessibility, but DNase I digestion revealed that HS4 does form when linked to the β-globin gene (not shown). MscI also cuts just 5′ to the proximal CACCC box, and was used to probe β-globin promoter structure. The β-globin promoter was accessible to MscI whether or not it was linked to an HS and whether or not it was transcriptionally active. Several conclusions can be drawn from these results. First, a globin promoter can be in a relatively open chromatin conformation and not be transcriptionally active. Second, the accessibility and structure of HS3 is strongly influenced by the gene to which it is linked. Third, although EKLF is required for activation of β-globin transcription by HS2 in minichromosomes, in this assay it does not affect the structure of HS2 or of the promoter. In addition, we find no evidence that either the structure or activity of HS3 is affected by EKLF expression in K562 cells.
Structural and functional interactions between HS2 and HS3
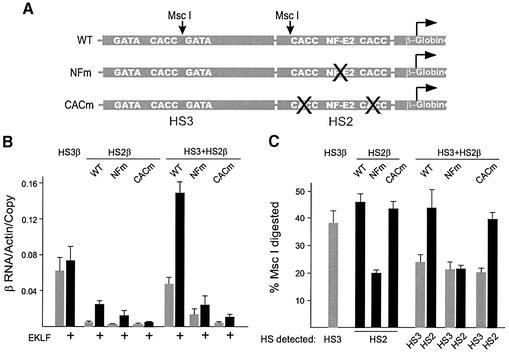
To begin addressing whether the LCR HSs interact with each other in chromatin, we inserted both HS2 and HS3 upstream of the β-globin gene (HS3+HS2β) in minichromosomes. To control for promoter competition and potential spacing effects on HS3 activity due to interposing HS2 between HS3 and the β-globin gene, we also created HS3+HS2β minichromosomes in which the HS2 tandem NF-E2 sites, or both CACCC sites, had been destroyed by clustered point mutations. These mutations markedly impair HS2 function when linked to the ε-globin gene (24,35); they also strongly adversely affect HS2 function when positioned upstream of the β-globin gene (see below). Figure 7A schematically depicts the HS3+HS2 constructs including the positions of transcription factor binding sites of interest and the MscI restriction sites.
Figure 7.
HS2 and HS3 structurally and functionally interact to stimulate β-globin transcription. (A) Depiction of MscI sites with respect to transcription factor motifs within HS2 and HS3 (not drawn to scale). The × indicates which motifs in HS2 were mutated by clustered point mutations. (B) Graphical representation of the results of RNA protection assays performed on RNA from clones of HS2β, HS3β and HS3+HS2β as described in the legend to Figure 3. The clones co-expressed EKLF from a second minichromosome (black bars) or had a vector-only minichromosome (gray bars). (C) Graphical representation of analysis of the chromatin structure of HS2 and HS3 when alone or linked in minichromosomes. Restriction enzyme accessibility at the MscI sites present in both HS2 (black bars) and HS3 (gray bars) was determined as described in the legend to Figure 6.
In the absence of EKLF, HS3+HS2β transcription differs little from that of HS3β either in the presence or absence of EKLF (Fig. 7B). However, HS3 and HS2 together resulted in a synergistic stimulation of β-globin transcription in the presence of EKLF. The sum of HS3β and HS2β RNA/actin/copy number individually is 0.096 compared with 0.143 for the HS3+HS2β minichromosome. These observations suggest that HS3 and HS2 interact directly or indirectly to stimulate transcription of the β-globin gene.
Before assaying the activity of constructs containing mutated HS2 core elements joined to HS3, we first confirmed that the clustered point mutations in the NF-E2 and CACCC sites of HS2 impaired HS2 function when linked to the β-globin gene as they do when linked to ε-globin. Even in the presence of EKLF, the NF-E2 mutation reduced the transcriptional activity of the HS2 β-globin construct by ∼50%, and the CACCC mutation essentially eliminated HS2 stimulation of β-globin transcription (Fig. 7B). Strikingly, when either mutation is present in a HS3+HS2β construct, transcription of the β-globin promoter is reduced below the level seen with HS3 alone, to ∼30% of HS3β for the NF-E2 mutant and to less than 15% for the CACCC mutant. These data further support the ideas that HS2 and HS3 interact functionally in the context of the β-globin promoter and that a mutant HS2 can dominantly interfere with the ability of HS3 to activate the β-globin gene.
