Abstract
Finasteride, a 5-α-reductase inhibitor, dramatically suppresses the production of dihydrotestosterone in men; thus, attention has turned to this agent for the treatment of benign prostatic hyperplasia (BPH). A number of randomized clinical trials have studied finasteride’s effects on prostate size, BPH symptoms, flow rate, and prostate-specific antigen (PSA) level. Although the decrease in symptoms with finasteride therapy has been modest compared with more invasive treatments, its use has resulted in sustained reductions in prostatic volume and PSA level with minimal adverse effects. Fewer surgeries for BPH, as well as a decreased incidence of acute urinary retention, have also been seen with finasteride therapy. More research is needed to maximize the effectiveness of such medical therapy for BPH.
Key words: Finasteride, Dihydrotestosterone, Benign prostatic hyperplasia, Acute urinary retention
The impetus for the development of a 5-α-reductase inhibitor to reduce dihydrotestosterone (DHT) production stemmed from an “experiment in nature,” whereby men with congenital 5-α-reductase deficiency were found to have the absence of a palpable prostate.1,2 A later study in a cohort of men from New Guinea with 5-α-reductase deficiency revealed undetectable prostate-specific antigen (PSA) levels, only a rudimentary central prostatic zone on transrectal ultrasound, and no identifiable prostatic epithelial glandular tissue on biopsy.3 Moreover, studies showed that intervention with luteinizing hormone-releasing hormone agonists can reduce prostate size by approximately 25% to 30%, even in men with established prostatic enlargement.4,5 In addition, interference with DHT function utilizing a number of compounds was shown to alter prostatic glandular cell formation and function in dogs.6 Although there are species differences in prostatic 5-α-reductase among dogs, rats, and humans, the stage was set for the use of a 5-α-reductase inhibitor in humans.
Early Studies of Finasteride
Early studies of finasteride in healthy men showed a dramatic decrease in circulating DHT without clinical side effects or biochemical changes.7 Subsequently, McConnell and colleagues8 found similar reductions in DHT in men with benign prostatic hyperplasia (BPH) who received finasteride at dosages ranging from 1 mg/d to 100 mg/d. To further investigate the effects of finasteride, the Finasteride Study Group was formed. The group conducted 6- and 12-month randomized, double-blind studies of finasteride, 5 mg/d, in an open protocol of 67 men with BPH. A number of important observations were made. First, the mean decrease in DHT level (approximately 80%) was sustained over a 12-month period. Second, there was no significant change in circulating testosterone levels and minimal side effects. Third, the decrease in prostate volume, approximately 20%, was also maintained throughout the year. Finally, a modest increase in maximum urinary flow rate, 4 mL/s, was demonstrated and symptoms were improved.9
The results of this 1-year study were confirmed by a large US and international cooperative study, in which both 1-mg and 5-mg daily doses of finasteride were evaluated.10 The reduction in symptoms, as measured by Boyarsky symptom score, was 2.7 points in the 5-mg group; the 1-mg group did not have a significant reduction. Accordingly, the 5-mg dose was chosen for subsequent open extension and additional study. The reduction in symptoms was related to severity of baseline symptoms: patients with the lowest baseline symptom scores did not show a significant response (Figure 1). This result is compatible with the American Urological Association (AUA) Guidelines, which recommend only lifestyle modification and observation in patients with an AUA symptom score of less than 7.11
Figure 1.
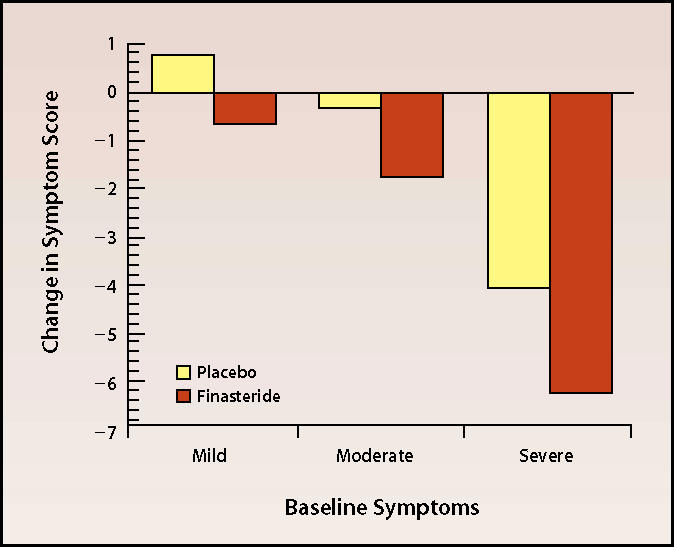
Reduction in symptoms, as measured by Boyarsky symptom score. Adapted, with permission, from Gormley GJ et al. N Engl J Med. 1992; 327:1185–1191.10 ©1992 Massachusetts Medical Society. All rights reserved.
