Abstract
ING-1(heMAb), a Human Engineered™ monoclonal antibody to epithelial cell adhesion molecule (Ep-CAM), was evaluated for its in vitro and in vivo activity. The dissociation constant of ING-1(heMAb) for binding to Ep-CAM on HT-29 human colon tumor cells was 2 to 5 nM, similar to chimeric ING-1. In antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity assays, ING-1(heMAb) caused a concentration -dependent lysis of BT-20 breast, MCF-7 breast, HT-29 colon, and CACO-2 colon tumor cells, with maximum cytolysis at approximately 1 µg/ml. After an intravenous injection in rats, plasma ING-1(heMAb) levels declined with an alpha half-life of 8 to 11 hours, and a beta half-life of 20 days, typical of an IgG in a species without the target for ING-1. In nude mice with human HT-29 colon tumors, plasma ING-1(heMAb) levels declined more rapidly than in non-tumor-bearing mice, suggesting an enhanced clearance via the tumor-associated human Ep-CAM. In nude mice, intravenous treatments with ING-1(heMAb) twice a week for 3 weeks significantly suppressed the growth of human HT-29 colon and PC-3 prostate tumors in a dose-dependent manner, with 1.0 mg/kg providing the greatest benefit. These results indicate that Human Engineered™ ING-1(heMAb) is a high-affinity antibody with potent in vitro activity that targets and suppresses the growth of human tumors in vivo.
Keywords: ING-1, ADCC, xenograft, pharmacokinetics, anti-Ep-CAM
Introduction
Monoclonal antibodies that target tumor-associated antigens have been developed for the imaging and treatment of human neoplasias. Because many of these antibodies are of murine origin, a number of difficulties, including a strong human anti-mouse antibody response and rapid clearance in patients, have limited their therapeutic potentials [1,2]. These problems are overcome or reduced by using chimeric antibodies that retain the specificity of the mouse variable region and add the effector function of the human constant region [3,4]. However, some studies indicate that chimeric antibodies may remain immunogenic [5,6]. More recently, murine-derived antibodies have been humanized to further reduce these complications [7], or fully human antibodies have been produced [8,9]. A number of techniques have been utilized to humanize murine variable regions, commonly by grafting murine complementarity-determining region (CDR) loops onto human framework regions [10].
One tumor-associated antigen that is often targeted by chimeric and humanized antibodies is epithelial cell adhesion molecule (Ep-CAM), also known as the 17-1A antigen [11], KSA [12], EGP [13], EGP40 [14], and GA733-2 [15]. Ep-CAM is a 40-kDa glycoprotein expressed on the basolateral surface of many, but not all, human epithelial cells and most human adenocarcinomas [16]. Murine antibodies to Ep-CAM were originally described by Herlyn et al. [11] in 1979 and chimeric versions of anti-Ep-CAM antibodies were subsequently developed [17]. Early chimeric antibodies inhibited tumor growth in animal models, most likely via antibody-dependent cellular cytotoxicity (ADCC) [18–20]. Subsequently developed high-affinity antibodies have even greater in vivo activity, perhaps due to enhanced ADCC [21–24]. The murine and chimeric versions of 17-1A have been studied in patients with adenocarcinomas [5,25]. In addition, a humanized version of 323/A3 has received initial clinical evaluation [26]. These studies have established safe doses for these antibodies and have suggested benefits in some patients [25].
In 1990, Liao et al. [27] described a high-affinity chimeric monoclonal antibody to Ep-CAM, called ING-1, that was derived from the murine antibody B38.1 [28], also described as BA-Br-1 or Br-1 [29]. Chimeric ING-1 demonstrated potent ADCC and complement-dependent cytotoxicity (CDC) against a variety of tumor cell lines in vitro [29]. The variable region of ING-1 has now been modified to further reduce the potential for immunogenicity in humans using the Human Engineering™ technology developed by Studnicka et al. [30]. This technology is an alternate approach to humanization of murine antibodies that takes advantage of the conserved nature of the variable region structure. By this approach, each amino acid within the variable regions is analyzed and classified based on the benefit of achieving more human-like sequences compared with the risk of adversely affecting binding. Low-risk changes from murine to corresponding human residues represent changes made to surface-located amino acids not directly involved in binding or variable region structure. Moderate risk changes may further reduce immunogenicity but may potentially impact binding. High-risk changes are those that either directly impact binding or affect the proper folding or association of the variable regions. The Human Engineered™ version of ING-1 that has resulted from this approach, ING-1(heMAb), has completed preclinical and initial clinical evaluations. ING-1(heMAb) is thus the first antibody developed with this Human Engineering™ technology to be tested in patients. The clinical results available describe the safety and immunogenicity of ING-1(heMAb) [31,32]. No antibody response to the administration of ING-1(heMAb) was detectable in 17 of 19 patients and only minimal responses were detected in two patients. The minimal immunogenicity of ING-1(heMAb) in patients represents the initial validation of the Human Engineering technology. However, in order for the Human Engineering™ approach to be truly useful, it is necessary to provide evidence that antibodies generated from this approach demonstrate biological activity, in addition to low immunogenicity. Thus, we describe here the in vitro activity, in vivo efficacy, and pharmacokinetics of ING-1(heMAb), hereafter referred to as ING-1.
