Abstract
A simple theoretical model for the cellular pharmacodynamics of cisplatin is presented. The model, which takes into account the kinetics of cisplatin uptake by cells and the intracellular binding of the drug, can be used to predict the dependence of survival (relative to controls) on the time course of extracellular exposure. Cellular pharmacokinetic parameters are derived from uptake data for human ovarian and head and neck cancer cell lines. Survival relative to controls is assumed to depend on the peak concentration of DNA-bound intracellular platinum. Model predictions agree well with published data on cisplatin cytotoxicity for three different cancer cell lines, over a wide range of exposure times. In comparison with previously published mathematical models for anticancer drug pharmacodynamics, the present model provides a better fit to experimental data sets including long exposure times (∼100 hours). The model provides a possible explanation for the fact that cell kill correlates well with area under the extracellular concentration-time curve in some data sets, but not in others. The model may be useful for optimizing delivery schedules and for the dosing of cisplatin for cancer therapy.
Keywords: chemotherapy, cellular pharmacodynamics, cisplatin, cellular pharmacokinetics, area under the curve
Introduction
The drug cisplatin, used in anticancer therapy for decades [1], has significant adverse side effects. The optimization of the dosing and delivery schedule can potentially minimize adverse effects while maintaining efficacy [2]. A consensus on the optimal schedule of the administration of cisplatin does not appear to have been reached. Kurihara et al. [3] stated that “Standard therapy with DDP [cisplatin] has been a single bolus injection because the antitumor activity of this drug has been considered to be dependent on its peak plasma concentration.” However, Drewinko and Gottlieb [4] concluded that a greater degree of killing was elicited with treatment extended over a period of 30 hours. Several clinical studies have compared continuous-infusion cisplatin with bolus administration. Hayashi et al. [5] found no improvement in response and no decrease in the toxicity for the cancer of the esophagus treated with a 5-day continuous infusion, whereas Salem et al. [6] reported the toxicity of this schedule to be “mild” but were unable to assess the antitumor efficacy. Dose-limiting toxicities have been associated both with peak plasma levels [7] and with plasma area under the concentration-time curve (AUC) [8]. Theoretical pharmacodynamic models that predict tumor cell survival for a given time course of drug exposure provide a rational basis for the optimization of administration schedules, which involves maximizing tumor cell kill under the constraint that host toxicity must remain tolerable.
For cell cycle nonspecific drugs such as cisplatin, it has been proposed that the AUC determines cytotoxicity, independent of exposure time [9]. Table 1 lists experimental studies that have provided data permitting assessment of the dependence of cisplatin cytotoxicity on exposure time for a given AUC. In some of these studies, when survival relative to controls is graphed as a function of AUC, curves for different exposure times clearly differ (e.g., Troger et al. [2]), implying that AUC alone is not predictive of survival. Similarly, AUC at 50% survival (relative to controls) can vary with exposure time [10]. In other studies, however, graphs of survival versus AUC nearly coincide for all exposure times [11,12], implying that AUC is predictive of cytotoxicity over the range considered. In two cases [3,13], the authors concluded that AUC predicted cytotoxicity, but their data imply a dependence on exposure time. All of the studies that showed AUC alone as being predictive were for exposure times of 24 hours or less. Taken together, these data indicate that the assumption that cell kill depends on AUC, independent of exposure time, does not adequately represent the pharmacodynamics of cisplatin.
Table 1.
Summary of Literature Studies on the Predictive Value of Extracellular AUC for the Survival of Human Cancer Cell Lines After Exposure to Cisplatin.
| Source | Cell/tumor type | AUC predicted cell survival? |
Range of exposure times (hours) | Replot needed* |
| Troger et al. [2] | Head and neck cancer | No | 1–121 | No |
| Nozue et al. [10] | Human gastric cancer | No | 1–72 | Yes |
| Matsushima et al. [33] | Human lung carcinoma PC-7 | No | 1–24 | Yes |
| Levasseur et al. [11] | Human ovarian and colon carcinomas | Yes | 1–24 | Yes |
| Rupniak et al. [34] | Human ovarian carcinoma | Maybe | 1–18 | Yes |
| Ma et al. [12] | IGROV1 ovarian | Yes | 1–20 | Yes |
| Kurihara et al. [3] | Human gastric carcinoma | No | 1–25 | Yes |
| Los et al. [35] | CC531 (colon carcinoma) | No | 1–4 | Yes |
| Erlichman et al. [13] | MGH-U1 human bladder cancer | No | 1.3–4.4 | No |
Data are plotted versus extracellular concentration in the cited study.
