Abstract
The treatment of benign prostatic hyperplasia (BPH) has changed dramatically over the past 10 years. Phase 3 studies of the safety and effectiveness of α-blockers (eg, terazosin and doxazosin) and 5-α-reductase inhibitors (eg, finasteride) for the treatment of BPH began to appear in the literature in 1992. This article reviews the results of landmark studies of these agents, either separately as monotherapy or as combined therapy, for the treatment of BPH. The relationship between prostate size and lower urinary tract symptoms (LUTS) is discussed. Although prostate volume is not as strongly correlated with these symptoms as was once believed, it has been shown to be an important predictor of risk for developing acute urinary retention. α-Blockers represent an effective treatment for LUTS independent of prostate volume; the clinical benefit of finasteride for LUTS is limited primarily to men with large prostates. Finasteride decreases the risk of progression to acute urinary retention and the requirement for surgical intervention; this benefit is greatest in men with enlarged prostates.
Key words: Benign prostatic hyperplasia, Lower urinary tract symptoms, Acute urinary retention, Prostate volume, Finasteride, Terazosin, Doxazosin
The treatment of benign prostatic hyperplasia (BPH) has changed dramatically over the past 10 years. Before 1992, sporadic reports in the literature claimed that various α-blockers and agents inhibiting the actions of androgens on the prostate were effective for the treatment of BPH.1 These studies generally enrolled small numbers of subjects at single institutions and had poorly defined enrollment criteria and outcome measurements. The effectiveness of α-blockers and androgen suppression observed in these studies validated the overarching hypothesis of the day—that the pathophysiology of BPH comprised a dynamic component related to prostate smooth muscle tension and a static component related to prostate size.2 These preliminary clinical observations provided the rationale for pharmaceutical companies to embark on multicenter, randomized, placebo-controlled trials evaluating the safety and effectiveness of α-blockers and 5-α-reductase inhibitors for the treatment of BPH.
5-α-Reductase Inhibitors
Finasteride, a 5-α-reductase inhibitor, was the first hormonal agent critically evaluated for the treatment of BPH. A landmark, multicenter, placebo-controlled, phase 3 trial that examined the safety and effectiveness of finasteride for the treatment of BPH was published in The New England Journal of Medicine in 1992.3 The theoretic advantage of finasteride over previously studied hormonal agents is its ability to selectively achieve androgen suppression in the prostate by blocking the conversion of testosterone to dihydrotestosterone.4 Serum testosterone is minimally increased in response to finasteride, thereby eliminating the consequences of castrate levels of testosterone achieved by gonadotropin-releasing hormone analogues5 and the estrogenic effects associated with antiandrogens, such as gynecomastia and breast tenderness, which result from marked upregulation of testosterone and its aromatization to estrogens.6
In the landmark study, reported by Gormley and colleagues,3 895 men were randomized to receive finasteride, 1 mg or 5 mg, or a placebo, once daily for 12 months. The inclusion criteria for enrollment in the study specified an enlarged prostate, although a specific prostate volume threshold was not established. In retrospect, the study enrolled men with larger prostates compared with those of the men in subsequent α-blocker studies. Targeting men with enlarged prostates was a reasonable strategy, because one would expect limited clinical advantage from shrinking a prostate that was not enlarged. The inclusion criteria were also designed to identify men with bothersome urinary symptoms and low urinary flow rates (ie, men with bladder outlet obstruction [BOO]). The primary outcome measures were improvement in symptom score and increase in peak urinary flow rate. The primary end points were consistent with the therapeutic goals, which were to relieve lower urinary tract symptoms (LUTS) and decrease BOO arising from the enlarged prostate.