Materials and Methods
Materials
The Human Engineered™ ING-1 variable region was derived from the murine B38.1 antibody by the method of Studnicka et al. [30]. Briefly, DNA encoding 13 surfaceexposed amino acids in the murine heavy chain variable region, and 6 in the light chain variable region were modified to encode residues derived from human consensus sequences. These 19 residues were selected after all variable region residues had been assigned a risk value (low, moderate, or high) as described [30]. These amino acids were then modified to residues found in human light and heavy chains at positions that had low risk for interfering with either antigen binding or protein folding. ING-1 was produced from Chinese hamster ovary (CHO) cells containing synthetic heavy and light chain genes encoding the modified variable regions linked to human IgG1 and kappa constant region cDNA, respectively. ING-1 was purified and then formulated in 20 mM sodium phosphate, 0.15 M sodium chloride, and 0.005% polysorbate 80. Cell culture media, DME/F12, RPMI 1640, and trypsin-EDTA were obtained from Life Technologies (Rockville, MD). Soluble Ep-CAM was produced by CHO-K1 cells transfected with cDNA encoding the extracellular region of Ep-CAM.
Binding Studies
In preparation for binding studies, HT-29 cells were grown to confluency in 96-well plates. 125I-labeled ING-1 (0.1 nM) was mixed with unlabeled chimeric or Human Engineered™ ING-1 that was two-fold serially diluted from 1 µM to 0.24 nM in 100 µl of McCoy's 5A medium supplemented with 1% BSA and 10 mM HEPES (MCM buffer). Samples were incubated at 2 to 8°C for 5 hours and then washed three times with ice-cold MCM buffer. Bound radioactivity in the wells was removed by adding 100 µM NaOH and counted in a LKB gamma counter. The results are depicted as the mean of three replicate samples.
ADCC and CDC Lysis Assays
Target cells for lysis assays were cultured in DME/F12 supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). For labeling, cells were harvested with trypsin-EDTA, resuspended in RPMI 1640 at 5x106 ml-1 (1–2 ml), and incubated with 100 µCi/ml 51Cr (NEN, Boston, MA) for 45 to 60 minutes at 37°C. Cells were washed twice with RPMI 1640 and resuspended in the appropriate medium before use.
ADCC assays were performed with peripheral blood mononuclear cells (PBMCs) prepared from blood obtained from healthy volunteers using acid citrate dextrose as an anticoagulant. Sources included blood collected in Vacutainer collection tubes (Becton Dickinson, Franklin Lakes, NJ), buffy coat cells obtained from the blood bank (American Red Cross Blood Services, Oakland, CA), and lymphapheresis cells (HemaCare, Sherman Oaks, CA). PBMCs were isolated on a Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) step gradient and suspended in RPMI 1640 supplemented with 10% normal human AB serum (ABS; Sigma, St. Louis, MO). PBMCs (8x105) were mixed with labeled target cells (104) and varying concentrations of ING-1, diluted in RPMI 1640 plus 10% ABS in roundbottomed 96-well assay plates. The plates were centrifuged for 1 minute at 250g then incubated at 37°C. After 4 hours, the plates were centrifuged for 5 minutes at 550g and the supernatant medium was collected with a Skatron harvestor.
CDC assays were performed with pooled human serum collected from four healthy volunteers. Labeled target cells were suspended in RPMI 1640 at 4x105 cells/ml. Target cells (2x104) were mixed with serum and varying concentrations of ING-1, diluted in RPMI 1640 in round-bottomed 96-well microtiter plates. Assay plates were incubated at 37°C for 3 hours, centrifuged at 550g for 5 minutes, and the supernatant liquid was collected with a Skantron harvestor.
Percent lysis was calculated by the equation:
where Spontaneous CPM was determined from wells containing no ING-1 and Maximum CPM was determined from wells where target cells were lysed with 1 M HCl.
Pharmacokinetic Studies
Male CD rats (Charles River, Hollister, CA) weighing 280 to 320 g, or male athymic nude mice (NCR nu/nu; Simonsen Laboratories, Gilroy, CA) weighing 20 to 30 g were housed in conventional cages. The animals received standard laboratory chow and water ad libitum in an environmentally controlled animal room with 12-hour light-dark cycles.
Five male rats received 50 mg/kg ING-1 (50 mg/ml), and another five male rats received 0.5 mg/kg of ING-1 (0.5 mg/ml) via the tail vein. Blood was collected on days 0 to 91 via the retro-orbital sinus under methoxyflurane anesthesia. Six male rats received 5 mg/kg ING-1 (5 mg/ml) subcutaneously at a single location. Blood samples were collected from days 0 to 196 after dose injection. In all rat experiments, approximately 200 µl of blood was collected at each time point into microcentrifuge tubes containing sodium citrate. Plasma was extracted and stored at -70°C until assayed.
Ten male nude mice received 5 mg/kg ING-1 (0.5 mg/ml) via a tail vein. In order to minimize volume depletion, blood (100 µl) was collected from five mice after dose injection, and at 6 hours, 3, 14, 28, 42, and 84 days later. Blood was obtained from another five mice after dose injection, and then 1, 7, 21, 35, 56, and 112 days later. Ten male nude mice with HT-29 tumors received 5mg/kg ING-1 (0.5 mg/ml) via the tail vain. Blood collection was staggered among the mice as before.
Measurement of ING-1 in Plasma
ING-1 was measured in rat plasma by enzyme-linked immunosorbent assay (ELISA). Microtiter plates were coated with the capture reagent, soluble Ep-CAM (XOMA, Berkeley, CA), diluted in 0.25 µg/ml phosphate buffered saline (PBS). The detection system consisted of alkaline phosphatase-conjugated goat anti-human IgG antibody (Zymed Laboratories, South San Francisco, CA) with p-nitrophenylphosphate as substrate. Color development was allowed to proceed for 1 hour at room temperature and then terminated with 100 µl of 1 N NaOH. The absorbance at 405 nm was determined for all wells using a Vmax Plate Reader (Molecular Devices, Menlo Park, CA). A standard curve was generated and samples were quantified by interpolation from the standard curve. Plasma standards were prepared by adding known amounts of ING-1 to plasma. These standards were used to calculate the proportion of ING-1 recovered by the assay in plasma. A linear regression of ING-1 concentration measured by ELISA versus added ING-1 concentration was performed, and the calculated slope was used as the fractional recovery. The plasma concentrations of ING-1 in the samples were then corrected for the recovery.
