Nuclear resonance vibrational spectroscopy (NRVS) is an emerging site-specific probe of active site vibrational dynamics in metalloproteins.1,2 NRVS is a synchrotron-based technique that uniquely targets the vibrations of a Mössbauer nucleus, such as 57Fe, without interference from vibrations of other atoms, and reveals not only the frequency, but also the (mean squared) amplitude,1b,2a of all vibrations of the probe nucleus along the direction of the incident X-ray beam. Quantitative characterization of vibrational modes involving a reactive probe atom can illuminate mechanisms of complex biomolecules.
Reactions with heme proteins mediate the physiological effects of nitric oxide (NO). The proposed trigger for activation of soluble guanylate cyclase (sGC) is rupture of the covalent Fe-His bond3a,b in the heme-containing domain3c,d upon NO binding to the Fe. A thermodynamic consequence of NO-induced weakening of a trans Fe-imidazole bond, as observed in several heme systems, is that imidazole binding should weaken a trans Fe-NO bond. Model compound structures support such a reciprocal negative trans interaction,4a although protein structural data4b-d may not have sufficient precision to resolve the 3 pm increase in Fe-NO bond length due to imidazole binding.
On the other hand, vibrational frequencies respond sensitively to bond length changes of this magnitude, and it is therefore puzzling that the frequency attributed to stretching of the Fe-NO bond in six-coordinate imidazole-ligated heme proteins5 is higher, rather than lower, than the frequencies observed for five-coordinate iron nitrosyl hemes. For example, the assigned Fe-NO stretching frequencies of the 5- and 6- coordinate NO complexes with myoglobin (MbNO) are 521 cm−1 and 552 cm−1, respectively.5c
NRVS measurements on 6-coordinate MbNO suggest reexamination of this issue (Fig. 1A). The Fe-weighted vibrational density of states (VDOS) D(ν) samples the vibrational kinetic energy distribution (KED), with each mode contributing an area eFe2 equal to the fraction of mode energy associated with Fe motion.2a,d The eFe2 = 0.11 area of the feature at 547 cm−1 is well below the eFe2 = 0.23-0.33 range that we observed for the FeNO stretching mode in a series of 5-coordinate nitrosyl porphyrins,2d but is clearly visible because of the distinctly improved signal quality compared to previously published NRVS measurements on myoglobin.1a,b,d In contrast, a mode with an area eFe2 = 0.25 appears at 443 cm−1, near a mode in the MbNO Raman spectrum previously shown to be sensitive to NO isotope substitution.5b,c
Figure 1.
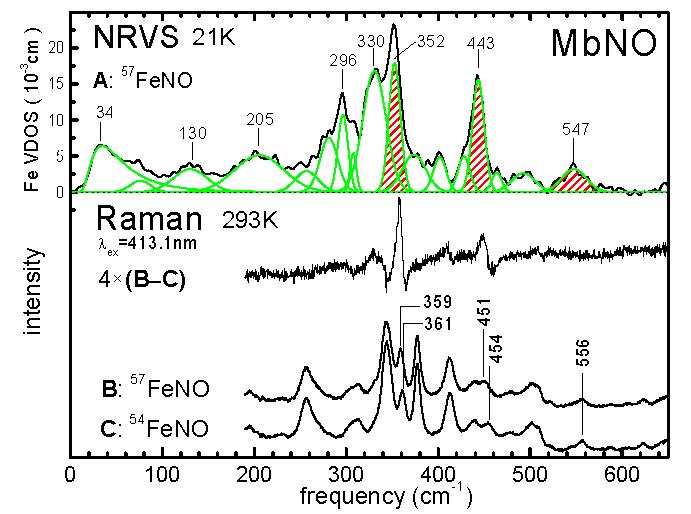
Fe-weighted VDOS determined from NRVS data on 57Fe-enriched horse MbNO at 21 K (A) using the program PHOENIX and Raman data on 57Fe- and 54Fe-enriched MbNO at ambient temperature (B, C) reveal modes with significant Fe contribution. An expanded trace shows the calculated Raman difference spectrum between B and C.
