Abstract
Benign prostatic hyperplasia (BPH) is the most common neoplastic condition afflicting men and constitutes a major factor impacting male health. Clinical evaluation to assess the presence and degree of voiding dysfunction and/or the role of BPH in its presence has an increasingly broad spectrum of treatment goals. The goals of the evaluation of such men are to identify the patient’s voiding or, more appropriately, urinary tract problems, both symptomatic and physiologic; to establish the etiologic role of BPH in these problems; to evaluate the necessity for and probability of success and risks of various therapeutic approaches; and to present the results of these assessments to the patient so he can make an informed decision about management recommendations and available alternatives.
Key words: Benign prostatic hyperplasia, Lower urinary tract symptoms, Clinical evaluation, Urinary flow rate
Benign prostatic hyperplasia (BPH) is the most common neoplastic condition afflicting men and constitutes a major factor impacting the health of the American male. This pathologic change is important because of the intimate anatomic relationship between the prostate and the bladder neck. The association of BPH with aging has been demonstrated repeatedly in autopsy studies using calculated or actual weight, prostate volume, or histologic criteria. Using histologic evidence, Randall1 and Harbitz and Haugen2 found the incidence of definite or probable BPH to exceed 50% in men older than 50 years. This occurrence rises to 75% as men enter their eighth decade.
From a clinical standpoint, Lytton and colleagues3 found the incidence of BPH requiring surgical intervention to increase progressively with age to a maximum of 10.9 per 1000 men older than 80 years. Berry and associates4 reviewed the major reports in the literature relating to the growth rate of human BPH with age. Their analysis implies that the growth of BPH is probably initiated in the third or fourth decade. The calculated doubling time for the weight of BPH also varies with age, being 4.5 years in men aged 30 to 50 years, 10 years in men aged 51 to 70 years, and over 100 years in those older than 70 years. Community- and practice-based studies suggest that men with lower urinary tract symptoms (LUTS) can expect slow progression of these symptoms over time.5,6 Among men with symptoms of BPH, rates of acute urinary retention requiring surgical relief range from 1% to 2% per year.6–8
Environmental and hereditary factors also influence the development of clinical BPH. The incidence of BPH is reported to be much lower in Chinese and Japanese men living in Asia than in white populations. Data on the racial background of patients subjected to prostatectomy in Hawaii also provide evidence suggesting a relatively lower incidence of BPH in Chinese and Japanese men than in white men. These and other studies support a genetic factor in the development of these lesions.9,10 Whatever its etiology, BPH greatly impacts the health of the male population of the United States. Indeed, associated bladder neck obstruction (BNO) and its sequelae are responsible for more than 160,000 operations per year.11
The prostate, the major accessory male sex gland, has an exocrine, but no established endocrine, secretory function. Its secretions provide fluid that constitutes less than 20% of the ejaculate. Aside from producing a volume-expanding vehicle for sperm, no definitive clinical function in reproduction has been identified for the prostate. The prostate, prostatic urethra, and bladder neck play critical roles in normal delivery of sperm during the reproductive act. However, the major clinical interest in the growth and function of the prostate results from the frequency with which it is the site of benign and malignant neoplasms and infection. The intimate anatomic relationship of the gland with the bladder neck and urethra increases the importance of these pathologic changes.
Clinical Evaluation
Clinical evaluation to assess the presence and degree of voiding dysfunction and/or the role of BPH in its presence has an increasingly broad spectrum of treatment goals. These include providing information on a range of epidemiologic studies, selecting patients for drug or interventional studies, and providing information and advice to individual patients. Treatment goals often play a significant role in the character and extent of a patient’s evaluation. The discussion of clinical evaluation that follows focuses on the management of the individual patient.
The goals of the clinical evaluation of men with voiding dysfunction caused by BPH are to identify the patient’s voiding or, more appropriately, urinary tract problems, both symptomatic and physiologic; to establish the etiologic role of BPH in these problems; to evaluate the necessity for and probability of success and risks of various therapeutic approaches; and to present the results of these assessments to the patient so he can make an informed decision about management recommendations and available alternatives. The clinical evaluation centers on an evaluation of symptoms, physical findings, and results of laboratory and selected imaging and endoscopic studies.
Symptoms
Most patients with medical problems caused by BPH present with symptoms of dysfunctional voiding. This symptom complex is nonspecific and is identified by a variety of terms, including the currently favored nonspecific designation LUTS; the traditional term, prostatism, which implies an established etiologic relationship; and BPH voiding dysfunction, once the etiologic relationship with the prostate warrants serious consideration.