Measurement of Antibodies to ING-1
Antibodies to ING-1 in the rat were assayed by ELISA. Microtiter plates were coated with ING-1 to which rat plasma samples were added. The signaling system consisted of biotin-conjugated ING-1 to which was added alkaline phosphatase- conjugated streptavidin (Zymed) as the enzyme for the substrate p-nitrophenylphosphate. Standards of different concentrations of goat anti-human IgG (Sigma) were assayed to convert the absorbance measurements of rat samples into goat anti-human IgG microgram-permilliliter equivalents.
Pharmacokinetic Analysis
Data were entered on an Alpha 3000, model 600 computer (Compaq, Maynard, TX), and analyzed using a validated software system developed at XOMA. Data of individual animals were fitted by nonlinear least squares analysis using the pharmacokinetic biexponential disposition function to describe the change in the concentration of ING-1 with time, with the inverse of the square of the model concentration as the weighting. The curve fits yielded four primary pharmacokinetic parameters: volume of distribution of the central compartment, the alpha half-life, the beta half-life, and the coefficient to the beta half-life. Secondary pharmacokinetic parameters were calculated from the primary parameters in accordance with Gibaldi and Perrier [33].
Mouse Xenograft Models
Male athymic nude mice (NCR nu/nu; Simonsen Laboratories ), 20 to 25 g, were maintained in a pathogen-free facility. The mice were kept in filter-topped cages and handled under a laminar flow hood. Each mouse received a subcutaneous injection of 3x106 HT-29 colon tumor cells or 5x106 PC-3 prostate cells in a flank region. After 24 hours, groups of 10 mice received ING-1 at 0.1, 0.3, or 1.0 mg/kg. Control mice received 1 mg/kg human IgG1. Dosing was continued for 3 weeks, at two doses per week. After tumors could be palpated, length and width measurements were obtained twice a week with microcalipers. Tumor volumes were calculated as LW2/2. Differences in mean tumor volumes between groups were analyzed by a one-way analysis of variance with repeated measures. Post-hoc analysis was performed with Tukey's honest significant difference test.
Results
ING-1 Binding to Ep-CAM
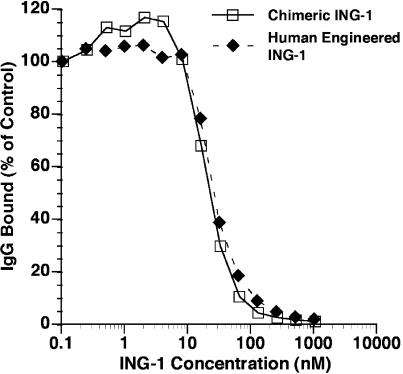
The ability of increasing concentrations of unlabeled ING-1 to compete with a fixed concentration of 125I-labeled chimeric ING-1 for binding to Ep-CAM on HT-29 human colon tumor cells was evaluated. Both ING-1 and chimeric ING-1 competed similarly with radiolabeled chimeric ING-1 for binding to Ep-CAM (Figure 1). Scatchard analysis of the data from four independent experiments was used to calculate an antibody dissociation constant (Kd) of 2 to 5 nM for ING-1. The number of antigen sites on HT-29 cells was estimated to be 1.5x106 per cell. The affinity of ING-1 for Ep-CAM on HT-29 colon tumor cells appeared to be indistinguishable from that previously described for the chimeric ING-1 antibody [29].
Figure 1.
Competition binding assay. HT-29 colon tumor cells (2x105 cells/well) were incubated at 4°C for 5 hours in the presence of 0.1 nM 125I-labeled chimeric ING-1 and increasing concentrations of unlabeled ING-1 or chimeric ING-1. The cells were then washed and counted.
ADCC and CDC Assays
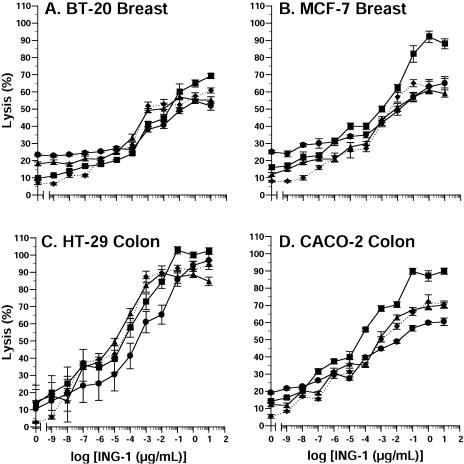
PBMCs from four separate donors and 51Cr-labeled human tumor target cells (80:1 ratio) were incubated with increasing concentrations of ING-1. ING-1 caused a concentration-dependent lysis of BT-20 breast (Figure 2A), MCF-7 breast (Figure 2B), HT-29 colon (Figure 2C), and CACO-2 colon (Figure 2D) tumor cells. Although maximal killing occurred at approximately 1 µg/ml, significant cytotoxicity was observed at much lower concentrations. In other experiments, ING-1 caused similar levels of lysis of non small cell lung (NCI-H1568), prostate (PC-3), and pancreatic (HPAF-II) tumor cells (data not shown).
Figure 2.
ADCC activity of ING-1 against human BT-20 breast (A), MCF-7 breast (B), HT-29 colon (C) and CACO-2 colon (D) tumor cells. Increasing concentrations of ING-1 were added to wells containing human blood mononuclear cells and 51Cr-labeled human tumor cells in an 80:1 ratio. After incubation for four hours at 37°C, cell lysis was determined by counting 51Cr released into the medium. Data are from four human donors. Each symbol represents a single donor.