A number of other theoretical models have been proposed to predict the dependence of cytotoxicity on the time course of exposure. For cells exposed to a constant extracellular concentration, the AUC is equal to CT, where C is the concentration and T is the exposure time. An alternative, more general, predictor of cell kill is CnT [14,15], where n may depend on the drug and the tumor type. Curves for survival S relative to controls, when plotted versus either extracellular AUC or CnT, typically have a sigmoidal shape that can be approximated by a Hill-type equation:
| (1) |
where A and m are constants. In the following, Eq. (1) with x=AUC will be referred to as the “extracellular AUC model”, and with x=CnT as the “extracellular CnT model”. For concentration varying with time, CnT can be replaced by the time integral of C(t)n. Levasseur et al. [11] proposed a model with several additional parameters, combining the dependence of cell kill on CnT with a Hill-type equation. They fitted the model to experimental results for several drugs, including cisplatin. Because an exponent in their model has a quadratic dependence on T, their model is not applicable for exposures longer than 24 hours—the maximum in their data set. Their model has no clear generalization to the case of time-varying concentration. Gardner [16] developed an “exponential kill” model based on a consideration of the kinetics of cell kill. This model contains some assumptions that restrict its applicability. It implies that the AUC required for a given level of cell kill decreases with exposure time to cell cycle-nonspecific drugs, whereas many drugs (including cisplatin) show the opposite effect [17]. These four models are summarized in Table 2. The survival relative to controls S is defined as the number of surviving cells after a single cycle of exposure, divided by the number of surviving cells at the same time point in untreated controls. This measure, which does not describe the kinetics of cell kill during the treatment period, is the most relevant for optimizing therapy.
Table 2.
Previous and Proposed Models for Cisplatin Cellular Pharmacodynamics.
| Name of model | Reference | Equations | Number of free parameters |
| Extracellular AUC model | Ozawa et al. [9] |
S=1/(1+A(AUC)m) where |
2 |
| Extracellular CnT model | Skipper [14] and Adams et al. [15] |
S=1/(1+A(AUCn)m) where |
3 |
| Levasseur et al. model | Levasseur et al. [11] |
IC50=(k/T)1/n γ=γ0+γ1T+γ2T2 |
7 |
| Gardner model | Gardner [16] | S=Exp[1-b(1-e-aCe)T] | 2 |
| Peak intracellular model | Present study | and Eq. (2) | 2 or 4* |
| Peak-bound intracellular model | Present study | and Eqs. (2) and (3) | 3 or 5* |
The two parameters relating to cellular uptake may be obtained separately (see text).
Cisplatin acts by binding to cellular DNA, and must enter cells to be lethal. The dependence of cell kill on the time course of extracellular exposure must therefore reflect the kinetics of cellular drug uptake and binding to intracellular targets. As yet, no theoretical model for the pharmacodynamics of chemotherapy drugs that meets the following requirements has been developed: (i) it is based on a consideration of the kinetics of drug entry into cells and binding within cells; (ii) it can be used to predict the response to an arbitrary time course of extracellular exposure (as occurs in actual cancer therapy); and (iii) it is applicable to all data sets with long exposure times (>100 hours). The goal of the present work is to develop such a model, and to compare its predictions with those of the previously proposed models listed in Table 2.
Cisplatin reacts both inside and outside the cell to produce a number of platinum species, and transport across the cellular and nuclear membranes has different kinetics for each species. Presently available data do not permit the determination of the individual kinetics of each of these reaction and transport processes. However, in kinetic systems, it is often possible to lump several species together and obtain a useful model, and this approach is taken here. The resulting model is relatively simple but yields predictions of cytotoxicity that are consistent with observations over a range of different exposure conditions.