Since we observed structural interdependencies between the HS2 and HS3 core elements and the β- and ε-globin promoters, we next asked whether we could also observe structural interdependencies between juxtaposed HS2 and HS3 core elements. Hence, we assayed MscI accessibility at HS2 and HS3 linked to the β-globin gene, either alone or in combination (Fig. 7C). MscI accessibility at HS3 in HS3+HS2β is substantially altered compared with the accessibility of HS3 alone. The change in accessibility does not require wild-type HS2 transactivation function (compare HS3 accessibility in the wild-type HS3+HS2 construct with its accessibility in either the NF-E2 or the CACCC mutant HS3+HS2 construct) and occurs even when the structure of HS2 itself is altered by the NF-E2 mutation. These observations are consistent with the conclusion that HS2 and HS3 interact structurally.
DISCUSSION
The question of how the globin genes are regulated either individually or collectively by the LCR HSs remains open. We have begun experiments in replicating minichromosomes to examine the interactions between the β-globin LCR HSs and different globin promoters using a reassembly approach. Our experiments indicate that the structure and function of HSs are dependent on the other regulatory elements they interact with, including the other HSs and globin gene promoters. We propose that the basis of this variability is structural and that the LCR HSs and globin promoters form a fluid transcriptional complex whose overall conformation depends on the specific components and transcription factors present. Recent, novel in vivo experiments provide direct evidence of preferential and close interaction between particular LCR HSs, in particular HS2, HS3 and HS4, and the murine β-globin Hbb-b1 and Hbb-b2 genes (45). Given that the sequence of the individual HSs and organization of the LCR as a whole are highly conserved within and across species in comparison with the globin promoters (3), this model focuses attention on the individual globin genes as key regulatory sites for stage-specific developmental regulation.
Individual LCR HSs, EKLF and the globin genes
Our experiments indicate preferential interdependencies in structure and function between the ε- and β-globin genes and the HSs we tested in the K562 embryonic/fetal milieu. For example, our previous studies of HS2 linked to the embryonic ε-globin gene on minichromosomes indicated that HS2 had a highly accessible chromatin structure and transactivated the ε-globin gene over 100-fold (35). In contrast, and consistent with experiments using stably integrated constructs in K562 cells (3), neither HS3 nor HS4 stimulated transcription from the ε-globin gene in episomes. We found that HS3 was highly resistant to restriction enzyme cleavage when linked to the ε-globin gene, similar to its nuclease-resistant state in the chromosomal locus in K562 cells (32,46). Others have reported that HS3 function is dependent on EKLF (23,28). The DNase I sensitivity of HS3 was also found to be diminished following genetic ablation of EKLF in mice (27). However, we found that EKLF overexpression alone was insufficient to alter HS3 chromatin structure either on minichromosomes or at the endogenous locus. The reason that EKLF alone does not restore HS3 structure in K562 cells may reflect differences in the transcription factor milieu between K562 cells and hematopoietic cells. Possibly, an ancillary factor(s) is missing in K562 cells relevant to formation of HS3, and possibly to interaction between HS3 and the ε-globin gene. Interestingly, HS4 does form both at the chromosomal locus and on minichromosomes when linked to the ε-globin gene (not shown), but it does not activate transcription of the gene in our experiments. This suggests a component relevant to transcription activation by HS4 is deficient or lacking in K562 cells. Work by others using transgenic mice suggests that HS4 function may require the regulatory environment of definitive erythroid cells (10,12).
HS2, HS3 and HS4 all formed nuclease-accessible structures when linked to the β-globin gene. Transactivation of the β-globin gene by HS2 was EKLF dependent. Both the β-globin promoter CACCC box and the EKLF activation domain are required for this activation. However, transactivation did not reflect an alteration of HS2 chromatin structure mediated by EKLF. Therefore, EKLF may stabilize promoter–HS2 interactions or may participate in recruiting basal transcription factors required for β-globin activation (44). HS3 does not appear to be a target for EKLF in K562 cells (regardless of whether it is linked to the ε- or β-globin gene), consistent with previous work (30). We found that the strong transactivation of the β-globin gene by HS3 was EKLF independent, while, similarly to HS2, its chromatin structure was unaltered by EKLF. Interestingly, HS3 was relatively sensitive to restriction enzyme cleavage when linked to the β-globin gene in contrast to its structure in the endogenous K562 chromosome and when linked to the ε-globin gene. Perhaps the closer linkage of the β-globin gene and HSs on minichromosomes (2 kb in contrast to 60 kb) influences the structure of HS3. Once HS3 structure is formed, it may no longer need EKLF to interact with the β-globin gene.