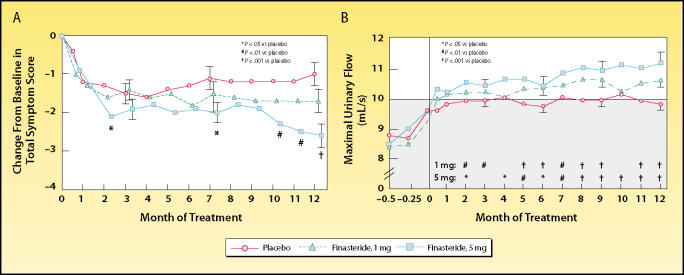
The overall treatment-related benefit of finasteride, 5 mg, relative to placebo was a 16.3% reduction in symptom score and a 14.6% increase in peak flow rate (Figure 1). The treatment-related reduction in prostate size was 16.9%. Although the study was designed to enroll men with “enlarged” prostates, LUTS, and BOO, men with minimally enlarged prostates were included. The relatively modest treatment-related effectiveness of finasteride observed in this study was likely attributable to the enrollment of these men, who had little to gain from prostate volume reduction. Finasteride was shown to be extremely well-tolerated, with rare adverse events related to sexual function.
Figure 1.
Trial results comparing placebo and 2 dosing regimens of finasteride in men with benign prostatic hyperplasia. (A) Men who received finasteride, 5 mg, had a significant decrease in symptom scores at months 2, 7, 10, 11, and 12, compared with placebo. Men who received finasteride, 1 mg, had no significant change in symptom scores. (B) At 6 and 12 months, the maximal flow rates in both finasteride-treated groups were significantly higher than baseline values and rates of the placebo group. Shaded area indicates range in which urinary flow was considered to be obstructed. Reproduced, with permission, from Gormley GJ et al. N Engl J Med. 1992;327:1185–1191.3
α-Blockers
Lepor and colleagues7 published results of the first phase 3, multicenter, randomized, double-blind, placebo-controlled study examining the safety and effectiveness of a long-acting, selective α1-blocker for the treatment of BPH. A total of 285 men were randomized to receive placebo or 2 mg, 5 mg, or 10 mg of terazosin daily. The enrollment criteria were similar to those of the phase 3 finasteride study, with the exception that no effort was made to identify a subset of men with enlarged prostates. The mean prostate volume in the terazosin study was 36.9 cm3, which is approximately 40% lower than that in the phase 3 finasteride study.
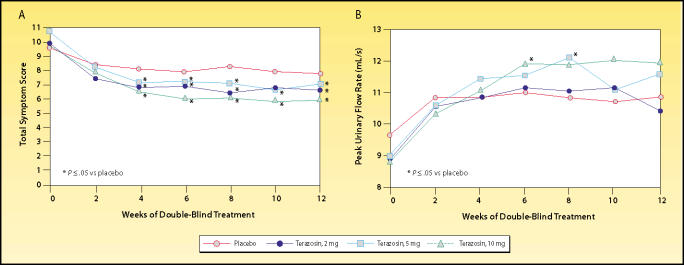
Treatment-related improvement with terazosin was shown to be dose-dependent (Figure 2). Subjects in the 10-mg terazosin group experienced a 22.4% reduction in symptom score and a 24.4% increase in peak flow rate.7 Similar treatment-related efficacy has been demonstrated for other selective α1-blockers.8,9 Terazosin was also shown to have a favorable safety profile, with dizziness and asthenia representing the most common adverse events.
Figure 2.
Trial results comparing placebo and 3 dosing regimens of terazosin in men with benign prostatic hyperplasia. The effects of terazosin on (A) American Urological Association symptom score and (B) peak urinary flow rate were found to be dose-dependent. Reproduced, with permission, from Lepor H et al. J Urol. 1992;148:1467–1474.7
A comparison of the phase 3 finasteride and terazosin data suggests that α1-blockers are more effective at relieving symptoms. Both drugs have a modest effect on improving urinary flow rates; both are well-tolerated; and finasteride is easier to administer, because of the lack of titration or potential cardiovascular effects. Because the overwhelming majority of men seek treatment for relief of LUTS, α1-blockers initially gained greater acceptance for the treatment of symptomatic BPH.
Veterans Affairs Cooperative Study 359
The Veterans Affairs (VA) Cooperative Study represented the first time an α1-blocker and a 5-α1-reductase inhibitor were directly compared and, also, the first time that the potential advantage of combination therapy was explored.10 A total of 1229 men were randomized to receive placebo, terazosin, finasteride, or a combination of terazosin and finasteride for 1 year. Men with LUTS and diminished urinary flow rates were enrolled independent of any prostate volume criteria. It is not surprising that, without an effort to identify men with enlarged prostates, the mean prostate volume was 37.3 cm3, similar to that of the phase 3 terazosin study.