Materials and Methods
The elements of the model for drug uptake and DNA binding are shown schematically in Figure 1. The quantities ce, ci, ck, and refer to concentrations of platinum species pools: ce, extracellular concentration; ci, intracellular concentration; ck, concentration bound to DNA; , concentration released from DNA as a result of DNA repair. Platinum species released during DNA repair are no longer available for binding [18]. The intracellular concentration ci includes platinum that is aquated, hydrolyzed inside the nucleus, bound to RNA, bound to other non-DNA proteins, and so on, but excludes the concentrations ck and . Experimental data [19,20] imply that DNA-bound intracellular platinum is a small fraction of total intracellular platinum. Therefore, both ck and are assumed to be much smaller than ci.
Figure 1.
Schematic representation of proposed model. Concentrations of platinum are: ce, extracellular; ci, intracellular non-DNA bound; ck, intracellular DNA-bound; , released from DNA as a result of DNA repair. Arrows indicate transport or reaction processes. The concentration ci is much larger than ck and .
A reversible exchange of drug between extracellular and intracellular compartments is assumed, and cellular uptake is therefore described by:
| (2) |
where t is time, and k1 and are constants. The uptake rate is assumed to be linear in ce based on numerous studies showing such a linear relation at any time [2,21–25]. The removal of platinum from the intracellular pool by DNA binding is neglected in this equation because ck and are much smaller than ci. Eq. (2) was solved analytically to give an expression for ci as a function of time.
The values for the constants k1 and were determined by minimizing the mean square deviation of predicted intracellular concentration ci (neglecting the part bound to DNA) from measured values for cellular uptake of cisplatin by the ovarian cancer cell line 2008 [26], using the software package Mathematica (Wolfram Research, Champaign, IL). In these data, extracellular concentration is 1.0 µmol/l cisplatin=0.195 µg/ml Pt, based on one Pt atom (atomic weight=195) per cisplatin molecule. Intracellular concentrations, given in picomoles of Pt per milligram of protein, are converted to micrograms of Pt per milliliter, based on 1 mg of protein=3 µl of cell volume [26]. The same model, with the same values of k1 and , was used to fit the data of Troger et al. [2] for a head and neck cancer cell line with the cell volume, which is used to convert intracellular concentrations from units of nanograms of Pt per 106 cells, treated as a free parameter.
This cellular pharmacokinetic model was used as a basis for developing a cellular pharmacodynamic model. Because cisplatin kills cells by binding to DNA in the cell nucleus, it is reasonable to assume that cell kill correlates more directly with measures of intracellular exposure than with measures of extracellular exposure. Survival relative to controls S, as defined previously, is then given by Eq. (1), with x replaced by a measure of intracellular exposure. Intracellular AUC is one such measure. As already mentioned, cellular uptake is assumed to be linear in the extracellular concentration ce, based on several experimental studies, which implies that intracellular AUC is linearly related to extracellular AUC. Using intracellular AUC as a measure is therefore mathematically equivalent to the extracellular AUC model as already described, for which results are presented below. The alternative possibility that cell kill is related to peak intracellular levels was therefore explored. Choosing x as the peak intracellular concentration of platinum (i.e., the maximum value of ci achieved over time) gives the “peak intracellular model” (Table 2).
Cisplatin kills cells by binding to cellular DNA and forming adducts, whose formation may take some hours [27]. This suggests a pharmacodynamic model that correlates cytotoxicity with the peak level of ck, the DNA-bound platinum, rather than ci. According to the model (Figure 1), the DNA-bound platinum concentration is governed by:
| (3) |
where k2 is a rate constant for binding to DNA, and k3 is a rate constant for DNA repair. Eq. (3) was solved analytically to give an expression for ci as a function of time. The survival relative to controls S is then given by Eq. (1), where x is the peak value of ck achieved over time, denoted (“peak-bound intracellular model”; Table 2).
