Abstract
Activity-dependent neuroprotective protein (ADNP, ∼123562.8 Da), is synthesized in astrocytes and expression of ADNP mRNA is regulated by the neuroprotective peptide vasoactive intestinal peptide (VIP). The gene that encodes ADNP is conserved in human, rat and mouse, and contains a homeobox domain profile that includes a nuclear-export signal and a nuclear-localization signal. ADNP is essential for embryonic brain development, and NAP, an eight-amino acid peptide that is derived from ADNP, confers potent neuroprotection. Here, we investigate the subcellular localization of ADNP through cell fractionation, gel electrophoresis, immunoblotting and immunocytochemistry using α-CNAP, an antibody directed to the neuroprotective NAP fragment that constitutes part of an N-terminal epitope of ADNP. Recombinant ADNP was used as a competitive ligand to measure antibody specificity. ADNP-like immunoreactivity was found in the nuclear cell fraction of astrocytes and in the cytoplasm. In the cytoplasm, ADNP-like immunoreactivity colocalized with tubulin-like immunoreactivity and with microtubular structures, but not with actin microfilaments. Because microtubules are key components of developing neurons and brain, possible interaction between tubulin and ADNP might indicate a functional correlate to the role of ADNP in the brain. In addition, ADNP-like immunoreactivity in the extracellular milieu of astrocytes increased by ∼1.4 fold after incubation of the astrocytes with VIP. VIP is known to cause astrocytes to secrete neuroprotective/neurotrophic factors, and we suggest that ADNP constitutes part of this VIP-stimulated protective milieu.
Keywords: ADNP, homeobox, brain development, microtubules, secreted proteins
INTRODUCTION
Astrocytes are two-way communication partners in the CNS. They receive signals from neighboring neurons and respond to these signals with the release of neuroactive substances (Brenneman et al., 1990; Gozes et al., 1991; Araque et al., 1999; Fields and Stevens-Graham, 2002). Vasoactive intestinal peptide (VIP) released from neurons (Martin and Magistretti 1989a; Martin and Magistretti 1989b; Brown, 2000) stimulates glia to generate neurotrophic factors including activity-dependent neurotrophic factor (Brenneman and Gozes, 1996), protease nexin-1 (Houenou et al., 1995), interleukin-1, interleukin-3, granulocyte colony stimulating factor, tumor necrosis factor-α, macrophage colony stimulating factor and interleukin-6 (Brenneman et al., 1992; Brenneman et al., 1995; Brenneman et al., 2003).
Activity-dependent neuroprotective protein (ADNP) is synthesized in astrocytes and expression of ADNP mRNA is regulated by VIP (Bassan et al., 1999). In human and mouse brains, ADNP is expressed predominantly in the cerebellum, hippocampus and cerebral cortex (Bassan et al., 1999; Zamostiano et al., 2001). The gene that encodes ADNP is conserved in human, rat and mouse (∼90% homology). ADNP contains an eight-amino acid peptide sequence, NAPVSIPQ (termed NAP), which provides potent neuroprotection in vitro and in vivo (Bassan et al., 1999; Gozes et al., 2000a; Zamostiano et al., 2001; Alcalay et al., 2004; Divinski et al., 2004). Additionally, ADNP contains 9 zinc fingers, a homeobox domain profile, and a bipartite nuclear-localization signal, which indicate transcription factor activity (Zamostiano et al., 2001). ADNP exhibits sequence homology to the engrailed homeoprotein (Joliot et al., 1998), containing a conserved nuclear export signal and a nuclear localization signal (Gozes et al., 2000b; Maizel et al., 1999). ADNP-knockout mouse embryos do not survive beyond embryonic day 9.5. In ADNP-knockout embryos, the expression of Pax6, which is essential for formation of the cerebral cortex, is abolished in the brain primordial tissue and the cranial neural tube fails to close (Pinhasov et al., 2003).
In the present study, we show that ADNP is localized to the nucleus of astrocytes, but there is some immunoreactivity in the cytoplasm and in the extracellular milieu following stimulation with VIP. Furthermore, ADNP might colocalize with tubulin, an important cytoskeletal protein.