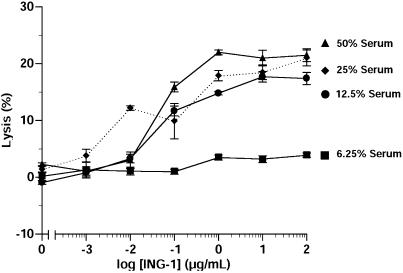
In order to determine if ING-1 mediates CDC, 51Cr-labeled HT-29 colon tumors cells were incubated with different amounts of serum. The ability of increasing concentrations of ING-1 to lyse the tumor cells was measured as a release of 51Cr into the supernatant. ING-1 caused a dosedependent lysis of the tumors cells with maximal killing occurring at approximately 1 µg/ml (Figure 3).
Figure 3.
CDC activity of ING-1. Increasing concentrations of ING-1 were added to wells containing 2x104 51Cr-labeled HT-29 colon tumor cells and different amounts of human serum. After 24 hours of incubation at 37°C, 51Cr was counted.
Pharmacokinetics in Rats
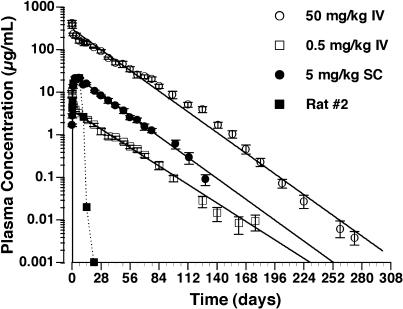
The decline in plasma concentration with time of intravenously administered ING-1 in an antigen-negative species (rats) could be described by a biexponential pharmacokinetic disposition function (Figure 4 and Table 1). The alpha-phase half-lives were approximately 6 and 13 hours for 0.5 and 50 mg/kg, respectively, whereas the beta-phase half-lives were approximately 18 and 17 days, respectively (Table 1). The clearance was approximately 4.5 ml/kg per day at the two doses. Thus, the clearance of ING-1 was dose-independent over the dose range studied. The plasma concentration-time profile did not reveal a change of kinetics at 10 to 14 days or later, suggesting that there was no host antibody production that altered ING-1 clearance.
Figure 4.
Plasma clearance of ING-1 in rats. ING-1 was administered intravenously (0.5 and 50 mg/kg) or subcutaneously (5 mg/kg), and plasma levels were measured by ELISA.
Table 1.
Pharmacokinetic Parameters in Rats.
| Dosing (mg/kg) | Vc (ml/kg | Vss (ml/kg) | Cl (ml/kg per day) | MRT (hours) | α (hours) | β (days) |
| 0.5, i.v. | 49.2±2.9 | 108±7 | 4.43±0.33 | 24.5±1.5 | 12.9±6.2 | 17.7±0.9 |
| 50, i.v. | 75.2±8.1 | 114±9 | 4.56±0.37 | 25.0±0.8 | 5.85±0.90 | 17.4±0.6 |
| Dosing (mg/kg) | Tmax (hours) | Cmax (µg/ml) | Cl (ml/kg per day) | MRT (hours) | α (hours) | β (days) |
| 5.0, s.c. | 4.94±0.41 | 21.5±0.7 | 7.95±0.43 | 25.9±1.2 | 29.4±3.5 | 16.7±0.8 |
Vc, volume of distribution of the central compartment; Vss, steady state volume of distribution; Cl, clearance; MRT, mean residence time; α, alpha-phase half-life; β, beta-phase half-life; Fβ, fraction of clearance attributable to the beta-phase half-life; Tmax, time to maximal concentration; Cmax, maximal concentration.
After subcutaneous administration of 5.0 mg/kg ING-1, plasma concentrations increased to a peak concentration of 21.5±0.7 µg/ml by 4.94±0.41 days. Thereafter, the plasma ING-1 levels declined with a half-life of 16.7±0.8 days, similar to the beta half-life observed after intravenous administration. The bioavailability of subcutaneously administered ING-1 relative to intravenously administered ING-1 was calculated to be 57±4%. In one of the rats, the ING-1 plasma level declined rapidly after day 7, and was below detection by day 14. As a result of this observation, plasma from all rats was assayed for anti-human antibodies. Antibodies were detected in the plasma of the one rat with altered clearance 21 and 70 days after injection, but not in the predose sample. No detectable levels of anti-human antibody were measured in the other rats.
Pharmacokinetics in Nude Mice
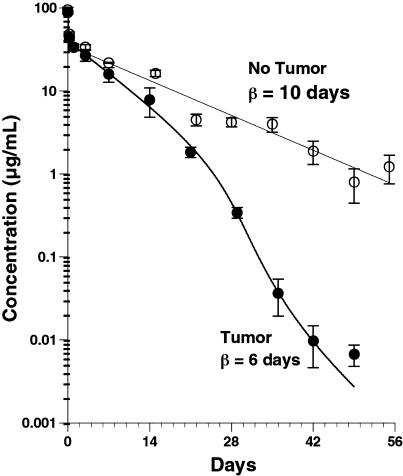
Thirteen male nude mice with HT-29 human colon tumors that averaged 195 mm3 received an intravenous bolus of 5 mg/kg ING-1. By 49 days postdose, the average tumor volume was 2200 mm3. An additional 13 mice that did not bear tumors received an intravenous bolus of 5 mg/kg ING-1. In mice without tumors, the decline in plasma concentration of ING-1 with time could be described by a biexponential pharmacokinetic disposition function (Figure 5). The average alpha-phase half-life was 2.8±2.4 hours and the beta-phase half-life was 10.1±0.5 days. The central compartment volume of distribution was 53±17 ml/kg, similar to plasma volume, and the clearance was 9.7±1.1 ml/kg per day. In tumor-bearing mice, the decline in plasma concentration of ING-1 with time could be described by a biexponential pharmacokinetic disposition function for the first 14 days after dosing. The average alpha-phase half-life was 1.9±0.7 hours and the beta-phase half-life was 5.7±0.4 days. The central compartment volume of distribution was 56±5 ml/kg and the clearance, based on the first 14 days, was 15.1±0.7 ml/kg per day. After 14 days, the plasma concentration of ING-1 declined more rapidly to near detection levels (5 ng/ml) on day 49, with an effective half-life of about 2 days.