The frequency shift upon isotopic substitution of atom j provides an indirect estimate2a of , assuming an unaltered mode composition. Raman measurements on isotopically enriched MbNO at ambient temperature (Fig. 1B, C) reveal a 3 cm−1 54Fe/57Fe isotope shift for a mode at 451 cm−1, and no significant Fe isotope sensitivity for the 556 cm−1 feature, in qualitative concord with the NRVS results. Furthermore, the value eFe2 = 0.29 calculated2a from the isotope shift of the 451 cm−1 mode is in reasonable quantitative agreement with that determined for the 443 cm−1 mode observed in the NRVS data. The value eFe2 = 0.18 determined from the isotope shift of an additional Fe-sensitive Raman feature at 360 cm−1 can be reconciled with a significantly larger value for the corresponding NRVS feature (Table 1) if both consist of two unresolved modes. These may be the (nominally degenerate) in-plane Fe-Npyr vibrations, which we previously observed to contribute prominently to the Fe VDOS of deoxyMb1b and of iron porphyrins.2 NRVS and Raman isotope shift measurements reveal the KED (eFe2, eN2, and eO2) over the FeNO fragment (Table 1).
Table 1.
Vibrational kinetic energy distributions on the FeNO fragment in MbNO
The 556 cm−1 feature is characteristic for six-coordinate hemes with NO and imidazole as axial Fe ligands, and has successfully monitored interconversion between five- and six-coordinate states.7a-c One component of this complex feature undergoes a large frequency shift upon 14→15NO substitution (Fig. S2), and has previously been identified as Fe-NO stretching.5 The KED (Table 1) indicates that 80-90% of the mode energy is localized on the FeNO fragment, but with the majority associated with motion of the NO nitrogen atom. This contrasts with the relatively uniform distribution of kinetic energy among all three atoms predicted2d for Fe-NO stretching in five-coordinate iron nitrosyls. On the other hand, Fe motion accounts for 25-30% of the 451 cm−1 mode energy, similar to the value eFe2 = 0.30 determined experimentally for the Fe-NO mode in Fe(TPP)(NO).2d
NRVS measurements on oriented samples provide additional insight into vibrational mode character.2b,1d Fig. 2 compares the NRVS excitation probability measured on a powder and on a single crystal of Fe(TPP)(1-MeIm)(NO), oriented to enhance the contribution of modes involving Fe motion perpendicular to the mean plane of the porphyrin, and suppress in-plane Fe vibrations.
Figure 2.
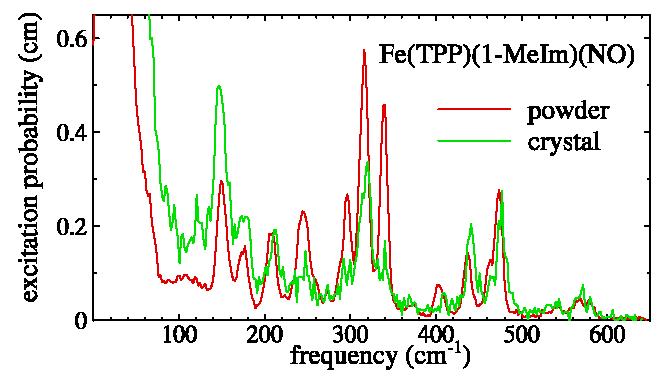
Excitation probabilities determined from NRVS measurements on a powder (13 K, red) and on a crystal (82 K, green) of Fe(TPP)(1-MeIm)(NO), oriented with the X-ray beam incident along the {001} direction, which lies 13.8° from the normal of all porphyrins. The single crystal spectrum only includes motion along the X-ray beam, so that modes with Fe motion perpendicular to the porphyrin plane are therefore enhanced, and in-plane modes suppressed, in the crystal spectrum relative to the powder spectrum.