OBJECTIVES
VIP is secreted from neurons and binds to receptors on astrocytes which, in turn, secrete neuroprotective factors. ADNP is a recently discovered protein that has a role in brain development and contains a homeobox domain that is homologous to that of engrailed. ADNP mRNA increases in response to incubation of astrocytes with VIP. The objective of the present study was to analyze the distribution of ADNP in astrocytes to aid further understanding of the endogenous mechanism of action of ADNP and determine whether it is secreted from glial cells following stimulation with VIP.
METHODS
Rat cortical astrocyte cultures
Rat cortical astrocytes were prepared as described previously (McCarthy and de Vellis, 1980; Gozes et al., 1991). These cells represent a superior source of astroglia because of their rapid growth characteristics and established cellular composition (∼98% purity) (McCarthy and de Vellis, 1980; McCarthy and Partlow, 1976; Gozes et al., 1991). Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Sigma) containing 10% fetal calf serum (Biological Industries). Astroglial cells were subcultured after 10 days and then incubated for 2 weeks.
Pheochromocytoma cell (PC12) cultures
Rat PC12 cells (Sigalov et al. 2000; Steingart et al., 2000) were grown in 75 mm flasks in DMEM containing 8% fetal calf serum, 8% donor horse serum (Biological Industries), 2 mM L-glutamine (Biological Industries) and penicillin–streptomycin solution (Biological Industries).
Astrocyte-conditioned media
Astrocyte-conditioned media (CM) was prepared from confluent cultures as described previously (Brenneman and Gozes, 1996). Briefly, two weeks after the cells were subcultured, cultures were washed with phosphate buffered saline (PBS; Biological Industries) and incubated at 25°C for 3 hours with either PBS alone or with PBS containing 0.1 nM VIP (synthesized by Prof. M. Fridkin and Ms. S. Rubinraut at the Weizmann Institute of Science; Gozes et al., 1996). After 3 hours, the CM was collected, centrifuged at 3000×g for 10 minutes, dialyzed against water and lyophilized. Protein concentrations were determined using the BCA-200 Protein Assay Kit (Pierce).
Protein extraction
After collecting the CM, cells were harvested and cytoplasmic and nuclear fractions prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce). Proteins were quantified using BCA-200 Protein Assay Kit (Pierce).
ADNP antibody
The ADNP antibody, α-CNAP, recognizes the epitope between residues 354 and 361 of rat and human ADNP (Sigalov et al., 2000; Zamostiano et al., 2001). A purified IgG fraction was prepared using AffinityPak prepacked columns of immunopure plus immobilized protein A (Pierce).
Immunoprecipitation of ADNP in PC12 cells
PC12 cells were cultured as described above and incubated for 10 minutes at 4°C in lysis buffer containing 50 mM Tris.Cl, 300 mM NaCl, 5 mM EDTA, 0.02% (w/v) sodium azide and 1% Triton X 100 supplemented with protease inhibitors. The cell lysate was then incubated at 4°C for 30 minutes followed by centrifugation at 13 000×g at 4°C for 10 minutes. The supernatant was used for ADNP immunoprecipitation. α-CNAP (22 μg) was added to PC12 cell lysate and the sample rotated overnight at 4°C. Pre-cleared Protein A/G Plus agarose beads (50 μl; Santa Cruz Biotechnology) were added to the sample and incubated for 1 hour at 4°C. The protein sample was then spun at 2000×g for 1 minute to pellet the beads and the super-natant removed. The beads were washed four times with lysis buffer, followed by a wash with PBS. Protein-gel sample-buffer was added to the beads and the sample boiled for 10 minutes. The sample was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), as described below.
Recombinant ADNP
Human ADNP (Zamostiano et al., 2001) was cloned into the Voyager vector (Invitrogen). Briefly, the vector contains the Herpes virus protein, VP22, upstream of the insert gene, and a c-myc tag and a His tag downstream of the insert. The vector was transformed into E. coli and protein expression induced by isopropyl-beta-D-thiogalactopyranoside (Sigma). The fusion protein was purified on a nickel column (Ni-AT; Qiagen). Western analysis and α-CNAP and α-cMyc antibodies were used to determine the size of the purified protein. The construct was established as follows:
The insert contains the full-length ADNP cDNA including the initiation codon and excluding the termination codon such that ADNP is fused with the His tag.
SDS–PAGE and Western analysis
SDS–PAGE for ADNP was performed essentially as described (Zamostiano et al., 2001). Proteins (5 μg lane−1) were separated by electrophoresis on a 10% (w/v) polyacrylamide gel (BioRad) containing 0.1% SDS. Molecular weights were determined using Wide Range (6.5–205 kD) Multicolored Protein Markers (NEN Life Science Products). Gel staining was performed using Gelcode Blue Staining Reagent (Pierce) according to manufacturer's instructions.