As emphasized by Blaivas,12 the clinically recognizable bladder response to various stresses or pathologic changes may be the result of overactive (frequency, nocturia, urgency, urge incontinence) or underactive (hesitancy, intermittency, weak stream, urinary retention) detrusor activity. The symptoms may be associated with primary diseases of the bladder; neurogenic and metabolic disorders; a variety of diseases of the cardiovascular-renal system; use of pharmacologic agents, including antihistamines and antidepressants; markedly increased or abnormal fluid intake; and apparently normal aging (Table 1). A small group of patients with “silent prostatism” has severe physiologic sequelae of BPH-induced BNO, such as bladder atony or renal failure, with minimal or no voiding problems.
Table 1.
Differential Diagnosis of Benign Prostatic Hypertrophy: Additional Causes of Bladder Outlet Obstruction
|
Separation of LUTS into those related to voiding (delivery) and those related to storage seems reasonable.13 Voiding symptoms include hesitancy, delay in initiating micturition, intermittency, involuntary interruption of voiding, weak urinary stream, straining to void, sensation of incomplete emptying, and terminal dribbling. Storage symptoms include frequency (normal, every 3 or more hours; abnormal, every 2 hours or less), nocturia (awakening to void), urgency (an increasingly strong desire to void), incontinence (urge, stress, overflow, anatomic), and bladder pain (pain in pelvis without voiding) or dysuria (pain and discomfort with voiding). Of these storage symptoms, nocturia, urgency, urge incontinence, and frequency correlate with increasing age.
Jepsen and Bruskewitz13 reviewed patients’ bother from LUTS and found that nocturia was the most bothersome, and urgency the second most bothersome, urinary symptom, confirming a long-standing urologic dictum. Selectively supplementing this information with a voiding diary and knowledge of habits, such as those related to food and fluid intake, sleep patterns, and medication history, can be invaluable in directing appropriate diagnostic and therapeutic approaches in patients in whom BPH-induced voiding dysfunction is suspected.
Patients with BPH-induced bladder outlet obstruction (BOO) may present with related complications, including acute urinary retention; manifestations of chronic urinary retention, such as overflow incontinence and/or renal failure; or urinary tract infection. Acute urinary retention is signaled by the sudden onset of a persistent ineffectual urge to void and severe unremitting bladder pain. Retention may be precipitated early in the course of BPH voiding dysfunction by ingestion of decongestants containing an α-agonist, antihistamines, or a variety of medications with parasympatholytic properties. Its occurrence postoperatively is well recognized. A retention episode may also be precipitated by a forced and prolonged delay in voiding, by a precipitous increase in urinary output caused by ingestion of ethanol or diuretics and, possibly, by chilling.
Persistent or chronic urinary retention may manifest its presence by causing overflow incontinence or renal failure. Patients with a decompensated bladder caused by BPH-induced BNO may present with problems of constant dribbling (overflow) incontinence accompanied by evidence of a persistently distended urinary bladder. Similarly, laboratory or clinical manifestations of renal failure may be the initial evidence of a significant upper-tract effect of lower urinary tract obstruction. Both constant dribbling incontinence and renal failure have several potential etiologies. However, the possibility of lower urinary tract obstruction as the cause is easily evaluated.
BPH BOO-induced urinary stasis and accompanying bladder stones or diverticula may predispose to development of urinary tract infection. The predominance of symptoms such as dysuria, stranguria, urgency, or other irritative voiding symptoms often delays consideration of the role of BPH-induced abnormalities in development of the infection. BPH is the most common cause of gross hematuria in men older than 60 years. Usually, hematuria from BPH is “initial” or “terminal,” but it can present as a significant bleeding problem requiring catheter placement or other acute intervention. Carcinoma of the prostate rarely presents in this manner.
Symptom Indices
In the past decade, the construction and use of symptom indices to evaluate patients with BOO caused by BPH have escalated. An increasingly scientific approach to targeting and evaluating appropriate symptoms resulted in the American Urological Association (AUA)-sponsored symptom index in 1992.14 Development of this index was preceded by the Boyarsky, Madsen-Iversen, and Maine Medical Assessment symptom indices. An International Prostate Symptom Score questionnaire and other assessment tools have been developed essentially concomitantly with the AUA symptom index. The AUA index questions target changes in symptoms; they are not meant as diagnostic screening instruments for BPH or BOO (Table 2). Several studies failed to document strong correlations between the AUA symptom index and anatomic and physiologic measurement of BPH effects.15–17 Despite this fact, US and international guidelines recommend use of the symptom indices to compare results of research protocols and for the initial patient evaluation in an office setting.17
Table 2.