Figure 5.
Plasma clearance of ING-1 in nude mice. Nude mice with or without human HT-29 colon tumors were administered 5 mg/kg ING-1, i.v., and plasma levels were measured by ELISA.
Efficacy in Mouse Xenograft Models
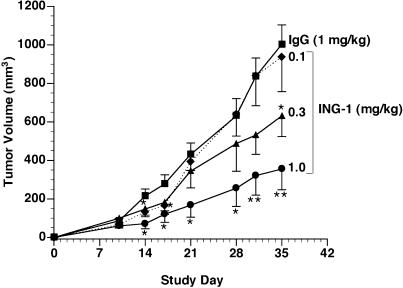
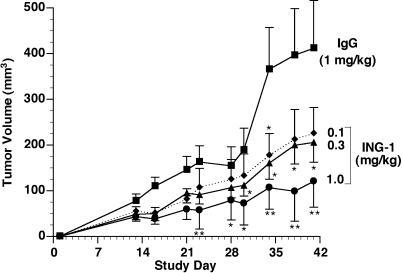
Figure 6 illustrates the effect of ING-1 on growth of HT-29 colon tumors in nude mice. ING-1 treatment two times per week, intravenously for 3 weeks, resulted in a dosedependent reduction in tumor size relative to control. A dose of 1 mg/kg ING-1 resulted in a 64% reduction in tumor size at the end of the experiment. Figure 7 shows that similar results were obtained in nude mice with PC-3 prostate xenografts. ING-1 treatment, at 1 mg/kg administered twice a week for 3 weeks, resulted in a 71% reduction in tumor volume.
Figure 6.
HT-29 colon tumor growth in nude mice treated with ING-1. Groups of 10 mice received ING-1 or human IgG 24 hours after tumor cell implantation. Data are mean±SEM. *P<.05 vs IgG; **P <.01.
Figure 7.
PC-3 tumor growth in nude mice treated with ING-1. Groups of 10 mice received ING-1 or human IgG 24 hours after tumor cell implantation. Data are mean±SEM *P< .05 vs IgG; **P <.01.
Discussion
This study provides the first description of the in vitro and in vivo activity of the Human Engineered™ monoclonal antibody ING-1 that targets the human cell adhesion molecule Ep-CAM. The data indicate that our Human Engineering technology did not alter the functional characteristics of the antibody relative to chimeric ING-1 as indicated by the retention of high binding to Ep-CAM and potent ADCC and CDC activity. Here we also provide the first detailed description of the in vivo efficacy and pharmacokinetics of ING-1.
The binding data illustrated in Figure 1 demonstrate that ING-1 is in the high-affinity class of anti-Ep-CAM antibodies, with a dissociation constant of 2 to 5 nM — a level of affinity similar to chimeric ING-1. In addition, the Kd of ING-1 is similar to that reported for other high-affinity chimeric or humanized anti-Ep-CAM antibodies, including 323/A3 (2 nM) [34] and USB-54 (5 nM) [8]. It has been suggested that high affinity (Kd>108) increases the likelihood of antibody uptake into a tumor [35,36], although it has been argued by others that low-affinity antibodies may penetrate deeper into a tumor nodule [34]. Indeed, autoradiographic studies suggest that 323/A3 may preferentially localize near blood vessels, whereas lowaffinity 17-1A exhibits a homogenous distribution within tumors [21,34]. Regardless, several other factors, including antigen distribution and density as well as tumor perfusion, affect the therapeutic benefit of these antibodies in experimental studies [37].
One of the factors believed to be especially important for benefits with anti-Ep-CAM antibodies is an interaction with host effector cells. A number of studies indicate that high-affinity anti-Ep-CAM antibodies may exhibit greater activity in ADCC assays compared to the low-affinity murine 17-1A, and that these antibodies demonstrate CDC activity as well [7,8,21,24]. Our results indicate that ING-1 also exhibits potent ADCC and CDC activity that is not apparently different from that demonstrated previously by chimeric ING-1 [29]. The results illustrated in Figure 2 demonstrate ADCC activity against breast and colon tumor cells, with maximal lysis approaching 100% against one cell line. Furthermore, although maximal killing was observed at approximately 1 µg/ml, as much as 50% lysis was evident at concentrations as low as 10 ng/ml. Although CDC activity was demonstrated, the activity observed was modest. This result is similar to results with other anti-Ep-CAM antibodies [8] and is probably due to the presence of complement regulatory proteins on tumor cells. In addition, the source of complement was human serum, which may have varying degrees of complement activity in vitro.
In vivo benefit with anti-Ep-CAM antibodies requires that the drug circulate at sufficiently high levels to interact with tumor cells and engage effector cells to evoke cytotoxic responses. Our pharmacokinetic results clearly demonstrate that ING-1 reaches adequate levels (>1 µg) for sufficient time to be evaluated as a potential therapeutic agent. The results also demonstrate that the clearance profile of ING-1 in nude mice without tumors, and in rats, is dose-independent and is similar to that of a typical native IgG1 without a specific host target site. The terminal half-lives of 10 days for ING-1 in nude mice without tumors and 20 days in rats were similar to that previously reported for native IgG1 [38,39].