For Western analysis, gels were not stained but following electrophoresis, the proteins were transferred to polyvinylidene fluoride filters (NEN Life Science Products). Non-specific antigen sites were blocked using a solution containing 5% non-fat dried milk (w/v) in 10 mM Tris pH 8, 150 mM NaCl and 0.05% Tween 20. Antigen detection was performed using α-CNAP (1:5000, final concentration 0.55 μg ml−1). Antibody–antigen complexes were detected using horseradish peroxidase conjugated secondary antibody (1:25 000, Sigma) and visualized by ECL Plus Western blotting detection system (Amersham Pharmacia Biotech). To ascertain antibody specificity, recombinant ADNP, prepared as described above, was added to the blocking solution in a 100-fold higher concentration (w/w) than the primary antibody. For detection of cMyc antigen, α-cMyc monoclonal mouse IgG (1:500, Sigma) was used and antibody–antigen complexes detected using peroxidase-conjugated affinity pure goat anti-mouse IgG (1:25 000, Jackson ImmunoResearch).
Densitometric measurements were performed using the B.I.S. BioImaging System 202D (Dynco-Renium) and the Tina 2.0 Software (Ray Test).
Fluorescent immunostaining
Rat cortical astrocytes were fixed using 4% paraformaldehyde 14 days after being subcultured and fluorescent, double immunostaining performed as follows: Triton X 100 (0.2%) was added to allow cellular penetration by the antibody. After washing with PBS followed by 2% bovine serum albumin in PBS, non-specific antibody binding was blocked using 50 μg ml−1 of goat IgG (Sigma). The primary antibodies used for labeling were (1) rabbit polyclonal α-CNAP (1:20, antibody final concentration, 136 μg ml−1), and (2) mouse monoclonal tubulin antibodies (1:20 TUB 2.5) (Gozes and Barnstable, 1982). Secondary antibodies were (1) Cy2-conjugated affinity-pure goat anti-rabbit IgG (green fluorescence, 1:250; Jackson ImmunoResearch) and (2) rhodamine-labeled secondary goat antimouse IgG (red fluorescence, 1:250; Jackson ImmunoResearch). Phalliodin (red fluorescence, 1:2000; Sigma) was used to stain actin. Fluorescently stained cells were analyzed using Zeiss confocal laserscanning microscope. Zeiss LSM 410 inverted (Oberkochen) is equipped with a 25-milliwatt krypton–argon laser (488 nm and 568 nm maximum lines). A 40×1.2W Apochromat water-immersion lens (Axiovert 135M; Zeiss) was used for all imaging.
RESULTS
Localization of ADNP in astrocytes
ADNP-like immunoreactivity was localized in astrocytes using two independent methods, Western blot analysis and immunocytochemistry. Fractionation of astrocytes followed by Western analyses using α-CNAP, identified ADNP-immunoreactive protein bands in cell nuclei and the cytoplasm (Fig. 1A). Three major protein bands were observed at the higher molecular-weight of the gel (∼116 kDa). ADNP-like immunoreactivity was enriched in the nuclear fraction. Incubation with VIP did not result in a major change in the intracellular distribution of ADNP. Furthermore, densito-metric scanning of the blots (n=3), indicated no significant increase in ADNP concentration following incubation with VIP. To ascertain antibody specificity, recombinant ADNP (100-fold higher concentration than primary antibody) was added to the blocking solution of a duplicate membrane (Fig. 1B). Binding of the antibody to the membrane was mostly inhibited, which showed that the antibody bound specifically to the immobilized ADNP. The band that almost completely disappeared as a consequence of incubation with recombinant ADNP was the middle protein band in the cytoplasmic fractions of Fig. 1A, which indicates that this band represents intact ADNP. Protein staining of a duplicate gel confirmed that the same amount of total protein was loaded in cytoplasmic and nuclear fractions (Fig. 1C), which allowed comparative analysis of the resulting Western blots. In addition, separation of recombinant ADNP (VP22–ADNP–cMyc-His*6) by gel electrophoresis showed an α-CNAP immunoreactive band at 114 kDa (Fig. 2A). Again, binding of α-CNAP antibodies to the membrane was abolished by adding recombinant ADNP at a 100-fold higher concentration than the primary antibody to the blocking solution (Fig. 2B). The identity of the ADNP-like immunoreactive band was further verified using the α-cMyc antibody (Fig. 2C).