The differential interactions we observed between the LCR HSs and the β- and ε-globin genes in K562 cells contrast with HS deletion studies in the mouse chromosomal globin locus where preferential interactions between particular HSs and globin promoters are not obvious (reviewed in 47). However, we suggest that preferential but non-exclusive interactions between the HSs and globin promoters and among the HSs may become apparent when the HSs are tested individually rather than in the context of the globin locus. In the intact LCR, preferential interactions could be masked by complementation among the linked HSs. The differences between promoter–HS interactions may be most easily understood in terms of an interaction model in which the particular arrays of transcription activators within the HSs and promoters create complementary structures that mediate preferential crosstalk between these elements (24,48). Possible candidate molecules for effecting such communication include the erythroid factors GATA-1, EKLF and NF-E2 (7,8,49).
Interactions among the LCR HSs
Since other laboratories have reported synergistic transcriptional interactions between HSs and the β- and γ-globin promoters (50–54), we linked HS3 and HS2 to the β-globin gene in minichromosomes. We observed modest synergistic stimulation of β-globin transcription by HS3+HS2 in the presence of forced expression of EKLF. Moreover, when we replaced the wild-type HS2 in the HS3+HS2β minichromosome with either of two different HS2 elements containing clustered point mutations that impair the ability of HS2 to transactivate the β- and ε-globin promoters, the level of transcription from the HS3+HS2β-globin promoter fell below the level seen with HS3 alone even though HS3 by itself stimulates transcription more strongly than HS2. The dominant interference of HS2 in activation by HS3 supports the idea that HS3 and HS2 interact with one another functionally. This interaction apparently does not require NF-E2 or the CACCC sites in HS2.
We also observed, somewhat surprisingly, that linking HS3 to either a wild-type or a mutated HS2 element decreased the accessibility of HS3 to MscI digestion in isolated nuclei. We infer from this reproducible alteration in accessibility that a structural change in HS3 has occurred, although from this data we cannot determine the nature of the alteration: for example, the association of additional factors with the complex might occlude the site, or structural interaction with HS2 might reorganize HS3 moving the MscI site into the interior of the complex. We cannot exclude the possibility that the reduction of HS3 accessibility simply results from the steric effect of juxtaposing HS2 to HS3 rather than from alterations in the structure of HS3; however, Lee et al. (30) observed that HS2 facilitated the recruitment of EKLF to a linked HS3, which also suggests that structural communication between the two elements occurs. It will be important to determine whether such interactions also occur with physiological spacing between the cores, inclusion of sequences flanking them, and/or a chromosomal context.
Promoter–HS communication
In this work we provide further evidence of communication between HSs and globin promoters since the extent of restriction enzyme accessibility of HS3 varies markedly depending on whether it is linked to the ε- or β-globin gene. These data extend our previous observation that mutations in the promoter of the ε-globin gene alter the structure of a linked HS2 element (24). Thus, there also appear to be structural interdependencies between HSs and different globin promoters.
Although the different HSs clearly have some ability to complement each other functionally in the context of the intact LCR (17–20), they also appear to display preferential interactions with the different β-like globin genes that may be important for correct developmental regulation. Our studies were conducted in the fetal/embryonic milieu of K562 cells, but at other stages of development the interactions we observed are likely to be modulated. A comparison of our results with those obtained in an environment where the β-globin gene is actively transcribed, such as murine MEL cells, would be desirable. However, EBV episomes become chromosomally integrated in rodent cells, requiring an entirely new approach, perhaps using BPV episomes, to such experiments. In our studies, the ability of HS2 to alter the structure of a linked HS3 and of a defective HS2 to impair the transactivating potential of a linked HS3 indicate that HS2, HS3 and the β-globin promoter interact with one another structurally and functionally, perhaps forming a transcription enhancing complex including HSs and a target promoter which would then recruit chromatin remodeling and modifying complexes, perhaps as part of the transcription apparatus, to the promoter. Conservation of the structure of the LCR would then be expected to reflect important structural interactions not only among the HSs themselves, but also between the HSs and the globin promoters.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr James Bieker for kindly providing EKLF expression vectors and antibodies, and Drs Jane Little, Cecelia Trainor and James Bieker for critical reading of the manuscript.
REFERENCES
- 1.Stamatoyannopoulos G. and Grosveld,F. (2001) Hemoglobin switching. In Stamatoyannopoulos,G., Majerus,P.W., Perlmutter,R.M. and Varmus,H. (eds), The Molecular Basis of Blood Diseases. W.B. Saunders, Philadelphia, PA, pp. 135–182.