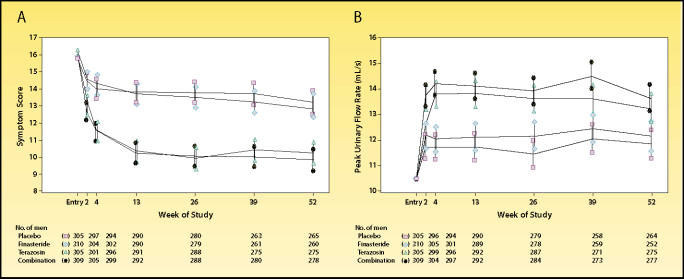
Overall treatment-related symptom improvement was 21.2%, 5.7%, and 23.5% in the terazosin, finasteride, and combination groups, respectively. Treatment-related increase in peak urinary flow rate was 12.2%, 1.6%, and 17.3% in the terazosin, finasteride, and combination groups, respectively. Overall, the treatmentrelated improvement with finasteride in this study was not significantly better than that with placebo (Figure 3). In addition, finasteride added little advantage over terazosin monotherapy for relieving symptoms or increasing peak flow rates. Both drugs were well tolerated: only 5.9%, 4.8%, and 7.8% of subjects receiving terozosin, finasteride, or combination therapy, respectively, discontinued owing to adverse events. A similar comparative trial that substituted doxazosin for terazosin has recently reported identical results.11
Figure 3.
Comparison of finasteride, terazosin, and combined dosing regimens for the treatment of benign prostatic hyperplasia. Symptom scores and flow rates are expressed as adjusted means and 95% confidence intervals. (A) American Urological Association symptom scores, according to treatment group. Symptom scores of subjects who received terazosin or combination therapy were significantly lower from baseline, as well as from those in the placebo and finasteride groups, at all follow-up visits. (B) Mean peak urinary flow rates were significantly higher in the terazosin and combination therapy groups than in the placebo and finasteride groups at all follow-up visits. Reproduced, with permission, from Lepor H et al. N Engl J Med. 1996;335:533–539.10
A subset analysis of the VA study demonstrated that the effectiveness of terazosin for both relieving symptoms and increasing flow rate was independent of prostate size.12 The treatment-related symptom improvements with finasteride in men with prostate volumes of 40 cm3 or less, 40.1 to 50.0 cm3, and greater than 50 cm3 were 0.30, 0.80, and 1.1 symptom units, respectively. Symptom improvement in men with prostate volumes of 40 cm3 or greater was similar to that in the finasteride phase 3 study.3
Collectively, the phase 3 finasteride and terazosin studies, the VA Cooperative Study, and other combination and comparative trials have demonstrated that α1-blockers are more effective than 5-α-reductase inhibitors at relieving LUTS in men with symptomatic BPH. In men with smaller prostates, finasteride has a negligible clinical benefit at relieving symptoms. In men with larger prostates, finasteride effectively relieves LUTS.
Pathophysiology of LUTS
Before the early 1990s, most experts, including myself, embraced the paradigm that an enlarged prostate causes BOO, which leads to LUTS.1,2 Therefore, pharmacologically induced reduction of prostate volume would be expected to both relieve BOO and improve symptoms. A strategy to treat symptomatic BPH by causing selective androgen suppression at the level of the prostate was ingenious.3 The problem, as we have subsequently learned, is that the paradigm is flawed.
A study by Barry and colleagues13 showed little correlation between prostate volume and the severity of LUTS (r2 = 0.008). Similarly, Girman and colleagues,14 in a cohort of men aged 40 to 80 years living in Olmsted County, Minnesota, demonstrated little correlation between the severity of LUTS and prostate volume (r2 = 0.045). Essentially, only 4.5% and 0.8% of the variability of LUTS in men aged 40 to 80 years in the general population and in men with symptomatic BPH, respectively, can be explained by prostate volume. Factors other than prostate volume are obviously responsible for LUTS in men.