American Urological Association Symptom Index
| Not at All | Less Than 1 Time in 5 | Less Than Half the Time | About Half the Time | More Than Half the Time | Almost Always | |
|---|---|---|---|---|---|---|
| 1. Over the past month, how often | ||||||
| have you had a sensation of not | ||||||
| emptying your bladder completely | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| after you finished urinating? | ||||||
| 2. Over the past month, how often | ||||||
| have you had to urinate again | ||||||
| less than 2 hours after you | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| finished urinating? | ||||||
| 3. Over the past month, how often | ||||||
| have you found you stopped and | ||||||
| started again several times when | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| you urinated? | ||||||
| 4. Over the past month, how often | ||||||
| have you found it difficult to | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| postpone urination? | ||||||
| 5. Over the past month, how often | ||||||
| have you had a weak urinary stream? | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| 6. Over the past month, how often | ||||||
| have you had to push or strain to | □ 0 | □ l | □ 2 | □ 3 | □ 4 | □ 5 |
| begin urination? | ||||||
| 7. Over the past month, how many times did you most typically get up to urinate from the time you went to bed at night until | ||||||
| the time you got up in the morning. | ||||||
| □ 0 none | □ 1 1 time | □ 2 2times | □ 3 3 times | □ 4 4 times | □ 5 5 or more times | |
| AUA symptom score = sum of questions 1 to 7. | ||||||
From Barry MJ et al. J Urol. 1992;148:1549–1557.14
Physical Examination
The physical examination should be systematic and meticulous; it should be appropriately expanded based on patient history or observed physical abnormalities. The patient’s general appearance and any externally apparent abnormalities should be noted. The abdomen and genitalia should be examined by inspection, palpation, and appropriate percussion to identify any organomegaly, asymmetry, tenderness, or mass. Ordinarily, the bladder must contain at least 150 mL of fluid to allow its detection by percussion; residual urine in excess of 500 mL usually produces a visibly distended bladder.18 Eliciting a sense of urgency by suprapubic pressure tends to confirm the nature of the identified mass. Ultrasound provides a noninvasive procedure to clarify the nature of a lower abdominal mass.
A properly performed rectal examination provides essential information in evaluating patients with voiding dysfunction. In men, the genitourinary aspects of the examination are usually enhanced by examining the supported, bent-over patient from behind. After inspection of the anal area, assessment of rectal sphincter tone by insertion of the examining finger provides evidence of the functional status of the somatic, sensory, and motor components of the sacral reflex arc and indirect evidence of parasympathetic input to the lower urinary tract.19 A thorough examination of the rectum, including the sacral hollow, may identify unsuspected rectal pathology. On examining the prostate, its size and consistency as well as the integrity of its landmarks (median furrow, lateral sulci) should be noted and recorded.
Enlargement of the prostate may manifest through increases in its normal width (4.4 cm), length (3.4 cm), or thickness. A universally accepted nomenclature describing prostatic size is not available. An estimation of dimensions (width, length, vertical prominence) may constitute the most reproducible form of assessment. The consistency and symmetry of the prostate should be noted, as well as the presence, character, and location of nodules and induration (slight, moderate, stony), particularly with regard to distorting or compromising the median furrow and lateral sulci. Palpable identification of the normally nonpalpable seminal vesicles or bladder base requires determination of a cause.
Laboratory Evaluation
Additional tests may aid in formulating the final clinical impression and treatment plan. These include blood and urine analyses, urodynamic evaluation, selected radiologic and ultrasound imaging studies, and cystourethroscopy. The complexity, risk, and cost of these tests determine their selective employment. Urine should be tested for glucose, protein, occult blood, and pH with a multiparameter dipstick, ideally complemented by gross inspection and microscopic analysis of an initial (10–30 mL) and midstream sample of a freshly voided urine specimen. Urine culture, localization studies, and urinary cytology warrant selective considerations.
Blood studies may be desirable at the initial evaluation. AUA guide-line- recommended prostate-specific antigen (PSA) and serum creatinine measurements should be performed. Serum PSA level should be determined in men in the BPH age group, preferably before the rectal examination. A careful history taking and physical examination will usually identify patients at risk for bleeding tendencies. In fact, the best screening test for disorders of hemostasis in surgical patients is a carefully acquired history taking soliciting examples of bleeding tendencies in the patient and in his close relatives.20 In the absence of clinical evidence of a hemostatic disorder, there is only a 0.008% probability that a given patient will have a non-drug-induced intraoperative clotting disorder.21,22 Obviously, all surgical candidates should be questioned regarding their use of aspirin, nonsteroidal antiinflammatory drugs, and other agents capable of altering normal hemostasis.