- 2.Reik A., Telling,A., Zitnik,G., Cimbora,D., Epner,E. and Groudine,M. (1998) The locus control region is necessary for gene expression in the human β-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol. Cell. Biol., 18, 5992–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardison R., Slightom,J.L., Gumucio,D.L., Goodman,M., Stojanovic,N. and Miller,W. (1997) Locus control regions of mammalian β-globin gene clusters: combining phylogenetic analyses and experimental results to gain functional insights. Gene, 205, 73–94. [DOI] [PubMed] [Google Scholar]
- 4.Forrester W.C., Thompson,C., Elder,J.T. and Groudine,M. (1986) A developmentally stable chromatin structure in the human β-globin gene cluster. Proc. Natl Acad. Sci. USA, 83, 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuan D., Solomon,W., Li,Q. and London,I.M. (1985) The “beta-like-globin” gene domain in human erythroid cells. Proc. Natl Acad. Sci. USA, 82, 6384–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson P.D., Evans,T., Nickol,J.M. and Felsenfeld,G. (1989) Developmental modulation of protein binding to β-globin gene regulatory sites within chicken erythrocyte nuclei. Genes Dev., 3, 1860–1873. [DOI] [PubMed] [Google Scholar]
- 7.Andrews N.C., Erdjument-Bromage,H., Davidson,M.B., Tempst,P. and Orkin,S.H. (1993) Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature, 362, 722–728. [DOI] [PubMed] [Google Scholar]
- 8.Bieker J.J. and Southwood,C.M. (1995) The erythroid Kruppel-like factor transactivation domain is a critical component for cell-specific inducibility of a β-globin promoter. Mol. Cell. Biol., 15, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levings P.P. and Bungert,J. (2002) The human beta-globin locus control region. Eur. J. Biochem., 269, 1589–1599. [DOI] [PubMed] [Google Scholar]
- 10.Fraser P., Pruzina,S., Antoniou,M. and Grosveld,F. (1993) Each hypersensitive site of the human β-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev., 7, 106–113. [DOI] [PubMed] [Google Scholar]
- 11.Navas P.A., Peterson,K.R., Li,Q., Skarpidi,E., Rohde,A., Shaw,S.E., Clegg,C.H., Asano,H. and Stamatoyannopoulos,G. (1998) Developmental specificity of the interaction between the locus control region and embryonic or fetal globin genes in transgenic mice with an HS3 core deletion. Mol. Cell. Biol., 18, 4188–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navas P.A., Peterson,K.R., Li,Q., McArthur,M. and Stamatoyannopoulos,G. (2001) The 5′HS4 core element of the human β-globin locus control region is required for high-level globin gene expression in definitive but not in primitive erythropoiesis. J. Mol. Biol., 312, 17–26. [DOI] [PubMed] [Google Scholar]
- 13.Bungert J., Dave,U., Lim,K.C., Lieuw,K.H., Shavit,J.A., Liu,Q. and Engel,J.D. (1995) Synergistic regulation of human β-globin gene switching by locus control region elements HS3 and HS4. Genes Dev., 9, 3083–3096. [DOI] [PubMed] [Google Scholar]
- 14.Bungert J., Tanimoto,K., Patel,S., Liu,Q., Fear,M. and Engel,J.D. (1999) Hypersensitive site 2 specifies a unique function within the human β-globin locus control region to stimulate globin gene transcription. Mol. Cell. Biol., 19, 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G., Lim,K.C., Engel,J.D. and Bungert,J. (1998) Individual LCR hypersensitive sites cooperate to generate an open chromatin domain spanning the human beta-globin locus. Genes Cells, 3, 415–429. [DOI] [PubMed] [Google Scholar]
- 16.Ellis J., Tan-Un,K.C., Harper,A., Michalovich,D., Yannoutsos,N., Philipsen,S. and Grosveld,F. (1996) A dominant chromatin-opening activity in 5′ hypersensitive site 3 of the human β-globin locus control region. EMBO J., 15, 562–568. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiering S., Epner,E., Robinson,K., Zhuang,Y., Telling,A., Hu,M., Martin,D.I.K., Enver,T., Ley,T.J. and Groudine,M. (1995) Targeted deletion of 5′HS2 of the murine β-globin LCR reveals that it is not essential for proper regulation of the β-globin locus. Genes Dev., 9, 2203–2213. [DOI] [PubMed] [Google Scholar]
- 18.Hug B.A., Wesselschmidt,R.L., Fiering,S., Bender,M.A., Epner,E., Groudine,M. and Ley,T.J. (1996) Analysis of mice containing a targeted deletion of β-globin locus control region 5′ hypersensitive site 3. Mol. Cell. Biol., 16, 2906–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson K.R., Clegg,C.H., Navas,P.A., Norton,E.J., Kimbrough,T.G. and Stamatoyannopoulos,G. (1996) Effect of deletion of 5′HS3 or 5′HS2 of the human β-globin locus control region on the developmental regulation of globin gene expression in β-globin locus yeast artificial chromosome transgenic mice. Proc. Natl Acad. Sci. USA, 93, 6605–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bender M.A., Roach,J.N., Halow,J., Close,J., Alami,R., Bouhassira,E.E., Groudine,M. and Fiering,S.N. (2001) Targeted deletion of 5′HS1 and 5′HS4 of the β-globin locus control region reveals additive activity of the DNaseI hypersensitive sites. Blood, 98, 2022–2027. [DOI] [PubMed] [Google Scholar]