Fig. 1.
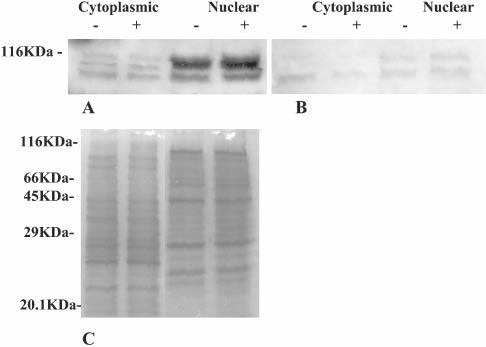
ADNP-like immunoreactivity localizes to the cytoplasm and nucleus of astrocytes. Western blot analysis of proteins extracted from astrocytes that were incubated either with (+) or without (−) VIP. Molecular weights were determined using molecular-weight markers separated with the tested samples. (A) ADNP immunoreactivity in cytoplasmic and nuclear fractions detected using α-CNAP. (B) As in A after incubation with excess of recombinant ADNP in the blocking solution (1:100, antibody:ADNP w/w). (C) Duplicate protein extracts were subjected to 10% polyacrylamide gel electrophoresis and stained with Gelcode Blue Staining Reagent, the resulting electropherogram is depicted. All experiments were repeated at least three times.
Fig. 2.

Separating recombinant ADNP using SDS–PAGE. Western blot analysis of recombinant ADNP (ADNP–VP22 fusion protein). Molecular weight markers were separated alongside the tested samples. (A) Detection of recombinant ADNP (1.7 μg) with α-CNAP. (B) As in A after incubation with excess recombinant ADNP in the blocking solution (1:100, antibody:ADNP w/w). (C) Detecting recombinant ADNP with α-cMyc.
The specificity of α-CNAP was also determined by immunoprecipitation of ADNP from pheochromocytoma (PC12) cell extracts (Fig. 3). PC12 cells have been shown previously to express ADNP mRNA by real-time polymerase chain reaction (Sigalov et al., 2000). These results indicated that PC12 cells are an abundant source of ADNP that is suitable for immunoprecipitation studies. The size of ADNP is estimated at 123562.8 Da, with an apparent molecular weight on SDS–PAGE of ∼114 000 Da (Zamostiano et al., 2001). Here, a single immunoprecipitated band was detected just above the 100 kDa molecular-weight marker, indicating intact ADNP.
Fig. 3.
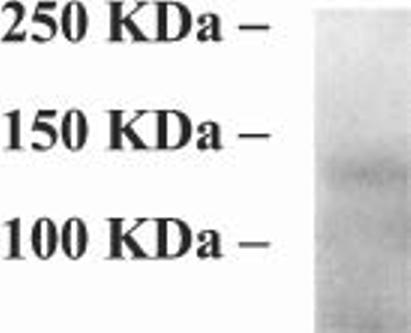
Immunoprecipitation of ADNP in PC12 cells. ADNP was immunoprecipitated from PC12 cells using the α-CNAP antibody, subjected to 10% polyacrylamide gel electrophoresis and stained with Gelcode Blue Staining Reagent. Experiments were repeated three times. A representative electropherogram is shown.
ADNP-like immunoreactivity colocalizes with tubulin
Fluorescent immunostaining with α-CNAP (Fig. 4A,D) also showed high concentrations of ADNP in the nucleus and lesser amounts in the cytoplasm, similar to the results of the Western analysis above. In the cytoplasm, the appearance of ADNP-like immunoreactivity indicated a possible interaction with cytoskeletal elements in selected astrocyte populations. Therefore, double labeling of ADNP and either microtubules (tubulin) (Fig. 4B,C) or microfilaments (actin) (Fig. 4E,F) was performed. Cytoplasmic staining of ADNP (Fig. 4A) and tubulin/microtubule staining (Fig. 4B) was similar and double-label immunofluorescence analysis showed the two to colocalize, in part (Fig. 4C). Higher magnification indicated ADNP-like immunoreactivity decorating microtubules (Fig. 4G). ADNP-like staining on microtubules was quantitated in three experiments (each in five different fields): in experiment one there were 164±9.3 dots on microtubules and 63±7.6 not on microtubules (P<0.001); experiment two had 104±5.6 dots on microtubules and 36.4±7.8 not on microtubules (P<0.001); and in experiment three there were 147±18 dots on microtubules and 41.6±6.0 not on microtubules (P<0.001). By contrast, there was no colocalization with actin filaments (Fig. 4F).