A urodynamic evaluation tailored to provide critical diagnostic information with the least risk, discomfort, and cost deserves consideration. Residual urine and urine flow rates can be determined noninvasively. An ultrasound assessment of postvoid residual urine in a patient who has been instructed to empty his bladder is a common urodynamic study. The postvoid residual has traditionally been used to assess bladder function, guide diagnostic inquiry, and evaluate efficacy of treatment efforts. Recently, these roles, particularly with regard to the evaluation of treatment efficacy, have been challenged because of numerically variable residual urine determinations, a feature shared with most urodynamic and other complex physiologic indicators.
The failure to determine residual urine volume and to use this information with judgment is questionable practice, especially with the availability of ultrasound to measure volume. The Olmsted County study clearly demonstrates that most men in all age groups empty their bladder with less than 12 mL of residual urine.23 Approximately 20% of men in each decade group from 40 to 80 had residuals of greater than 50 mL, and BPH was disproportionally represented in these men. Men with a postvoid residual greater than 50 mL had a 3-fold increased risk of developing acute retention. These considerations, along with evidence suggesting that residual urine volume measurements are at least as reproducible as other urodynamic determinations,24 deserve increased emphasis in evaluating the potential clinical roles of residual urine determinations.
Uroflowometry, or uroflow, is a simple, noninvasive, and useful test to detect the presence of BOO. Uroflowometry is the electronic recording of the urinary flow through the course of 1 voiding episode. It is the single best noninvasive test for evaluation of patients presenting with the symptoms of BNO. However, the results of uroflow are nonspecific for many causes. Classic examples include the presence of an enlarged prostate from BPH, urethral stricture, meatal stenosis, and weakness of the detrusor muscle itself.
Urinary flow rate is the representation of the interaction between detrusor contraction and urethral resistance. The integration of these 2 factors in a single simple test is the reason that uroflowometry is useful and commonly employed; however, it is also the ultimate reason for the low specificity of the test. Uroflow is characterized by several important parameters: mean flow rate (Qave), the volume of urine voided divided by the continuous flow time; peak flow rate (Qmax), the maximum recorded flow rate; voided volume; time to maximum flow rate; total flow time; and urinary flow pattern (Figure 1).
Figure 1.
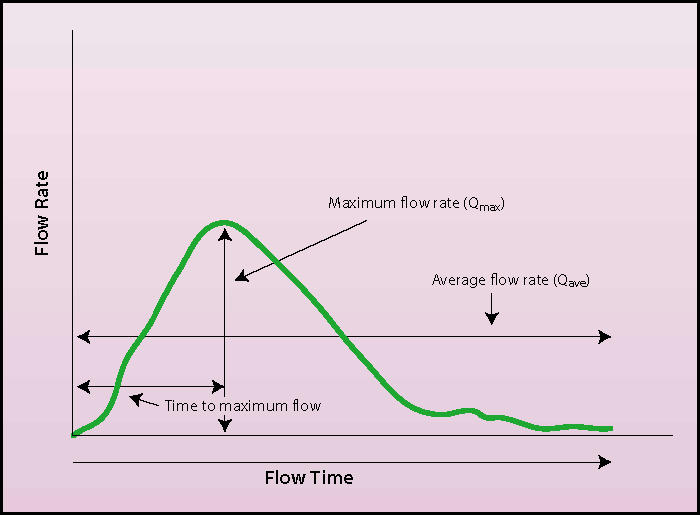
Typical uroflow demonstrating the important parameters of mean flow rate (Qave), peak flow rate (Qmax), time to maximum flow rate, and total flow time. Note the shape of the curve demonstrating an unobstructed pattern and a continuous-flow pattern.
Although no study has been able to characterize accepted normal or abnormal standards for comparison, it is generally acknowledged that certain urinary flow patterns are representative of normal and abnormal function (Figures 2 and 3). Flows that have a low rate and broad or ill-defined peak imply some type of BNO or impaired contractility. An intermittent flow rate may represent sphincter dyssynergia, detrusor instability, or abdominal straining artifact. A high flow rate implies, but is not pathognomonic of, a decreased outlet resistance. Uroflowometry needs interpretation, and caution should be used when interpreting results.
Figure 2.