The data in Fig. 2 identify a mode at 440 cm−1 with dominant Fe motion perpendicular to the mean porphyrin plane. Normal mode analyses2b,d,5a indicate significant vibrational mixing between Fe-NO stretching and FeNO bending, and the reduced amplitude (eFe2=0.16) of the 440 cm−1 mode determined from the VDOS of Fe(TPP)(1-MeIm)(NO) (Table S2) relative to the 443 cm−1 mode in MbNO and the 540 cm−1 mode in Fe(TPP)(NO) might reflect an enhanced contribution from FeNO bending. Nevertheless, the absence of any other mode above 200 cm−1 with dominant out-of-plane Fe motion supports a dominant Fe-NO stretching contribution. The 100 cm−1 frequency decrease from the 540 cm−1 frequency identified2a,b,d with Fe-NO stretching in the analogous 5-coordinate complex Fe(TPP)(NO) indicates that this mode is highly sensitive to the strength of the Fe-NO bond. DFT calculations on Fe(TPP)(NO) also revealed a strong sensitivity of the Fe-NO frequency to the Fe-N bond distance.2b
It would be desirable to confirm the negative trans interaction between the NO and Im ligands by monitoring the vibration of the Fe-Im bond in the presence and absence of NO. In fact, the Fe(TPP)(1-MeIm)(NO) single crystal data identify several modes below 200 cm−1 whose Fe motion is primarily orthogonal to the porphyrin plane. However, none approach the area eFe2 = 0.59 predicted for a two-body Fe-1-MeIm oscillator. Further work will be needed to determine whether any of these modes correlates with the strength of the Fe-His bond.
The vibrational data presented above identify modes with FeNO stretching character in the 440-450 cm−1 region for MbNO and Fe(TPP)(1-MeIm)(NO). The 70-100 cm−1 decrease relative to the Fe-NO frequencies in the corresponding 5-coordinate NO complexes2a,5 confirms the weakening of the Fe-NO bond in the presence of a trans imidazole ligand. These results support a proposed mechanism3a,b for NO activation of heme proteins, and underline the value of NRVS as a direct probe of metal reactivity in complex biomolecules. The revised assignment for the Fe-NO stretch mode may also facilitate attempts7b,d to identify correlations between Fe-NO and N-O stretching vibrations, analogous to the well-established vibrational Fe-CO/C-O anticorrelation7e in heme-CO complexes.
Supplementary Material
Acknowledgment
We acknowledge financial support from the National Institutes of Health (GM-52002 to J.T.S. and GM-38401 to W.R.S.) and the National Science Foundation (0240955 to J.T.S.). We are grateful for the loan of an Oxford cryocooler from BioCARS and for valuable operating assistance provided by Jay VonOsinski. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. W-31-109-Eng-38.
Footnotes
Supporting Information Available: Experimental information and fits to NRVS and Raman data. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Keppler C, Achterhold K, Ostermann A, van Burck U, Potzel W, Chumakov AI, Baron AQ, Ruffer R, Parak F. Eur. Biophys. J. 1997;25:221–224. doi: 10.1007/s002490050034. [DOI] [PubMed] [Google Scholar]; (b) Sage JT, Durbin SM, Sturhahn W, Wharton DC, Champion PM, Hession P, Sutter J, Alp EE. Phys. Rev. Lett. 2001;86:4966–4969. doi: 10.1103/PhysRevLett.86.4966. [DOI] [PubMed] [Google Scholar]; (c) Bergmann U, Sturhahn W, Linn DE, Jr., Jenney FE, Jr., Adams MWW, Rupnik K, Hales BJ, Alp EE, Mayse A, Cramer SP. J. Am. Chem. Soc. 2003;125:4016–4017. doi: 10.1021/ja028767+. [DOI] [PubMed] [Google Scholar]; (d) Achterhold K, Parak FG. J. Phys.: Condens. Matter. 2003;15:S1683–S1692. [Google Scholar]; (e) Chumakov AI, Rüffer R, Leupold O, Sergueev I. Structural Chemistry. 2003;14:109–119. [Google Scholar]