This model contains six unknown parameters, k1, , k2, k3, A, and m. The values of ck predicted by the model at each instant are proportional to k2. In the absence of measured values of ck, values of k2 and A cannot be deduced from survival data because S depends on them mathematically only through a combined parameter . Therefore, A was set to an arbitrary fixed value [1 (µg/ml)-m] in this model, giving five unknown parameters. Values of ck and predicted by the model are relative and cannot be compared in absolute terms with ci.
Table 2 summarizes the six models to be compared (i.e., the four previous models discussed above and the two proposed here). For each model, best fits were determined between predicted values of the survival S relative to controls and the measured values for human head and neck cancer cells [2], the human ovarian cancer cell line A2780 [11], and human gastric cancer cells [3]. The parameters were varied to minimize the root mean square deviation between calculated and experimental values of S, using Mathematica or a Fortran implementation of the method of steepest descent. Data for tumor cell survival and extracellular concentration were read from the published graphs. In one study [11], not all data points could be read because points for different exposure times overlapped. For the peak intracellular and peak-bound intracellular models, the values of k1 and determined from the cell uptake model were used to fit the Troger et al. [2] cell kill data. Cell uptake data are not available for the cell lines in the other studies [3,11], so k1 and were fit to the cytotoxicity data along with the other parameters.
Results
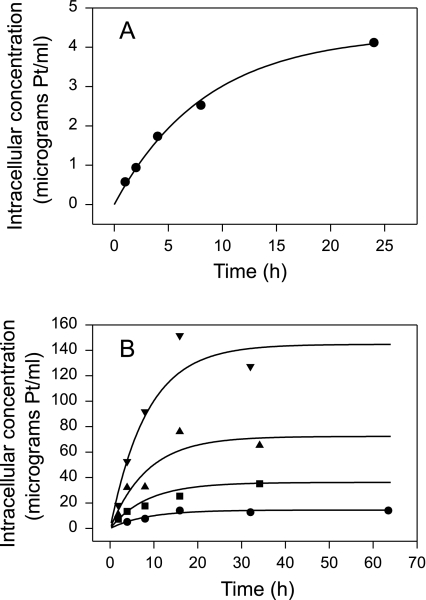
Figure 2 shows the fit of the cellular uptake model to the uptake data. A close fit to the data of Andrews et al. [26] was obtained with k1=2.635 hour-1 and =0.1184 hour-1. With these same values for k1 and , the optimal value of cell volume to fit the Troger et al. [2] drug uptake data was computed as 2.17 x 10-9 ml. This fits their drug uptake data well, and is a physiologically reasonable value.
Figure 2.
Fits of cellular pharmacokinetic model to data on the uptake of platinum by human cancer cells in vitro. Curves show model predictions. (A) Data of Andrews et al. [26] for ovarian carcinoma cells. Extracellular concentration: 0.195 µg/ml Pt. (B) Data of Troger et al. [2] for head and neck cancer cells. Extracellular concentrations: (●) 0.65 µg/ml Pt; (■) 1.62 µg/ml Pt; (▲) 3.2 µg/ml Pt; and (▼) 6.5 µg/ml Pt.
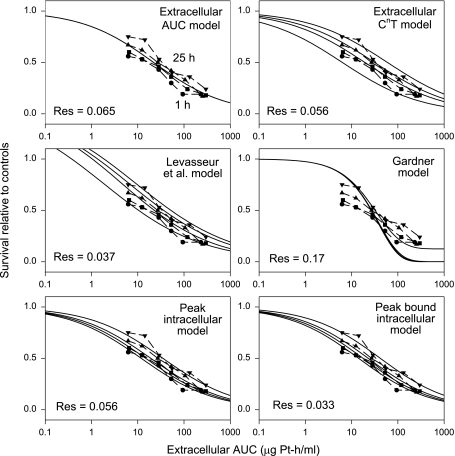
The best-fit parameter values for the six models, applied to the three cytotoxicity data sets, are summarized in Table 3. Figures 3–5 show the resulting fit of the models to the cytotoxicity data sets of Troger et al. [2], Levasseur et al. [11], and Kurihara et al. [3], respectively. The data of Kurihara et al. [3] are plotted in Figure 5 as a function of extracellular AUC to show the trend for survival (increasing with exposure time for a given AUC), contrary to the stated conclusion [3] that AUC predicts cell kill. For each plot in Figures 3–5, the residual (root mean square) deviation between measured and predicted values is shown within the plot area. For each data set considered, the peak-bound intracellular model yields the best fit, as indicated by the lowest residual.