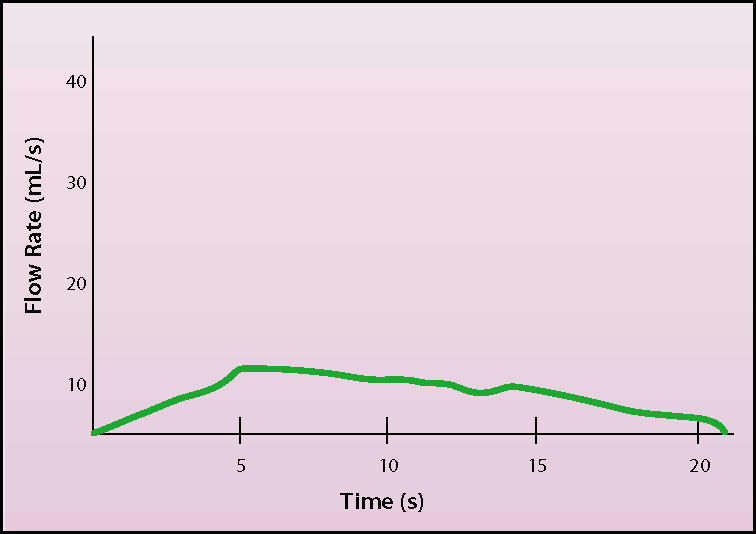
Uroflow demonstrating reduced rate of flow. The flat, elongated curve with low peak flow rate suggests outlet obstruction or reduced bladder detrusor contractility.
Figure 3.
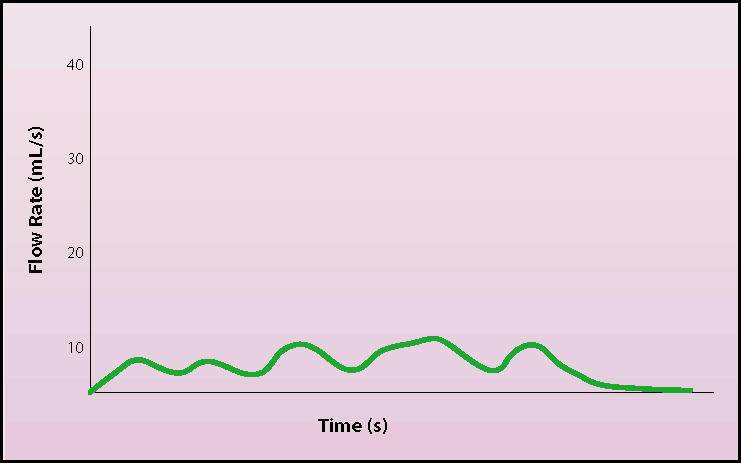
Uroflow demonstrating stream interruption by abdominal straining.
Peak urinary flow rate is generally accepted as the most useful parameter in a noninvasive evaluation of BPH. In men, “normal” Qave and Qmax are reported to be approximately 15 mL/s and 25 mL/s, respectively; normal values are slightly higher in women. In addition, Qave and Qmax in healthy men almost certainly decrease with age.
One of the more difficult issues in the use of uroflowometry is artificial elevations in the flow secondary to Valsalva maneuvers. To prevent this from affecting interpretation, it is suggested that a Qmax last a minimum of 2 seconds. This criterion will “weed out” uroflows that are influenced by abdominal straining.
The use of uroflowometry as a tool in the diagnosis of BPH remains a point of controversy. Although BNO is somewhat associated with a lower flow rate, it may also be associated with impaired bladder contractility. There is no parameter that can distinguish outflow obstruction from primary impaired detrusor contractility. Therefore, the ability to distinguish between BNO and primary problems of the bladder detrusor is not possible with uroflow alone. In addition, uroflows cannot distinguish among different types of BNO, such as stricture or BPH. Surprisingly, quantified symptom score analyses do not correlate strongly with uroflow results. Both are independent tools in the assessment of BNO.
Prognostic information based on uroflow data is a point of contention. Some investigators have found that preoperative uroflows in patients who had high flows and low residuals worsened after transurethral resection of the prostate (TURP).25–27 Patients with a Qmax under 10 mL/s were thought to be obstructed and the best candidates for TURP. Patients with a Qmax greater than 15 mL/s were considered unobstructed and not likely to benefit from the surgery. Such strict dichotomies in outcome have been challenged by many investigators.
Perhaps the most useful attribute of uroflow is that Qmax has become a primary objective parameter of treatment outcomes for various surgical and medical therapies for BPH. Once the diagnosis of BNO has been made and some type of treatment effected, comparisons between pre- and post-treatment Qmax are easily performed and analyzed. Most notably, unlike other prominent parameters of treatment outcome (eg, AUA symptom score), Qmax responds minimally to placebo, making it an objective tool when evaluating a patient’s response to therapy.