In 1999, the North American Finasteride Study Group and coworkers reported the results of an open-label extension study of finasteride, 5 mg, in 186 patients.12 At 5 years, prostate volume had decreased 22.7% from baseline; maximum flow rate had increased by 2.3 mL/s; and the quasi-AUA symptom score had decreased by 4.3 points. Of interest, there was no increase in the prevalence of adverse sexual effects, which was 10.1% at year 5.
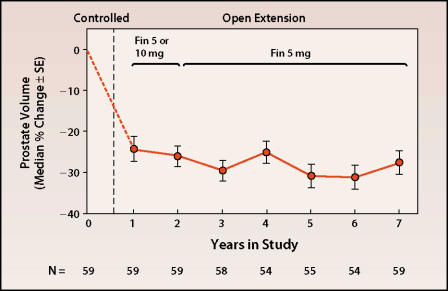
More recently, the long-term (7- to 8-year) results have been reported for more than 70 patients who were included in the early studies.13 Remarkably, all parameters remained similar to those originally reported. This group of patients had sustained decreases in DHT level (80%), PSA level (54%), and prostate volume (28%) (Figure 2) and an increase in flow rate of 2.5 mL/s.
Figure 2.
Change from baseline in prostate volume over time with finasteride treatment. Adapted, with permission, from Vaughan ED et al. Urology. 2002;60:1040–1044.13
Taken together, these studies show that the initial effects of finasteride are sustained without tachyphylaxis and with minimal side effects. The modest change in symptoms suggests the need for combined treatment strategies. In addition, the 28% reduction in prostate volume strongly suggests that other factors responsible for the maintenance of prostatic integrity in the setting of an 80% reduction in DHT need to be elucidated.
Finasteride and the Natural History of BPH
Unfortunately, during the era of surgical management of BPH, little information was collected concerning the natural history of this condition. It was known that about 20% of men with BPH would eventually undergo a surgical procedure.14 However, little was known about the increase in prostate size, change in symptoms or reduction in quality of life caused by BPH, or the incidence of acute urinary retention (AUR). The development of medical management and the need for randomized clinical trials provided the opportunity to learn more about the natural history of BPH and, more important, to determine whether medical intervention could impact this natural history.
Thus, a 4-year investigation was conducted in more than 3000 men with BPH. Subjects were randomized to receive placebo or finasteride, 5 mg/d, and the occurrence of outcome events was reported every 4 months. The most important findings were a 55% reduction in surgery for BPH and a 57% reduction in the development of AUR in the subjects who received finasteride. Mean decrease in symptom score was 3.3 in the finasteride group and 1.3 in the placebo group (P < .001); the patients who received finasteride also had significant improvement in urinary flow rates and reduced prostatic volume (P < .001).15
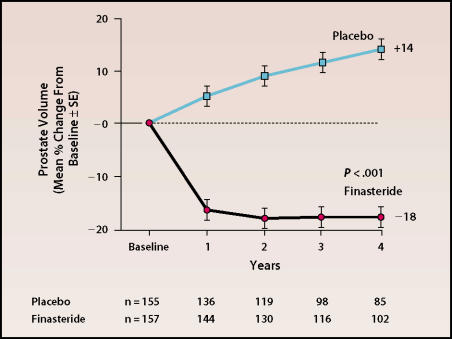
Moreover, for the first time, the increase in prostate size in patients not receiving treatment was documented over a 4-year period. Among men who completed the study, the overall increase in prostate size in the placebo group was 14%, whereas the finasteride group had an 18% decrease in prostate volume (Figure 3). Although the decrease in prostate size with finasteride treatment did not continue throughout the study period, neither did prostate size begin to increase. This was the first study to report a decrease in the incidence of AUR and the need for surgery in a large group of patients over time.
Figure 3.
Change in prostate volume over time in patients receiving finasteride or placebo. Adapted, with permission, from McConnell JD et al. N Engl J Med. 1998;338:557–563.B15 ©1998 Massachusetts Medical Society. All rights reserved.