- 21.Bender M.A., Mehaffey,M.G., Telling,A., Hug,B., Ley,T.J., Groudine,M. and Fiering,S. (2000) Independent formation of DnaseI hypersensitive sites in the murine β-globin locus control region. Blood, 95, 3600–3604. [PubMed] [Google Scholar]
- 22.Reitman M., Lee,E., Westfall,H. and Felsenfeld,G. (1993) An enhancer/locus control region is not sufficient to open chromatin. Mol. Cell. Biol., 13, 3990–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tewari R., Gillemans,N., Harper,A., Wijgerde,M., Zafarana,G., Drabek,D., Grosveld,F. and Philipsen,S. (1996) The human β-globin locus control region confers an early embryonic erythroid-specific expression pattern to a basic promoter driving the bacterial lacZ gene. Development, 122, 3991–3999. [DOI] [PubMed] [Google Scholar]
- 24.McDowell J.C. and Dean,A. (1999) Structural and functional cross-talk between a distant enhancer and the ε-globin gene promoter shows interdependence of the two elements in chromatin. Mol. Cell. Biol., 19, 7600–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuez B., Michalovich,D., Bygrave,A., Ploemacher,R. and Grosveld,F. (1995) Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature, 375, 316–318. [DOI] [PubMed] [Google Scholar]
- 26.Perkins A.C., Sharpe,A.H. and Orkin,S.H. (1995) Lethal β-thalassaemia in mice lacking the erythroid CACCC- transcription factor EKLF. Nature, 375, 318–322. [DOI] [PubMed] [Google Scholar]
- 27.Wijgerde M., Gribnau,J., Trimborn,T., Nuez,B., Philipsen,S., Grosveld,F. and Fraser,P. (1996) The role of EKLF in human beta-globin gene competition. Genes Dev., 10, 2894–2902. [DOI] [PubMed] [Google Scholar]
- 28.Gillemans N., Tewari,R., Lindeboom,F., Rottier,R., de Wit,T., Wijgerde,M., Grosveld,F. and Philipsen,S. (1998) Altered DNA-binding specificity mutants of EKLF and Sp1 show that EKLF is an activator of the β-globin locus control region in vivo. Genes Dev., 12, 2863–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tewari R., Gillemans,N., Wijgerde,M., Nuez,B., von Lindern,M., Grosveld,F. and Philipsen,S. (1998) Erythroid Kruppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5′HS3 of the β-globin locus control region. EMBO J., 17, 2334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.S., Lee,C.H. and Chung,J.H. (1999) The β-globin promoter is important for recruitment of erythroid Kruppel-like factor to the locus control region in erythroid cells. Proc. Natl Acad. Sci. USA, 96, 10051–10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donze D., Townes,T.M. and Bieker,J.J. (1995) Role of erythroid Kruppel-like factor in human γ- to β-globin gene switching. J. Biol. Chem., 270, 1955–1959. [DOI] [PubMed] [Google Scholar]
- 32.Dhar V., Nandi,A., Schildkraut,C.L. and Skoultchi,A.I. (1990) Erythroid-specific nuclease-hypersensitive sites flanking the human β-globin domain. Mol. Cell. Biol., 10, 4324–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Yates J.L., Warren,N. and Sugden,B. (1985) Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature, 313, 812–815. [DOI] [PubMed] [Google Scholar]
- 35.Gong Q.H., McDowell,J.C. and Dean,A. (1996) Essential role of NF-E2 in remodeling of chromatin structure and transcriptional activation of the ε-globin gene in vivo by 5′ hypersensitive site 2 of the β-globin locus control region. Mol. Cell. Biol., 16, 6055–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller I.J. and Bieker,J.J. (1993) A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol. Cell. Biol., 13, 2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown R.C., Pattison,S., van Ree,J., Coghill,E., Perkins,A., Jane,S.M. and Cunningham,J.M. (2002) Distinct domains of erythroid Kruppel-like factor modulate chromatin remodeling and transactivation at the endogenous β-globin gene promoter. Mol. Cell. Biol., 22, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong Q. and Dean,A. (1995) Enhancer dependent transcription of the human ε-globin gene on a stably maintained minichromosome. In Stamatoyannopoulos,G. (ed.), Molecular Biology of Hemoglobin Switching. Intercept, Andover, UK, pp. 279–288.