Fig. 4.
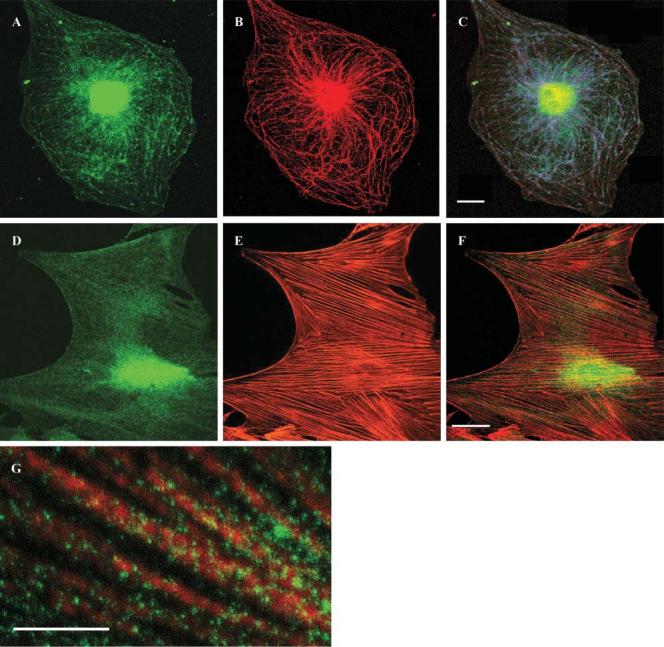
Tubulin, actin and ADNP-like immunoreactivity in astrocytes. (A,D) ADNP-like immunoreactivity (green) determined using α-CNAP. (B) Tubulin immunoreactivity (red) determined using α-tubulin antibodies. (C) Areas of colocalization of ADNP and tubulin are indicated in blue. (E) Actin microfilaments stained by phalloidin (red). (F) ADNP-like immunoreactivity (green) does not colocalize with actin microfilaments (red). (G) Higher magnification of C. Scale bar, 10 μm. Experiments were repeated at least three times.
ADNP is found in the extracellular milieu of astrocytes
It has been shown previously that VIP stimulates the secretion of trophic proteins from astroglial cells to provide a neurotrophic milieu (Gozes and Brenneman, 1996; Bassan et al., 1998; Brenneman et al., 2003) and ADNP was identified originally as a VIP-responsive gene in astrocytes (Bassan et al., 1999). We have shown before that in this model system there is no extracellular activity of lactate dehydrogenase, which indicates that there is no release from broken cells (Bassan et al., 1998). Here, we asked whether (1) ADNP occurs in the extracellular milieu of astrocytes and, if so, (2) whether ADNP secretion is stimulated by VIP. Results showed that α-CNAP detects an ADNP-like immunoreactive protein in conditioned media from astrocytes (Fig. 5A). Densitometric analysis showed that this extracellular, ∼114 000 kDa, ADNP-like immunoreactive band was enhanced ∼1.4 fold after incubation with VIP for 3 hours (Fig. 5A). ADNP-immunospecificity was demonstrated by immunoabsorption using a purified ADNP-recombinant protein (Fig. 5B).
Fig. 5.
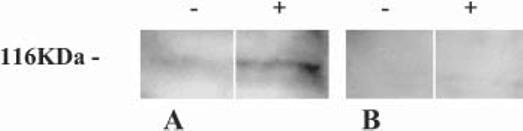
ADNP-like immunoreactivity in conditioned media from astrocytes. Western blot analysis on conditioned media from astrocytes incubated either with (+) or without (−) 0.1 nM VIP for 3 hours. To allow comparative analyses, 5 μg of total protein were loaded on each lane. (A) ADNP immunoreactivity determined using α-CNAP. (B) As in A after incubation with an excess of the recombinant ADNP in the blocking solution (1:100, antibody:ADNP w/w).
CONCLUSIONS
ADNP-like immunoreactivity is localized mainly to the nucleus of astrocytes.