Finasteride and Hematuria
Puchner and colleagues16 were the first to observe that finasteride reduced bleeding of prostatic origin. These early observations were confirmed with additional controlled studies involving larger numbers of patients. Finasteride is now used in patients with idiopathic prostatic bleeding, bleeding during anticoagulation, or bleeding after instrumentation.17
These clinical observations led to the interesting hypothesis that an additional action of finasteride is the inhibition of vascular endothelial growth factor (VEGF) in the prostate,17 leading to atrophy and programmed cell death. The implications of this theory give further credence to the hypothesis that 5-α-reductase inhibition may be a viable chemopreventive strategy for prostate cancer. It is known that prostate cancer is associated with increased microvascular density, which may be influenced by VEGF inhibition.
Limitations of 5-α-Reductase Therapy
Medical management of BPH with 5-α-reductase inhibitors results in a sustained beneficial effect, reducing not only symptoms but also the incidence of AUR and the need for surgical intervention. In addition, 5-α-reductase inhibitors have been shown to stabilize prostate size and alter the natural history of BPH. However, compared with more invasive therapies, the effect on symptom score and flow rate is modest. Incomplete involution of the prostate is obvious from pathologic studies, the 50% reduction of PSA level, and the approximate 20% reduction in prostate size.
The reason for lack of a more dramatic response remains unclear and may be attributed to several factors (Table 1). It is known that the pathologic appearance of BPH occurs in men in their thirties. Therefore, intervention trials to date may have been initiated too late to mimic the findings in men with 5-α-reductase deficiency. In addition, because most patients do not undergo formal urodynamic studies before the initiation of medical treatment, it is likely that studies have included patients with lower urinary tract symptoms who do not have bladder outlet obstruction.
Table 1.
Possible Factors Influencing 5-α-Reductase Therapy
|
LUTS, lower urinary tract symptoms; BOO, bladder outlet obstruction.
Furthermore, although data show that prostate size is an important variable and that patients with prostates larger than 40 g respond better to 5-α-reductase therapy,18 we do not currently have the means by which to discriminate between patients with predominately glandular BPH and those with primarily stromal disease. Thus, although PSA level may be used as a surrogate measurement of prostate size,19 what is needed is an index of the glandular component. Finally, precise lobar localization of 5-α-reductase in cohorts of patients who have or have not responded to 5-α-reductase therapy is needed.20
There are 2 different 5-α-reductase enzymes, and finasteride is a more potent inhibitor of type 2 than type 1.21 Although the type 2 enzyme is the predominant enzyme in prostatic basal epithelial and stromal cells in both normal prostates and BPH,22,23 the lack of type 1 enzyme inhibition may limit the effectiveness of finasteride. However, a recent clinical study with a 5-α-reductase type 1 and type 2 inhibitor showed clinical responses similar to those seen with finasteride, as well as a similar reduction in prostate size, despite a greater reduction in circulating DHT.24 Based on these results, failure to inhibit the type 1 enzyme does not appear to be a limiting factor of finasteride.
Future Strategies
The success of medical management of BPH is thus far encouraging and serves as a strong stimulus for further research (Table 2). Our understanding of the pathophysiology of BPH remains incomplete. Animal studies suggesting a role for estrogen or progesterone, as well as information about stromal-epithelial cell interaction and regulation of cell growth and programmed cell death, have not resulted in translational research leading to clinical trials.
Table 2.
Important Points for Future Research
| Physiology: |
|
| Patient selection: |
|
BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms; BOO, bladder outlet obstruction
In addition, we need better means by which to select patients appropriate for 5-α-reductase therapy, α-blockade, or new therapies (Table 2). The development of imaging techniques and the investigation of new serum markers for glandular BPH will perhaps aid in this task.
Main Points.
The Finasteride Study Group’s 1-year investigation demonstrated the key effects of the 5-α-reductase inhibitor finasteride: an 80% reduction in dihydrotestosterone level and a 20% reduction in prostate volume, both sustained throughout the year, along with a modest increase in maximum flow rate, no significant change in circulating testosterone levels, and minimal side effects.
Finasteride has been shown by subsequent studies to reduce the symptoms of benign prostatic hyperplasia (BPH) and to decrease levels of prostate-specific antigen; these effects were sustained at 7- to 8-year follow-up.
A 4-year study by McConnell and colleagues was the first to examine finasteride’s effect on the need for BPH-related surgery and the development of acute urinary retention; these outcomes were reduced by 55% and 57%, respectively.
More carefully controlled research is needed to elucidate the role of finasteride and other potential therapies for the treatment of BPH and prostate cancer.