- 2.(a) Sage JT, Paxson C, Wyllie GRA, Sturhahn W, Durbin SM, Champion PM, Alp EE, Scheidt WR. J. Phys.: Condens. Matter. 2001;13:7707–7722. [Google Scholar]; (b) Rai BK, Durbin SM, Prohofsky EW, Sage JT, Wyllie GRA, Scheidt WR, Sturhahn W, Alp EE. Biophys. J. 2002;82:2951–2963. doi: 10.1016/S0006-3495(02)75636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rai BK, Durbin SM, Prohofsky EW, Sage JT, Ellison MK, Roth A, Scheidt WR, Sturhahn W, Alp EE. J. Am. Chem. Soc. 2003;125:6927–6936. doi: 10.1021/ja028219w. [DOI] [PubMed] [Google Scholar]; (d) Leu BM, Zgierski MZ, Wyllie GRA, Scheidt WR, Sturhahn W, Alp EE, Durbin SM, Sage JT. J. Am. Chem. Soc. 2004;126:4211–4227. doi: 10.1021/ja038526h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Traylor TG, Sharma VS. Biochemistry. 1992;31:2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]; (b) Zhao Y, Brandish PE, Ballou DP, Marletta MA. Proc. Natl. Acad. Sci. USA. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J. Proc. Natl. Acad. Sci. USA. 2004;101:12854–12859. doi: 10.1073/pnas.0405188101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nioche P, Berka V, Vipond J, Minton N, Tsai AL, Raman CS. Science. 2004;306:1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wyllie GRA, Schulz CE, Scheidt WR. Inorg. Chem. 2003;42:5722–5734. doi: 10.1021/ic034473t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brucker EA, Olson JS, Ikeda-Saito M, Phillips GN., Jr. Proteins. 1998;30:352–356. [PubMed] [Google Scholar]; (c) Rich AM, Armstrong RS, Ellis PJ, Lay PA. J. Am. Chem. Soc. 1998;120:10827–10836. [Google Scholar]; (d) Copeland DM, West AH, Richter-Addo GB. Proteins. 2003;53:182–192. doi: 10.1002/prot.10495. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hu S, Kincaid JR. J. Am. Chem. Soc. 1991;113:2843–2850. [Google Scholar]; (b) Hu S, Kincaid JR. J. Am. Chem. Soc. 1991;113:9760–9766. [Google Scholar]; (c) Tomita T, Hirota S, Ogura T, Olson JS, Kitagawa T. J. Phys. Chem. B. 1999;103:7044–7054. [Google Scholar]; (d) Benko B, Yu N-T. Proc. Natl. Acad. Sci. USA. 1983;80:7042–7046. doi: 10.1073/pnas.80.22.7042. We note that earlier work. had attributed the 556 cm−1 mode to FeNO bending. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Morse RH, Chan SI. J. Biol. Chem. 1980;255:7876–7882. [PubMed] [Google Scholar]; (b) Hori H, Ikeda-Saito M, Yonetani T. J. Biol. Chem. 1981;256:7849–7855. [PubMed] [Google Scholar]
- 7.(a) Rodgers KR, Lukat-Rodgers GS, Tang LJ. Biol. Inorg. Chem. 2000;5:642–654. doi: 10.1007/s007750000150. [DOI] [PubMed] [Google Scholar]; (b) Thomas MR, Brown D, Franzen S, Boxer SG. Biochemistry. 2001;40:15047–15056. doi: 10.1021/bi011440l. [DOI] [PubMed] [Google Scholar]; (c) Andrew CR, George SJ, Lawson DM, Eady RR. Biochemistry. 2002;41:2353–2360. doi: 10.1021/bi011419k. [DOI] [PubMed] [Google Scholar]; (d) Coyle CM, Vogel KM, Rush TS, III, Kozlowski PM, Williams R, Spiro TG, Dou Y, Ikeda-Saito M, Olson JS, Zgierski MZ. Biochemistry. 2003;42:4896–4903. doi: 10.1021/bi026395b. [DOI] [PubMed] [Google Scholar]; (e) Ray GB, Li X-Y, Ibers JA, Sessler JL, Spiro TG. J. Am. Chem. Soc. 1994;116:162–176. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