There is some ADNP-like immunoreactivity in the cytoplasm of astrocytes.
ADNP-like immunoreactivity colocalizes with tubulin/microtubules but not actin filaments.
Secretion of ADNP from astrocytes is enhanced by incubation with VIP.
DISCUSSION
The current study detected for the first time increased ADNP-like immunoreactivity in the nucleus compared with the cytoplasm of astrocytes. Furthermore, ADNP-like immunoreactivity was identified in conditioned media from astrocytes, and the concentration increased after treatment with VIP. Previous studies utilizing antibodies directed against other epitopes of ADNP have detected a protein of a similar molecular weight in cancer cells (Zamostiano et al., 2001) and in developing mouse embryos (Pinhasov et al., 2003). The subcellular localization of the protein indicated that it might be directed to different pathways in the cell, with the largest concentration directed to the nucleus. Previous studies have shown that ADNP can be alternatively spliced (Zamostiano et al., 2001). In the case of parathyroid hormone-related peptide (Nguyen et al., 2001) and fibroblast growth factor (Bugler et al., 1991), nuclear localization and secretion is regulated by alternative splicing. Future studies will decipher if this is also the case for ADNP.
ADNP mRNA is regulated by VIP (Bassan et al., 1999), and here we demonstrate that incubation with VIP resulted in higher levels of ADNP-like immunoreactivity in conditioned media from astrocytes. VIP is known to produce a neurotrophic milieu (Brenneman et al., 1990; Gozes et al., 1991; Waschek, 1995; Brenneman and Gozes, 1996; Gozes and Brenneman 1996; Gressens, 1999; Brenneman et al., 2003), and it has been suggested that ADNP constitutes part of this protective environment. However, it is unclear whether ADNP is released into the medium by a constitutive or regulated process.
ADNP contains a homeobox profile that includes a leucine-rich sequence that is homologous to that of the homeoprotein engrailed (Gozes et al., 2000b). The leucine-rich sequence is necessary for the secretion (Joliot et al., 1998) and nuclear export (Maizel et al., 1999) of engrailed. In recent years, a growing number of proteins have been identified that can be either internalized or secreted and gain access to the cell cytoplasm and nucleus (Henderson, 1997; Prochiantz, 2000). Bioinformatics identifies an additional homology between the cytoplasmic-import sequence of engrailed and ADNP. The sequence SDIASHFSNKRKKCVR occurs in the homeodomain profile of ADNP and the homologous sequence in engrailed (SQIKIWFQNKRAKKIK) is important for the internalization of the protein into cells. It is our working hypothesis that ADNP might be internalized by other cells, such as neurons, after secretion from glial cells.
As indicated previously, ADNP has a role in the developing fetal mouse brain, and is associated with the activation of vital genes (Pinhasov et al., 2003). Colocalization of ADNP-like immunoreactivity with tubulin-like immunoreactivity (but not with actin microfilaments) was observed in selected astrocyte populations. Tubulin is an essential cytoskeletal protein that is important for cell shape, transport, motility and division (Gozes and Barnstable, 1982; Nogales, 2001), and microtubules are key subcellular components of the developing and mature brain (Gozes and Littauer, 1979; Gozes and Sweadner, 1981). It is possible that the proposed interaction between ADNP and microtubules is associated with ADNP transport between different domains of the cell. A similar colocalization of microtubules and the parathyroid hormone-related protein is involved in the transport of the latter into the nucleus (Lam et al., 2002). Recent studies have shown that the active peptide moiety of ADNP (NAP) interacts with tubulin (Divinski et al., 2004) to enhance cellular protection. Furthermore, NAP shares structural similarities with protein sequences that can penetrate the cell membrane at 4°C and at low pH, independent of a cellular receptor and are incorporated into cells.