The Olmsted County study did show that men with prostate volumes greater than 50 cm3 are 3 times more likely than men with volumes less than 50 cm3 to have moderate or severe LUTS, suggesting that prostate volume is not totally disassociated from LUTS.14 On the basis of these relationships, one would predict that reducing prostate volume would have a minimal effect on LUTS in men with small prostates and a modest effect in men with enlarged prostates. This is exactly what the clinical studies performed to date have demonstrated.
Proscar Long-Term Efficacy and Safety Study
The objective of the Proscar Long-Term Efficacy and Safety Study (PLESS) was to examine the longterm benefit of finasteride in men with symptomatic BPH.15 In this study, 3040 men were randomized to receive finasteride or placebo for 4 years. The PLESS trial represents the largest randomized, placebo-controlled trial of medical therapy for BPH published to date. Because the protocol was designed to enroll men with large prostates, the mean prostate volume was 54.5 cm3. This is slightly lower than that of the phase 3 finasteride study but considerably greater than those of the phase 3 terazosin study and the VA Cooperative Study.
Over 4 years, prostate volume in the placebo and finasteride groups increased 14% and decreased 18%, respectively. Prostate volume in the finasteride group reached a nadir at 1 year and remained at this level for 4 years. Symptom score and urinary flow rate in the finasteride group showed modest and progressive improvements, in contrast to those of the placebo group, which were unchanged between years 1 and 4 of the study.
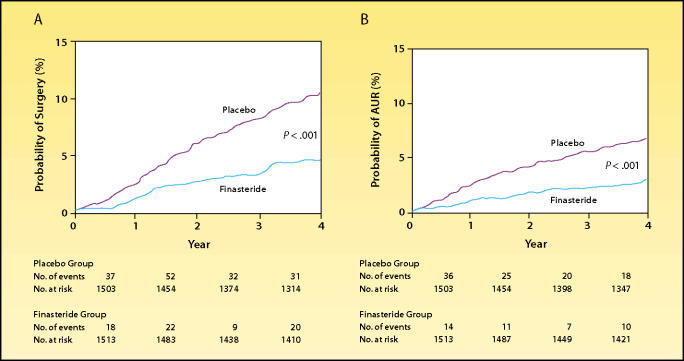
Because of the duration of the study, clinically important end points other than LUTS and urinary flow rate were analyzed. Over the 4-year study period, the finasteride group exhibited a 57% reduction in risk of episodes of acute urinary retention (AUR) and a 55% reduction in risk of progression to surgical intervention, relative to placebo (Figure 4).15 In men with very large prostates (58–150 mL), finasteride reduced the risk of AUR by 74%.16
Figure 4.
Probability of (A) surgical intervention or (B) the development of acute urinary retention (AUR) during a 4-year study of finasteride treatment in men with benign prostatic hyperplasia. Life-table analyses of the proportion of men with each outcome are shown. The numbers of events shown below the graphs are those that occurred during each 1-year interval. The numbers of men at risk are those at the beginning of the respective 1-year intervals. Differences in the rates of surgery and AUR were evident within 4 months. Reproduced, with permission, from McConnell JD et al. N Engl J Med. 1998;338:557–563.15
The PLESS trial demonstrated that finasteride prevents progression of BPH. Because of a lack of long-term, placebo-controlled trials, similar claims cannot be made for or against α1-blockers.
Pathophysiology of AUR
The factors predisposing persons to AUR are poorly understood. AUR has significant consequences, and, undoubtedly, many men at high risk for developing postvoid residual would proactively intervene to reduce this risk.
In the longitudinal Olmsted County study, prostate volume was an important predictor of risk of developing AUR.17 Men with prostate volumes greater than 30 cm3 had a 4 times greater risk of developing AUR than men with prostates smaller than 30 cm3. The PLESS trial confirmed the relationship between prostate volume and the development of AUR.16 Based on our knowledge of the pathophysiology of AUR, it is not surprising that finasteride was shown to reduce the risk of AUR and that this risk was highest in men with the largest prostates.