In contrast to the above experiments, the pharmacokinetics of ING-1 in tumor-bearing mice suggests that the presence of antigen-positive tumors results in more rapid clearance than was observed in tumor-negative mice. Figure 5 illustrates that, after 3 days, the increased clearance resulted in a nearly two-fold faster beta-phase half-life compared to mice without human tumors. Furthermore, after 21 days, there was an ever-increasing proportional decrease in clearance rate. Such a concentration-time profile is characteristic of a drug that is cleared by a slow, nonsaturable elimination mechanism at high concentrations, and a faster saturable elimination mechanism at lower concentrations, presumably represented by human Ep-CAM in the tumors. The concentration-time profile of ING-1 in tumor-bearing mice is also characteristic of proteins that bind to specific host target sites of low capacity and high affinity [40–43].
As the presence of a human target site is the best explanation for the altered clearance of ING-1 in tumorbearing mice, our pharmacokinetics suggests a direct interaction of ING-1 with human tumor cells in nude mice. Tumor penetration, as well as the potent cytotoxic activity of ING-1 in vitro, are the characteristics required for successful in vivo treatment of experimental tumors. Our efficacy studies clearly demonstrate that ING-1 suppresses the growth of HT-29 colon and PC-3 prostate tumors in nude mice. The effect we observed in this study was dosedependent with 1 mg/kg, i.v., twice a week providing significant efficacy at most time points and 0.3 mg/kg was also effective by the end of the experiments (Figures 6 and 7). The nude mouse, while almost devoid of T lymphocytes, is well endowed with other effector cells [44]. Thus, the mechanism by which ING-1 inhibited tumor growth in these experiments is most likely ADCC. In a series of studies by Herlyn et al., evidence was presented that the in vivo activity of murine 17-1A resulted from ADCC that is mediated by macrophages [17,45–47]. Since then, others have suggested that anti-Ep-CAM antibodies interact with natural killer cells and neutrophils as well as macrophages in vivo [19,48,49]. A prominent role for CDC in the in vivo activity of these antibodies is doubtful because complement depletion does not alter the effectiveness of 17-1A [18] and tumor cells are well endowed with membrane-bound complement inhibitory proteins [50,51].
Whereas these results demonstrate the in vitro and in vivo activity of ING-1, they also provide the first description of the biological activity of an anti-Ep-CAM antibody generated by the Human Engineering technology of Studnicka et al. [30]. These data thus support the use of this technology for generating antibodies with desirable characteristics for human use. An important advantage of the Human Engineering method over other humanization technologies is its relative simplicity. Despite the simplified nature of the technology, it generates antibodies with variable region sequences that are more human-like than murine variable region sequences and that retain the binding activity of the murine version. For example, this method is more direct than CDR grafting techniques that require replacement of the entire murine framework with a human framework followed by a series of “dehumanizing” steps to recover binding activity [10].
In summary, these results demonstrate that Human Engineering of ING-1 results in a high-affinity antibody that demonstrates potent in vitro and in vivo efficacy. As Ep-CAM is highly expressed in human adenocarcinomas, targeting this tumor antigen with ING-1 may provide a therapeutic tool that is useful for the treatment of patients with adenocarcinomas and that evokes minimal immunogenicity. This suggestion is supported by preliminary clinical studies demonstrating the safety and low immunogenicity of ING-1 in cancer patients [31,32].
Acknowledgements
The authors express their gratitude to Patrick Scannon, Steve Carroll, Janet McNicholas, and Robert Gundel for helpful suggestions in the writing of this manuscript.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- CDC
complement-dependent cytotoxicity
- CDR
complementarity-determining region
- Ep-CAM
epithelial cell adhesion molecule
- PBMC
peripheral blood mononuclear cell
References
- 1.Khazaeli MB, Saleh MN, Wheeler RH, Huster WJ, Holden H, Carrano R, LoBuglio AF. Phase I trial of multiple large doses of murine monoclonal antibody CO17-1A: II. Pharmacokinetics and immune response. J Natl Cancer Inst. 1998;80:937–942. doi: 10.1093/jnci/80.12.937. [DOI] [PubMed] [Google Scholar]
- 2.Shawler DL, Bartholomew RM, Smith LM, Dillman RO. Human immune response to multiple injections of murine monoclonal IgG. J Immunol. 1985;135:1530–1535. [PubMed] [Google Scholar]
- 3.LoBuglio AF, Wheeler RH, Trang J, Haynes A, Rogers K, Harvey EB, Sun L, Grayeb J, Khazaeli MB. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci USA. 1989;86:4220–4224. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiden PL, Breitz HB, Seiler CA, Bjorn MJ, Ratliff BA, Mallett R, Beaumier PL, Appelbaum JW, Fritzberg AR, Salk D. Rhenium-186-labeled chimeric antibody NR-LU-13: pharmacokinetics, biodistribution and immunogenicity relative to murine analog NR-LU-10. J Nucl Med. 1993;34:2111–2119. [PubMed] [Google Scholar]