In conclusion, ADNP contains motifs that are responsible for protein transport. Furthermore, ADNP is thought to have several roles that depend on subcellular localization. We suggest possible roles for ADNP in brain development and maintenance, through a potential tubulin–ADNP interaction. Furthermore, we suggest that ADNP is a nuclear transcription factor, and that it might function as a part of the neuroprotective extracellular milieu provided by astrocytes.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Leonid Mittelman for invaluable help with the confocal microscopy and Ms. I. Divinski for assistance with fluorescent immunostaining. We thank Prof. M. Fridkin and Ms. S. Rubinraut for VIP and Prof. C.J. Barnstable for the help with the tubulin antibodies, and Dr. R. Zamostiano and Ms. I. Zemlyak who helped in the initial stages of the work. This work is in partial fulfillment of the requirements towards the Ph.D. degrees of S.F. and S.M. This study was supported by the US–Israel Bi-National Science Foundation, the Neufeld Award, the Combined Program of the National Institutes of Health and the Sackler Faculty of Medicine/Tel-Aviv University in Women's Health, the Israel Science Foundation, the Institute for the Study of Aging, and Allon Therapeutics Inc. I.G. holds the Lily and Avraham Gildor Chair for the Investigation of Growth Factors. ADNP is under patent protection.
REFERENCES
- Alcalay RN, Giladi E, Pick CG, Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neuroscience Letters. 2004;361:128–131. doi: 10.1016/j.neulet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Giladi E, Davidson A, Wollman Y, Pitman J, Hauser J, Brenneman DE, Gozes I. The identification of secreted heat shock 60-like protein from rat glial cells and a human neuroblastoma cell line. Neuroscience Letters. 1998;250:37–40. doi: 10.1016/s0304-3940(98)00428-5. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. Journal of Neurochemistry. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Nicol T, Warren D, Bowers LM. Vasoactive intestinal peptide: a neurotrophic releasing agent and an astroglial mitogen. Journal of Neuroscience Research. 1990;25:386–394. doi: 10.1002/jnr.490250316. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. Journal of Neurochemistry. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Hill JM, Glazner GW, Gozes I, Phillips TW. Interleukin-1 alpha and vasoactive intestinal peptide: enigmatic regulation of neuronal survival. International Journal of Developmental Neuroscience. 1995;13:187–200. doi: 10.1016/0736-5748(95)00014-8. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide. Journal of Clinical Investigation. 1996;97:2299–2307. doi: 10.1172/JCI118672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Phillips TM, Hauser J, Hill JM, Spong CY, Gozes I. Complex array of cytokines released by vasoactive intestinal peptide. Neuropeptides. 2003;37:111–119. doi: 10.1016/s0143-4179(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Brown DR. Neuronal release of vasoactive intestinal peptide is important to astrocytic protection of neurons from glutamate toxicity. Mol. Cel. Neurosci. 2000;15:465–475. doi: 10.1006/mcne.2000.0840. [DOI] [PubMed] [Google Scholar]
- Bugler B, Amalric F, Pratz H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Molecular and Cellular Biology. 1991;11:573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. Journal of Biological Chemistry. 2004;279:28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Littauer UZ. The alpha-subunit of tubulin is preferentially associated with brain presynaptic membrane. FEBS Letters. 1979;99:86–90. doi: 10.1016/0014-5793(79)80255-0. [DOI] [PubMed] [Google Scholar]
- Gozes I, Sweadner KJ. Multiple tubulin forms are expressed by a single neuron. Nature. 1981;294:477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Gozes I, Barnstable CJ. Monoclonal antibodies that recognize discrete forms of tubulin. Proceedings of the National Academy of Sciences of the U.S.A. 1982;79:2579–2583. doi: 10.1073/pnas.79.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, McCune SK, Jacobson L, Warren D, Moody TW, Fridkin M, Brenneman DE. An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 1991;257:959–966. [PubMed] [Google Scholar]
- Gozes I, Bardea A, Reshef A, Zamostiano R, Zhukovsky S, Rubinraut S, Fridkin M, Brenneman DE. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proceedings of the National Academy of Sciences of the U.S.A. 1996;93:427–432. doi: 10.1073/pnas.93.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. Activity-dependent neurotrophic factor (ADNF). An extracellular neuroprotective chaperonin? Journal of Molecular Neuroscience. 1996;7:235–244. doi: 10.1007/BF02737061. [DOI] [PubMed] [Google Scholar]