Medical Therapy of Prostatic Symptoms Trial
The relative effectiveness and safety of α1-blockers and 5-α-reductase inhibitors for the treatment of clinical BPH was established by the VA Cooperative Study10 and the Prospective European Doxazosin and Combination Therapy (PREDICT) trial.11 The National Institutes of Health initiated the Medical Therapy of Prostatic Symptoms (MTOPS) trial in 1992.18 The MTOPS trial is the largest and longest clinical study of medical therapy for BPH to date. Its primary objective is to address the impact of medical therapy on the clinical progression of BPH. Clinical progression was defined as an increase of the American Urological Association symptom score of at least 4 points relative to baseline, development of AUR, development of renal insufficiency, recurrent urinary retention, or socially or hygienically unacceptable urinary incontinence.19
A total of 3047 men were randomized to receive placebo, finasteride, doxazosin, or combination therapy for a mean of 4.5 years. The final results of this study are pending publication and, therefore, reference can only be made to elements of the study previously presented in abstracts (Table 1).20
Table 1.
Disease Progression in the Medical Therapy of Prostatic Symptoms Trial
| Progression Rate/100 Patient-Years (% Risk Reduction) | ||||
|---|---|---|---|---|
| Clinical | Combination | |||
| Progression | Placebo | Doxazosin | Finasteride | Therapy |
| Overall | 4.5 | 2.7 (39) | 2.9 (34) | 1.5 (67) |
| AUA symptom score | 3.6 | 1.9 (45) | 2.5 (30) | 1.3 (64) |
| AUR | 0.6 | 0.4 (35) | 0.2 (68) | 0.1 (81) |
| Invasive therapy | 1.3 | 1.3 (3) | 0.5 (64) | 0.4 (67) |
AUA, American Urological Association; AUR, acute urinary retention
The overall reductions in risk of disease progression relative to placebo in the finasteride, doxazosin, and combination groups were 39%, 34%, and 67%, respectively. Finasteride and doxazosin monotherapies were significantly superior to placebo, and combination therapy was significantly superior to the monotherapies, at reducing overall risk of progression. The reductions in risk of AUR in the finasteride, doxazosin, and combination groups were 68%, 35%, and 81%, respectively. The risk reductions for surgical intervention in the finasteride, doxazosin, and combination groups were 64%, 34%, and 67%, respectively. The risk reductions for symptom progression were 30%, 45%, and 64% for finasteride, doxazosin, and combination therapy, respectively. Subset analyses are currently under way to identify those subgroups of patients that are at greatest risk for disease progression who would benefit most from finasteride therapy.
The MTOPS study confirmed the observation from PLESS that finasteride significantly reduces the risk of progression to AUR and the need for surgical intervention. Combination therapy is the superior option to decrease overall disease progression.
Medical Therapy: What Have We Learned in 15 Years?
Both finasteride and selective, long-acting α1-blockers are well-tolerated. The adverse events associated with both drugs are few and rarely result in termination of therapy.
The majority of men initially seek medical attention to relieve bothersome LUTS. α-Blockers represent an effective therapy for LUTS, independent of prostate volume. The clinical benefit of finasteride for LUTS is limited primarily to men with large prostates. If relieving LUTS represented the only relevant clinical therapeutic objective in the treatment of BPH, α-blockers would consistently be first-line therapy, and finasteride would be reserved for symptom relief in men with large prostates.
AUR and the requirement for surgical intervention are well-known and significant consequences of BPH that men would proactively seek to avoid. PLESS and the MTOPS trial provide compelling evidence that finasteride decreases the risk of progression to AUR and the requirement for surgical intervention. This benefit is greatest in men with enlarged prostates. Therefore, men with enlarged prostates should be counseled regarding their risk of BPH progression and should be offered finasteride, both to reduce the risk of progression and to achieve symptom improvement. In men with enlarged prostates and moderate to severe LUTS, combination therapy with an α1-blocker will likely provide optimal management.
Main Points.
Finasteride, a 5-α-reductase inhibitor, was the first hormonal agent critically evaluated for the treatment of benign prostatic hyperplasia (BPH); a landmark phase 3 study published in 1992 showed a 16.3% reduction in symptom score, a 14.6% increase in peak flow rate, and a reduction in prostate size of 16.9% with finasteride, 5 mg, relative to placebo.