- 5.Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, Woody JN. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 6.Khazaeli MB, Saleh MN, Liu TP, Meredith RF, Wheeler RH, Baker TS, King D, Secher D, Allen L, Rogers K. Pharmacokinetics and immune response of 131I-chimeric mouse/human B72. (human gamma 4) monoclonal antibody in humans. Cancer Res. 1991;15:5461–5466. [PubMed] [Google Scholar]
- 7.Graves SS, Goshorn SC, Stone DM, Axworthy DB, Reno JM, Bottino B, Searle S, Henry A, Pedersen J, Rees AR, Libby RT. Molecular modeling and preclinical evaluation of the humanized NRLU-13 antibody. Clin Cancer Res. 1999;5:899–908. [PubMed] [Google Scholar]
- 8.Huls GA, Heijnen IA, Cuomo ME, Koningsberger JC, Wiegman L, Boel E, van der uurst de Vries AR, Loyson SA, Helfrich W, van Berge Henegouwen G, van Meijer M, de Kruif J, Logtenberg T. A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nat Biotechnol. 1999;17:276–281. doi: 10.1038/7023. [DOI] [PubMed] [Google Scholar]
- 9.Naundorf S, Preithner S, Mayer P, Lippold S, Wolf A, Hanakam F, Fichtner I, Kufer P, Raum T, Riethmuller G, Baeuerle PA, Dreier T. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- 10.Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- 11.Herlyn M, Steplewski Z, Herlyn D, Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci USA. 1979;76:1438–4146. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez MS, Walker LE. Isolation and characterization of a cDNA encoding the KS1/4 epithelial carcinoma marker. J Hematol. 1989;142:3662–3667. [PubMed] [Google Scholar]
- 13.Strnad J, Hamilton AE, Beavers LS, Gamboa GC, Apelgren LD, Taber LD, Sportsman JR, Bumol TF, Sharp JD, Gadski RA. Molecular cloning and characterization of a human adenocarcinoma/epithelial cell surface antigen complementarity DNA. Cancer Res. 1989;49:314–317. [PubMed] [Google Scholar]
- 14.Simon B, Podolsky DK, Moldenhauer G, Isselbacher KJ, Gattoni-Celli S, Brand SJ. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci USA. 1990;87:2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, Linnenbach AJ. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci. 1990;87:3542–3546. doi: 10.1073/pnas.87.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzar M, Winter MJ, deBoer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 17.Shaw DR, Khazaeli MB, Lee KS, Ghrayeb J, Daddona PE, McKinney S, LoBuglio AF. Characterization of a mouse/human chimeric monoclonal antibody (17-1A) to a colon cancer tumor-associated antigen. J Immunol. 1987;138:4534–4538. [PubMed] [Google Scholar]
- 18.Herlyn D, Koprowski H. IgG2a monoclonal antibodies inhibit human tumor growth through interaction with effector cells. Proc Natl Acad Sci. 1982;79:4761–4765. doi: 10.1073/pnas.79.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masucci G, Lindemalm C, Frodin J-E, Hagstrom B, Mellstedt H. Effect of human blood mononuclear cell populations in antibody dependent cellular cytotoxicity (ADCC) using two murine (CO17-1A and BR55-2) and one chimeric (17-1A) monoclonal antibodies against a human colorectal carcinoma cell line (SW948) Hybridoma. 1988;7:429–440. doi: 10.1089/hyb.1988.7.429. [DOI] [PubMed] [Google Scholar]
- 20.Steplewski Z, Sun LK, Shearman CW, Ghrayeb J, Daddona P, Koprowski H. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc Natl Acad Sci. 1988;85:4852–4856. doi: 10.1073/pnas.85.13.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velders MP, Litvinov SV, Warnaar SO, Gorter A, Fleuren GJ, Zurawski VR, Jr, Coney LR. New chimeric anti-pancarcinoma monoclonal antibody and superior cytotoxicity-mediating potency. Cancer Res. 1994;54:1753–1759. [PubMed] [Google Scholar]
- 22.Velders MP, vanRhijn CM, Briaire IH, Fleuren GJ, Warnaar SO, Litvinov SV. Immunotherapy with low and high affinity monoclonal antibodies 17-1A and 323/A3 in a nude mouse xenograft carcinoma model. Cancer Res. 1995;55:4398–4403. [PubMed] [Google Scholar]
- 23.Velders MP, vanRhijn CM, Cornelissen IM, van Muijen GN, Briaire I, Hohlsten M, Fleuren GJ, Warnaar SO, Litvinov SV. The role of monoclonal antibody affinity in tumor immunotherapy evaluated in in vivo models for minimal residual disease. J Immunother. 1996;19:245–256. doi: 10.1097/00002371-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Velders MP, van Rhijn CM, Oskam E, Fleuren GJ, Warnaar SO, Litvinov SV. The impact of antigen density and antibody affinity on antibody-dependent cellular cytotoxicity: relevance for immunotherapy of carcinomas. Br J Cancer. 1998;78:478–483. doi: 10.1038/bjc.1998.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riethmuller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmmaier H, Hierche H, Pichlmayr R, Buggisch P, Witte J. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 26.Saleh MN, Posey JA, Khazaeli MB, Thurmond M, Khor SP, Lampkin TA, Wissel PS, LoBuglio AF. Phase I trial testing multiple doses of humanized monoclonal antibody (MAB) 3622W94. Proc ASCO. 1998;17:436a. [Google Scholar]
- 27.Liao S-K, Horton L, Flahart RE, O'Rear L, Crumpacker D, Imbaratto JW, Yannelli JR, Robinson RR, Oldham RK. Binding and functional properties of a mouse-human chimeric monoclonal antibody of the human IgG1 subclass with specificity for human carcinomas. Hum Antib Hybridomas. 1990;1:66–67. [PubMed] [Google Scholar]
- 28.Kufe DW, Nadler L, Sargent L, Shapiro H, Hand P, Austin F, Colcher D, Schlom J. Biological behavior of human breast carcinoma-associated antigens expressed during cellular proliferation. Cancer Res. 1983;43:851–857. [PubMed] [Google Scholar]
- 29.Robinson RR, Chartier J, Chang CP, Horwitz AH, Better M. Chimeric mouse-human anti-carcinoma antibodies that mediate different anti-tumor cell biological activities. Hum Antib Hybridomas. 1991;2:84–93. [PubMed] [Google Scholar]