- Gozes I, Giladi E, Pinhasov A, Bardea A, Brenneman DE. Activity-dependent neurotrophic factor: intranasal administration of femtomolar-acting peptides improve performance in a water maze. Journal of Pharmacology and Experimental Therapeutics. 2000a;293:1091–1098. [PubMed] [Google Scholar]
- Gozes I, Zamostiano R, Pinhasov A, Bassan M, Giladi E, Steingart RA, Brenneman DE. A novel VIP responsive gene: activity dependent neuroprotective protein. Annals of the New York Academy of Sciences. 2000b;921:115–118. doi: 10.1111/j.1749-6632.2000.tb06957.x. [DOI] [PubMed] [Google Scholar]
- Gressens P. VIP neuroprotection against excitotoxic lesions of the developing mouse brain. Annals of the New York Academy of Sciences. 1999;897:109–124. doi: 10.1111/j.1749-6632.1999.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Henderson JE. Nuclear targeting of secretory proteins. Molecular and Cellular Endocrinology. 1997;129:1–5. doi: 10.1016/s0303-7207(97)04021-5. [DOI] [PubMed] [Google Scholar]
- Houenou LJ, Turner PL, Li L, Oppenheim RW, Festoff BW. A serine protease inhibitor, protease nexin I, rescues motoneurons from naturally occurring and axotomy-induced cell death. Proceedings of the National Academy of Sciences of the U.S.A. 1995;92:895–899. doi: 10.1073/pnas.92.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Maizel A, Rosenberg D, Trembleau A, Dupas S, Volovitch M, Prochiantz A. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Current Biology. 1998;8:856–863. doi: 10.1016/s0960-9822(07)00346-6. [DOI] [PubMed] [Google Scholar]
- Lam MH, Thomas RJ, Loveland KL, Schilders S, Gu M, Martin TJ, Gillespie MT, Jans DA. Nuclear transport of parathyroid hormone (PTH)-related protein is dependent on microtubules. Molecular Endocrinology. 2002;16:390–401. doi: 10.1210/mend.16.2.0775. [DOI] [PubMed] [Google Scholar]
- Maizel A, Bensaude O, Prochiantz A, Joliot A. A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development. 1999;126:3183–3190. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- Martin J-L, Magistretti PJ. Release of Vasoactive intestinal peptide in mouse cerebral cortex: evidence for a role of arachidonic acid and metabolites. Journal of Neuroscience. 1989a;9:2536–2542. doi: 10.1523/JNEUROSCI.09-07-02536.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J-L, Magistretti PJ. Pharmacological evidence for a role of voltage-sensitive Ca2+ channels of the T-type in the release of Vasoactive intestinal peptide evoked by K+ in mouse cerebral cortical slice. Neuroscience. 1989b;30:423–431. doi: 10.1016/0306-4522(89)90262-5. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. Journal of Cell Biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, Partlow LM. Preparation of pure neuronal and non-neuronal cultures from embryonic chick sympathetic ganglia: a new method based on both differential cell adhesiveness and the formation of homotypic neuronal aggregates. Brain Research. 1976;114:391–414. doi: 10.1016/0006-8993(76)90962-8. [DOI] [PubMed] [Google Scholar]
- Nogales E. Structural insights into microtubule function. Annual Review of Biophysical and Biomolecular Structure. 2001;30:397–420. doi: 10.1146/annurev.biophys.30.1.397. [DOI] [PubMed] [Google Scholar]
- Nguyen MTA, He B, Karaplis AC. Nuclear forms of parathyroid hormone-related peptide are translated from non-AUG start sites downstream from the initiator methionine. Endocrinology. 2001;142:694–703. doi: 10.1210/endo.142.2.7944. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi A, Pittel Z, Goldsweig AM, Servoss SJ, Brenneman DE, Gozes I. Activity dependent neuroprotective protein: a novel gene essential for brain formation. Brain Research Developmental Brain Research. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Current Opinion in Cell Biology. 2000;12:400–406. doi: 10.1016/s0955-0674(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Sigalov E, Fridkin M, Brenneman DE, Gozes I. VIP-Related protection against lodoacetate toxicity in pheochromocytoma (PC12) cells: a model for ischemic/hypoxic injury. Journal of Molecular Neuroscience. 2000;15:147–154. doi: 10.1385/JMN:15:3:147. [DOI] [PubMed] [Google Scholar]
- Steingart RA, Solomon B, Brenneman DE, Fridkin M, Gozes I. VIP and peptides related to activity-dependent neurotrophic factor protect PC12 cells against oxidative stress. Journal of Molecular Neuroscience. 2000;15:137–145. doi: 10.1385/JMN:15:3:137. [DOI] [PubMed] [Google Scholar]
- Waschek JA. Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Developmental Neuroscience. 1995;17:1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. Journal of Biolological Chemistry. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]