Flow rate recording is the single best noninvasive urodynamic test to detect lower urinary tract obstruction. However, based on the current information, there is no recommended cutoff value to document the appropriateness of therapy. Although considerable uncertainty exists, patients with flow rates greater than 15 mL/s have a poorer treatment outcome after TURP than do patients with flow rates less than 15 mL/s. However, the majority of patients still improve. Patients with flow rates greater than 15 mL/s may benefit from pressure flow studies, provided they have bothersome symptoms suggestive of prostatism.
Maximum flow rate, flow pattern, and volume voided provide important information. Voided volumes less than 150 mL or greater than 500 mL provide suspicious information. Review of the available literature indicates that the prevalence of urine volumes greater than 200 mL varies with age.28 In a noteworthy article, Roehrborn24 cited data that indicate a significant variability (most, 1 SD; half, 2 SD) in repetitiously performed individual flow rates; a definite tendency toward a substantially increased flow rate with sequential determinations; and an appreciable decrease in maximum flow rate with age, with 69% of men aged 75 to 79 years in the Olmsted County study having rates under 15 mL/s and 35% having rates under 10 mL/s.
The evidence supporting the use of a peak flow rate of less than 15 mL/s to select patients for therapeutic trials of presumed BPH voiding dysfunction seems open to challenge, as does the presumed advantage of flow rate over residual urine measurements. Simultaneous pressure flow studies appropriately supplemented by video assessment provide the maximum opportunity to differentiate detrusor and outlet effects and have largely replaced the use of the cytometrogram. Data from initial and particularly repetitious urodynamic studies can provide useful diagnostic and therapeutic insights.
In the past, evaluation of the upper urinary tract by radiologic or ultrasound imaging was commonplace in patients with BNO. Now, these assessments are performed selectively in patients with a history, physical findings, or laboratory studies that indicate a significant possibility of an important independent or secondary urinary tract abnormality accompanying the BOO.
Cystourethroscopy to confirm the presence and effect of BNO from BPH is commonly perfomed with local anesthesia in an outpatient setting. The presence, configuration, and site of obstructive tissue, but not its physiologic effect, can usually be identified. The latter can be assessed to some degree by determination of residual urine and recognition of the presence and degree of trabeculation and bladder pathology such as diverticula and stones. Prostate size can be estimated crudely from the increase in length of the prostatic urethra, degree and length of the adenomatous occlusion, presence of posterolateral and anterior clefting, and thickness of the prostate when palpated rectally with the cystoscope in place.
Rectal or abdominal ultrasound may be used to assess prostate size and weight with accuracy to within 5%. An assessment of residual urine and possibly the thickness and configuration of the bladder wall may provide some supportive evidence for BNO.29,30 Cystography and retrograde and voiding urethrography can at times provide invaluable information regarding the diagnosis and evaluation of bladder or urethral diverticula. These studies warrant consideration in patients with clinical evidence of BNO who have an unusual course or unexplained findings.
Indications for Treatment
Acute urinary retention is often indicative of end-stage bladder decompensation requiring operative relief. The patient in whom retention is triggered by ingestion of drugs, such as α-agonists or anticholinergic agents, may void satisfactorily once the medication is stopped and the bladder drained for a time. A supervised attempt at decatheterization for minimally symptomatic patients who present with an incident-related (eg, postoperative or acute bacterial prostatitis) or spontaneous episode of retention is often reasonable. Use of α-adrenergic antagonists in conjunction with this voiding trial is worthwhile. For repeated episodes of retention, management by intermittent catheterization or continued catheter drainage is a possible, but often unacceptable, alternative to an operative approach.
The patient with urinary retention is usually treated satisfactorily by insertion of a urethral catheter. For difficult catheterizations, a percutaneous suprapubic cystotomy tube remains an appropriate alternative that is usually well-tolerated and associated with few catheter-related complications. If chronic retention is suspected, the bladder should be emptied gradually to avoid diffuse mucosal cracking and bleeding that may follow rapid decompression. Use of a variety of internalized catheters is currently being evaluated in selected high-risk patients.
Bilateral hydronephrosis with renal functional impairment requires relief of the obstruction to preserve the integrity of the upper tracts. On catheter insertion, a postobstructive diuresis may ensue, requiring meticulous fluid and electrolyte management. The patient’s general condition should be optimized before operative intervention is undertaken.
The presence of multiple bladder stones, prominent narrow-necked bladder diverticula, overflow incontinence, or other signs of end-stage bladder decompensation are indications for therapeutic intervention. Recurrent or chronic urinary tract infections caused by elevated residual urine are also an indication for considering intervention. Acute or chronic bacterial prostatitis should be excluded as a possible source of infection. A careful history taking, physical examination, and lower tract localization cultures should help to clarify this issue.