- 39.Dignam J.D., Lebowitz,R.M. and Roeder,R.G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang L., Chen,X. and Bieker,J.J. (1998) Regulation of erythroid Kruppel-like factor (EKLF) transcriptional activity by phosphorylation of a protein kinase casein kinase II site within its interaction domain. J. Biol. Chem., 273, 23019–23025. [DOI] [PubMed] [Google Scholar]
- 41.Chen X. and Bieker,J.J. (1996) Erythroid Kruppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J., 15, 5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins A. (1999) Erythroid Kruppel like factor: from fishing expedition to gourmet meal. Int. J. Biochem. Cell Biol., 31, 1175–1192. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W. and Bieker,J.J. (1998) Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl Acad. Sci. USA, 95, 9855–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong J.A., Bieker,J.J. and Emerson,B.M. (1998) A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell, 95, 93–104. [DOI] [PubMed] [Google Scholar]
- 45.Carter D., Chakalova,L., Osborne,C.S., Dai,Y. and Fraser (2002) Long-range chromatin regulatory interactions in vivo. Nature Genet., 32, 623–626. [DOI] [PubMed] [Google Scholar]
- 46.Guy L.G., Mei,Q., Perkins,A.C., Orkin,S.H. and Wall,L. (1998) Erythroid Kruppel-like factor is essential for β-globin gene expression even in absence of gene competition, but is not sufficient to induce the switch from γ-globin to β-globin gene expression. Blood, 91, 2259–2263. [PubMed] [Google Scholar]
- 47.Bulger M. and Groudine,M. (1999) Looping versus linking: toward a model for long-distance gene activation. Genes Dev., 13, 2465–2477. [DOI] [PubMed] [Google Scholar]
- 48.Huber M.C., Jagle,U., Kruger,G. and Bonifer,C. (1997) The developmental activation of the chicken lysozyme locus in transgenic mice requires the interaction of a subset of enhancer elements with the promoter. Nucleic Acids Res., 25, 2992–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans T. and Felsenfeld,G. (1989) The erythroid-specific transcription factor Eryf1: a new finger protein. Cell, 58, 877–885. [DOI] [PubMed] [Google Scholar]
- 50.Jackson J.D., Miller,W. and Hardison,R.C. (1996) Sequences within and flanking hypersensitive sites 3 and 2 of the β-globin locus control region required for synergistic versus additive interaction with the ε-globin gene promoter. Nucleic Acids Res., 24, 4327–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson J.D., Petrykowska,H., Philipsen,S., Miller,W. and Hardison,R. (1996) Role of DNA sequences outside the cores of DNase hypersensitive sites (HSs) in functions of the β-globin locus control region. Domain opening and synergism between HS2 and HS3. J. Biol. Chem., 271, 11871–11878. [DOI] [PubMed] [Google Scholar]
- 52.Bresnick E.H. and Tze,L. (1997) Synergism between hypersensitive sites confers long-range gene activation by the β-globin locus control region. Proc. Natl Acad. Sci. USA, 94, 4566–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sargent T.G., DuBois,C.C., Buller,A.M. and Lloyd,J.A. (1999) The roles of 5′-HS2, 5′-HS3, and the γ-globin TATA, CACCC, and stage selector elements in suppression of β-globin expression in early development. J. Biol. Chem., 274, 11229–11236. [DOI] [PubMed] [Google Scholar]
- 54.Molete J.M., Petrykowska,H., Bouhassira,E.E., Feng,Y.Q., Miller,W. and Hardison,R.C. (2001) Sequences flanking hypersensitive sites of the β-globin locus control region are required for synergistic enhancement. Mol. Cell. Biol., 21, 2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]