Another 1992 phase 3 study of the selective α1-blocker terazosin for the treatment of BPH showed dose-dependent, treatmentrelated improvements: terazosin, 10 mg, provided a 22.4% reduction in symptom score and a 24.4% increase in peak flow rate, with a favorable safety profile.
The phase 3 finasteride and terazosin studies, the Veterans Affairs Cooperative Study, and other combination and comparative trials demonstrated that, in men with large prostates, finasteride effectively relieves lower urinary tract symptoms.
The Medical Therapy of Prostatic Symptoms (MTOPS) trial showed that finasteride and doxazosin monotherapies were significantly superior to placebo, and combination therapy was significantly superior to the monotherapies, at reducing overall risk of BPH progression.
The Proscar Long-Term Efficacy and Safety Study and the MTOPS trial provide compelling evidence that finasteride decreases the risk of progression to acute urinary retention and the requirement for surgical intervention; this benefit is greatest in men with enlarged prostates.
Men with enlarged prostates should be counseled regarding their risk of BPH progression and should be offered finasteride, both to reduce the risk of disease progression and to achieve symptom improvement.
References
- 1.Lepor H. Alpha blockade in the therapy of benign prostatic hyperplasia. In: Lepor H, Lawson RK, editors. Prostate Diseases. Philadelphia: WB Saunders Company; 1993. pp. 170–181. [Google Scholar]
- 2.Lepor H. Nonoperative management of benign prostatic hyperplasia. J Urol. 1989;141:1283–1289. doi: 10.1016/s0022-5347(17)41282-1. [DOI] [PubMed] [Google Scholar]
- 3.Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 4.McConnell JD. Hormonal treatment of benign prostatic hyperplasia. Urol Clin North Am. 1995;22:387–400. [PubMed] [Google Scholar]
- 5.Eri LM, Tveter KJ. A prospective placebo-controlled study of the luteinizing hormone-releasing hormone agonist leuprolide as treatment for patients with benign prostatic hyperplasia. J Urol. 1993;150:359–364. doi: 10.1016/s0022-5347(17)35483-6. [DOI] [PubMed] [Google Scholar]
- 6.Berger BM, Naadimuthu A, Boddy A, et al. The effect of zanoterone, a steroidal androgen receptor antagonist, in men with benign prostatic hyperplasia. J Urol. 1995;154:1060–1064. [PubMed] [Google Scholar]
- 7.Lepor H, Auerbach S, Puras-Baez A, et al. A randomized multicenter placebo controlled study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148:1467–1474. doi: 10.1016/s0022-5347(17)36941-0. [DOI] [PubMed] [Google Scholar]
- 8.Fawzy A, Braun K, Lewis GP, et al. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–109. [PubMed] [Google Scholar]
- 9.Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 11.Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in the treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61:119–126. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- 12.Lepor H, Williford WO, Barry MJ, et al. The impact of medical therapy on bother due to symptoms, quality of life and global outcome, and factors predicting response. J Urol. 1998;160:1358–1367. [PubMed] [Google Scholar]
- 13.Barry MJ, Cockett ATK, Holtgrewe HL, et al. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 14.Girman CJ, Jacobsen SJ, Guess HA, et al. Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol. 1995;153:1510–1515. doi: 10.1016/s0022-5347(01)67448-2. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 16.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. Urology. 1999;53:473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 18.Bautista OM, Kusek JW, Nyberg LM, et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials. 2003;24:224–243. doi: 10.1016/s0197-2456(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 19.Kusek JW, Ahrens A, Burrows PK, et al. Recruitment for a clinical trial of drug treatment for benign prostatic hyperplasia. Urology. 2002;59:63–67. doi: 10.1016/s0090-4295(01)01454-6. [DOI] [PubMed] [Google Scholar]
- 20.McConnell JD. Benign prostatic hyperplasia (BPH): medical and hormonal therapy [abstract] J Urol. 2002;167(suppl 4):265. [Google Scholar]