Gross hematuria is an infrequent but legitimate indication for so-called prostatectomy, particularly when the episodes are multiple and associated with clot retention or significant blood loss. The usual limited initial hematuria associated with BPH is best managed conservatively. Antiandrogen measures, such as the use of finasteride, almost always have a favorable impact on recurrent prostatic bleeding.31
Obstructive and irritative symptoms that significantly interfere with the quality of life of the patient are common indications to consider prostatic surgery and other therapeutic approaches. The cause of the symptoms should be established with a high degree of probability. In the past, most patients have had multiple indications to support the decision to initiate therapy.32 Both the urologic surgeon and the patient must be clearly aware of the results that can be expected and the risks involved in achieving them.
Main Points.
Benign prostatic hyperplasia (BPH) is the most common neoplastic condition afflicting men and constitutes a major factor impacting the health of the male population in the United States.
The goals of the clinical evaluation of persons with voiding dysfunction caused by BPH are to identify the patient’s voiding or, more appropriately, urinary tract problems, both symptomatic and physiologic; to establish the etiologic role of BPH in these problems; to evaluate the necessity for and probability of success and risks of various therapeutic approaches; and to present the results of these assessments to the patient so he can make an informed decision about management recommendations and available alternatives.
Most patients with medical problems caused by BPH present with symptoms of dysfunctional voiding. An increasingly scientific approach to targeting and evaluating appropriate symptoms resulted in the American Urological Association-sponsored symptom index.
Additional tests may aid in formulating the final clinical impression and treatment plan. These include blood and urine analyses, urodynamic evaluation, selected radiologic and ultrasound imaging studies, and cystourethroscopy.
Uroflowometry is the single best noninvasive test used in evaluation of patients presenting with the symptoms of bladder neck obstruction. However, the results of uroflow are nonspecific for many causes. Classic examples include the presence of an enlarged prostate from BPH, urethral stricture, meatal stenosis, and weakness of the detrusor muscle itself.
Acute urinary retention is often indicative of end-stage bladder decompensation requiring operative relief. The presence of multiple bladder stones, prominent narrow-necked bladder diverticula, overflow incontinence, or other signs of end-stage bladder decompensation are indications for therapeutic intervention. Gross hematuria is an infrequent but legitimate indication for so-called prostatectomy, particularly when the episodes are multiple and associated with clot retention or significant blood loss. Obstructive and irritative symptoms that significantly interfere with the quality of life of the patient are common indications to consider prostatic surgery and other therapeutic approaches.
References
- 1.Randall A. Surgical Pathology of Prostatic Obstruction. Baltimore: Williams & Wilkins; 1931. [Google Scholar]
- 2.Harbitz TB, Haugen OA. Histology of the prostate in elderly men: a study in an autopsy series. Acta Pathol Microbiol Immunol Scand [A] 1972;80:756–777. doi: 10.1111/j.1699-0463.1972.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Lytton B, Emery JM, Harvard BW. The incidence of benign prostatic obstruction. J Urol. 1968;99:639–645. doi: 10.1016/S0022-5347(17)62763-0. [DOI] [PubMed] [Google Scholar]
- 4.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Fowler FJ, Jr, Bin L, et al. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol. 1997;157:10–14. [PubMed] [Google Scholar]
- 7.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 8.McConnell JD, Bruskewitz R, Walsh P, et al. for the Finasteride Long-Term Efficacy and Safety Study Group, authors. The effect of finasteride on the risk of acute urinary retention for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 9.Rotkin ID. Epidemiology of benign prostatic hypertrophy: review and speculations. In: Grayhack JT, Wilson JD, Scherbenske MJ, editors. Benign Prostatic Hyperplasia: IAMDD Workshop Proceedings; Feb 20–21, 1975; 1976. p. 105. US Department of Health, Education and Welfare publication no. (NIH) 76-1113. [Google Scholar]