References
- 1.Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;27:1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, Madden JD, Harrod MJ, et al. Familial incomplete male pseudohermaphroditism, type 2: decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974;291:944–949. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- 3.Imperato-McGinley J, Gautier T, Zirinsky K, et al. Prostate visualization studies in males homozygous and heterozygous with 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 119(75):1022–1026. doi: 10.1210/jcem.75.4.1400866. [DOI] [PubMed] [Google Scholar]
- 4.Peters CA, Walsh PC. The effect of nafarelin acetate, a leuteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med. 1987;317:599–604. doi: 10.1056/NEJM198709033171004. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilove JL, Levine AC, Kirschenbaum A, Droller M. Effect of a GnRH analogue (leuprolide) on benign prostatic hypertrophy. J Clin Endocrinol Metab. 1987;64:1331–1333. doi: 10.1210/jcem-64-6-1331. [DOI] [PubMed] [Google Scholar]
- 6.Brooks JR, Berman D, Garnes D, et al. Prostatic effects induced in dogs by chronic or acute oral administration of 5 alpha-reductase inhibitors. Prostate. 1986;9:65–75. doi: 10.1002/pros.2990090110. [DOI] [PubMed] [Google Scholar]
- 7.Gormley GJ, Stoner E, Rittmaster RS, et al. Effect of finasteride (MK-906) a 5 alpha-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990;70:1136–1141. doi: 10.1210/jcem-70-4-1136. [DOI] [PubMed] [Google Scholar]
- 8.McConnell JD, Wilson JD, George FW, et al. Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1992;74:505–508. doi: 10.1210/jcem.74.3.1371291. [DOI] [PubMed] [Google Scholar]
- 9.The MK-906 (Finasteride) Study Group, authors. One-year experience in the treatment of benign prostatic hyperplasia with finasteride. J Andrology. 1991;12:372–375. [PubMed] [Google Scholar]
- 10.Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 11.AUA Guideline on the Management of Benign Prostatic Hyperplasia. Baltimore: American Urological Association; 2003. AUA Practice Guidelines Committee. [Google Scholar]
- 12.Hudson PB, Boake R, Trachtenberg J, et al. Efficacy of finasteride is maintained in patients with benign prostatic hyperplasia treated for 5 years. Urology. 1999;53:690–695. doi: 10.1016/s0090-4295(98)00666-9. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan ED, Imperato-McGinley J, McConnell J, et al. Long-term (7 to 8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology. 2002;60:1040–1044. doi: 10.1016/s0090-4295(02)01971-4. [DOI] [PubMed] [Google Scholar]
- 14.Ball AJ, Feneley RC, Abrams PH. The natural history of untreated “prostatism”. Br J Urol. 1981;53:613–616. doi: 10.1111/j.1464-410x.1981.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Bruskewitz R, Walsh P. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 16.Puchner PJ, Miller MI. The effects of finasteride on hematuria associated with benign prostatic hyperplasia: a preliminary report. J Urol. 1995;154:1779–1782. [PubMed] [Google Scholar]
- 17.Kearney MC, Bingham JB, Bergland R. Clinical predictors in the use of finasteride for control of gross hematuria due to benign prostatic hyperplasia. J Urol. 2002;167:2489–2491. [PubMed] [Google Scholar]
- 18.Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomized clinical trials. Urology. 1996;48:398–405. doi: 10.1016/s0090-4295(96)00353-6. [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn CG, McConnell JD, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. J Urol. 2000;163:13–20. [PubMed] [Google Scholar]
- 20.Sherwood JB, McConnell JD, Vazquez DJ, et al. Heterogeneity of 5 alpha-reductase gene expression in benign prostatic hyperplasia. J Urol. 2003;169:575–579. doi: 10.1097/01.ju.0000051683.18898.77. [DOI] [PubMed] [Google Scholar]
- 21.Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120–125. doi: 10.1056/NEJM199401133300208. [DOI] [PubMed] [Google Scholar]
- 22.Silver RI, Wiley EL, Thigpen AE, et al. Cell type specific expression of steroid 5 alpha-reductase 2. J Urol. 1994;152:438–442. doi: 10.1016/s0022-5347(17)32758-1. [DOI] [PubMed] [Google Scholar]
- 23.Silver RI, Wiley EL, Davis DL, et al. Expression and regulation of steroid 5 alpha-reductase 2 in prostate disease. J Urol. 1994;152:433–437. doi: 10.1016/s0022-5347(17)32757-x. [DOI] [PubMed] [Google Scholar]
- 24.Boyle P, Roehrborn CG, Marks LS, et al. Early use of dutasteride arrests prostate growth, improves clinical parameters and prevents complications in men with benign prostatic hyperplasia. J Urol. 2003;169(4 suppl):477. Abstract 1789. [Google Scholar]