- 30.Studnicka GM, Soares S, Better M, Williams RE, Nadell R, Horwitz R. Human-engineered monoclonal antibodies retain full specific binding activity by preserving non-complementarity-modulating residues. Protein Eng. 1994;17:805–814. doi: 10.1093/protein/7.6.805. [DOI] [PubMed] [Google Scholar]
- 31.de Bono JS, Forero A, Hammond LA, Patnaik A, Takimoto C, Lobuglio A, Mays T, Rowinsky EK, Bauer R, Van Hove G, Tolcher A. Safety, tolerability, and maximum tolerated dose of an intravenously administered human engineered monoclonal antibody, ING-1(he-MAb), in patients with advanced adenocarcinomas. Proc ASCO. 2002;21:9a. [Google Scholar]
- 32.Better M, Bohmann KJ, White ML, Forero A, de Bono JS, Hamond LA, Patnaik A, Takimoto C, LoBuglio A, Rowinsky EK, Tolcher AW, Vanhove GF. Recombinant human engineered ING-1 monoclonal antibody, ING-1(heMAb), exhibits minimal immunogenicity in patients with advanced adenocarcinomas. Proc ASCO. 2002;21:20a. [Google Scholar]
- 33.Gibaldi N, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker; 1982. Multicompartment models; pp. 45–111. [Google Scholar]
- 34.Langmuir MK, Mendonca HL, Woo DV. Comparisons between two monoclonal antibodies that bind to the same antigen but have differing affinities: uptake kinetics and 125I-antibody therapy efficacy in multicell spheroids. Cancer Res. 1992;52:4728–4734. [PubMed] [Google Scholar]
- 35.Sung C, Shockley TR, Morrison PF, Dvorak HF, Yarmush ML, Dedrick RL. Predicted and observed effects of antibody affinity and antigen density on monoclonal antibody uptake in solid tumors. Cancer Res. 1992;52:377–384. [PubMed] [Google Scholar]
- 36.Shockley TR, Lin K, Sung C, Nagy JA, Tompkins RG, Dedrick RL, Dvorak HF, Yarmush ML. A quantitative analysis of tumor specific monoclonal antibody uptake by human melanoma xenografts: effects of antibody immunological properties and tumor antigen expression levels. Cancer Res. 1992;52:357–366. [PubMed] [Google Scholar]
- 37.Kievit E, Pinedo HM, Schluper HMM, Schluper HM, Haisma HJ, Boven E. Determination of tumor-related factors of influence on the uptake of the monoclonal antibody 323/A3 in experimental human ovarian cancer. Int J Cancer. 1997;71:237–245. doi: 10.1002/(sici)1097-0215(19970410)71:2<237::aid-ijc19>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Adams DO, Hall T, Steplewski Z, Koprowski H. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive for of antibody-dependent cytolysis. Proc Natl Acad Sci USA. 1984;81:3506–3510. doi: 10.1073/pnas.81.11.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herlyn DM, Steplewski Z, Herlyn MF, Koprowski H. Inhibition of growth of colorectal carcinoma in nude mice by monoclonal antibody. Cancer Res. 1980;40:717–721. [PubMed] [Google Scholar]
- 40.Johnson WJ, Steplewski Z, Matthews TJ, Hamilton TA, Koprowski H, Adams DO. Cytolytic interactions between murine macrophages, tumor cells, and monoclonal antibodies: characterization of lytic conditions and requirements for effector activation. J Immunol. 1986;136:4704–4713. [PubMed] [Google Scholar]
- 41.Masucci G, Wersall P, Nielsen J, Nielsen HK, Wigzell H, Mellstedt H. Lymphokine activated killer (LAK) cells in antibody dependent cellular cytotoxicity (ADCC) using Mab 17-1A: a combination of potential usefulness in tumor therapy. Hybridoma. 1989;8:507–516. doi: 10.1089/hyb.1989.8.507. [DOI] [PubMed] [Google Scholar]
- 42.Abdullah N, Greenman J, Pimenidou A, Topping KP, Monson JR. The role of monocytes and natural killer cells in mediating antibody-dependent lysis of colorectal tumour cells. Cancer Immunol Immunother. 1999;48:517–524. doi: 10.1007/s002620050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung N-KV, Walter EI, Smith-Mensah WH, Ratnoff WD, Tykocinski ML, Medof ME. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988;81:1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorter A, Block VT, Haasnoot WHB, Ensink NG, Daha MR, Fleuren MJ. Expression of CD46, CD55, and CD59 on renal tumor cell lines and their role in preventing complement-mediated tumor cell lysis. Lab Invest. 1996;74:1039–1049. [PubMed] [Google Scholar]
- 45.Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- 46.Waldmann TA, Strober W, Blaese RM. Variations in the metabolism of immunoglobulins measured by turnover rates. In: Merler E, editor. Immunoglobulins — biological aspects and uses. New York: National Academy of Sciences; 1970. pp. 33–51. [Google Scholar]
- 47.Bauer RJ, Gibbons JA, Bell DP, Luo ZP, Young JD. Nonlinear pharmacokinetics of recombinant human macrophage colony-stimulating factor (M-CSF) in rats. J Pharmacol Exp Ther. 1994;258:152–158. [PubMed] [Google Scholar]
- 48.Bauer RJ, Dedrick RL, White ML, Murray MJ, Garovoy MR. Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm. 1999;27:397–420. doi: 10.1023/a:1020917122093. [DOI] [PubMed] [Google Scholar]
- 49.Tanswell P, Heinzel G, Greischel A, Krause J. Nonlinear pharmacokinetics of tissue-type plasminogen activator in three animal species and isolated perfused rat liver. J Pharmacol Exp Ther. 1990;255:318–324. [PubMed] [Google Scholar]
- 50.Palm M, Mattsson CH. Pharmacokinetics of heparin and low molecular weight heparin fragment (Fragmin ®) in rabbits with impaired renal or metabolic clearance. Thromb Haemost. 1987;58:932–935. [PubMed] [Google Scholar]
- 51.Holub M. Immunology of Nude Mice. Boca Raton, FL: CRC Press; 1989. [Google Scholar]