- 10.Sanda MG, Beaty TH, Stutzman RE, et al. Genetic susceptibility of benign prostatic hyperplasia. J Urol. 1994;152:115–119. doi: 10.1016/s0022-5347(17)32831-8. [DOI] [PubMed] [Google Scholar]
- 11.Holtgrewe H. Transurethral resection of the prostate. In: Lepor H, editor. Prostatic Diseases. Philadelphia: WB Saunders Company; 2000. pp. 232–245. [Google Scholar]
- 12.Blaivas JG. Urinary symptoms and symptom scores. J Urol. 1993;150(5 pt 2):1714. doi: 10.1016/s0022-5347(17)35875-5. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen JV, Bruskewitz RC. Clinical manifestation and indications for treatment in prostatic diseases. In: Lepor H, editor. Prostatic Diseases. Philadelphia: WB Saunders Company; 2000. pp. 127–142. [Google Scholar]
- 14.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. for the Measurement Committee of the American Urological Association, authors. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ. Epidemiology and natural history of benign prostatic hyperplasia. In: Lepor H, Lawson RK, editors. Prostate Diseases. Philadelphia: WB Saunders Company; 1993. p. 96. [Google Scholar]
- 16.Barry MJ, Cockett AT, Holtgrewe HL, et al. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary MP, Barry MJ, et al. Evaluating symptoms and functional status. In: Grayhack JT, Wilson JD, Scherbenske MJ, et al., editors. Benign Prostatic Hyperplasia. Bethesda, MD: National Institutes of Health; 1996. pp. 139–147. [Google Scholar]
- 18.Carlton CE, Jr., et al. Initial evaluation including history, physical examination, and urinalysis. In: Harrison JH, Gittes RF, Perlmutter AD, et al., editors. Campbell’s Urology. 4th ed. Vol 1. Philadelphia: WB Saunders Company; 1978. p. 203. [Google Scholar]
- 19.Mundy AR. Clinical physiology of the bladder, urethra and pelvic floor. In: Mundy AR, Stephenson PP, Wein AJ, editors. Urodynamics: Principles, Practice, and Application. New York: Churchill Livingstone; 1984. p. 14. [Google Scholar]
- 20.Collins JA. Blood transfusions and disorders of surgical bleeding. In: Sabiston DC, editor. Textbook of Surgery-The Biological Basis of Modern Surgical Practice. 14th ed. Philadelphia: WB Saunders Company; 1991. pp. 85–102. [Google Scholar]
- 21.Eiseman B American College of Surgeons, editors. Care of the Surgical Patient, vol 2: Elective Care, section V: Initial Evaluation for Elective Surgery. New York: Scientific American; 1989. Necessary preoperative tests; pp. 1–9. [Google Scholar]
- 22.Suchman AL, Mushlin AI. How well does the activated partial thromboplastin time predict postoperative hemorrhage? JAMA. 1986;256:750–753. [PubMed] [Google Scholar]
- 23.Kolman C, Girman LJ, Jacobsen SJ, Lieber MM. Distribution of post-void residual urine volume in randomly selected men. J Urol. 1999;161:122–127. [PubMed] [Google Scholar]
- 24.Roehrborn CG. The role of guidelines in the diagnosis and treatment of benign prostatic hyperplasia. In: Lepor H, editor. Prostatic Disease. Philadelphia: WB Saunders Company; 2000. pp. 143–162. [Google Scholar]
- 25.Jensen KME, Jorgensen JB, Mogensen P. Urodynamics in prostatism. III. Prognostic value of medium-fill water cystometry. Scand J Urol Nephrol Suppl. 1988;114:78–83. [PubMed] [Google Scholar]
- 26.Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology. 1984;24:403–409. doi: 10.1016/0090-4295(84)90225-5. [DOI] [PubMed] [Google Scholar]
- 27.Meyhoff HH, Nordling J, Hald T. Clinical evaluation of transurethral versus transvesical prostatectomy: a randomized study. Scand J Urol Nephrol. 1984;18:201–209. doi: 10.3109/00365598409180184. [DOI] [PubMed] [Google Scholar]
- 28.Abrams P, Torrens M. Urine flow studies. Urol Clin North Am. 1979;6:71–79. [PubMed] [Google Scholar]
- 29.Abu-Yousef M, Narayana A. Transabdominal ultrasound in the evaluation of prostate size. J Clin Ultrasound. 1982;10:275–278. doi: 10.1002/jcu.1870100606. [DOI] [PubMed] [Google Scholar]
- 30.Smith EH, Raptopoulos V, et al. Radiology of the urinary tract. In: Walsh PC, Gittes RF, Perlmutter AD, et al., editors. Campbell’s Urology. 5th ed. Vol 1. Philadelphia: WB Saunders Company; 1986. p. 312. [Google Scholar]
- 31.Foley SJ, Soloman LZ, Wedderburn AW, et al. A prospective study of the natural history of hematuria associated with benign prostatic hyperplasia and the effect of finasteride. J Urol. 2000;163:496–498. [PubMed] [Google Scholar]
- 32.Mebust WK. Transurethral resection of the prostate and transurethral incision of the prostate. In: Lepor H, Lawson RK, editors. Prostate Disease. Philadelphia: WB Saunders Company; 1993. pp. 150–163. [Google Scholar]