Table 3.
Best-Fit Model Parameters for Cisplatin Cytotoxicity Data Sets.
| Model | Parameters for Troger et al. [2] data | Parameters for Levasseur et al. [11] data | Parameters for Kurihara et al. [3] data | |
| Extracellular AUC model | A=0.2033, m=1.229 | A=0.4047, m=1.882 | A=0.1786, m=0.5574 | |
| Extracellular CnT model | n=1.303, A=0.2988, m=0.9991 | n=0.8762, A=0.2603, m=2.171 | n=3.313, A=0.3920, m=0.1539 | |
| Levasseur et al. [11] model |
k=3.670, n=1.306, B=0*, Econ=0.9811, γ0=-1.621, γ1=0.05718 hour-1, γ2=-0.0004485 hour-2 |
k=1.855, n=0.8683, B=0.006507, Econ=0.9974, γ0=-1.856, γ1=-0.05292 hour-1, γ2=0.002680 hour-2 |
k=2.832, n=1.652, B=0*, Econ=1.514, γ0=-0.4066, γ1=-0.006071 hour-1, γ0=0.0002250 hour-2 |
|
| Gardner [16] model | a=0.0006284, b=271.0 | a=0.1156, b=3.691 | a=0.01265, b=2.077 | |
| Peak intracellular model | (k1=2.635 hour-1), ( 0=0.1184 hour-1), A=0.1477, m=1.212 |
k1=0.04629 hour-1, 0=0.0002569 hour-1, A=131.4, m=1.881 |
(k1=2.635 hour-1), =0.1184 hour-1), A=0.1573, m=0.5465 |
|
| Peak-bound intracellular model | (k1=2.635 hour-1), (=0.1184 hour-1), k2=0.2024 hour-1, |
k3=0.4707 hour-1, m=1.381 |
k1=0.3267 hour-1, =0.02413 hour-1, k2=3.745 hour-1, k3=1.441 hour-1, m=1.956 |
(k1=2.635 hour-1), (=0.1184 hour-1), k2/k3=0.02837, (k3=infinity), m=0.5715 |
Where units are not shown, concentrations are in micrograms per milliliter and time is in hours. Values in parentheses were determined from independent uptake data [2], or were chosen arbitrarily because they do not significantly affect the fit [3].
B represents the plateau value of survival (fraction of controls) in the high-concentration limit, and therefore cannot be less than 0. This constraint was included in the optimization process.
Figure 3.
Best fits of six cytotoxicity models to the data of Troger et al. [2] for human head and neck cancer cells. Exposure times: (●) 1 hour; (■) 2 hours; (▲) 11 hours; and (▼) 121 hours. Curves: model predictions. Res: root mean square residual deviation between experimental data and model.
Figure 4.
Best fits of six cytotoxicity models to the data of Levasseur et al. [11] for a human ovarian cancer cell line. Exposure times: (●) 1 hour; (■) 2 hours; (▲) 3 hours; (▼) 4 hours; (○) 6 hours; (□) 9 hours; (△) 12 hours; and (▽) 24 hours. Curves: model predictions. Res: root mean square residual deviation between experimental data and model.
Figure 5.
Best fits of six cytotoxicity models to the data of Kurihara et al. [3] for a human gastric cancer cell line. Exposure times: (●) 1 hour; (■) 5 hours; (▲) 10 hours; and (▼) 25 hours. Curves: model predictions. Res: root mean square residual deviation between experimental data and model.
Discussion
The uptake model used here has some similarities to that of Sadowitz et al. [18], which modeled cisplatin cell uptake, intracellular reaction with thiols, and binding to DNA, and distinguished between intracellular nuclear and extranuclear cisplatin. With some simplifications, their model contained four unknown parameters that were obtained by fitting data on DNA adducts as a function of extracellular cisplatin for a 2-hour exposure. However, the present uptake model (Eq. 2), with only two fitted parameters, provides an adequate fit to the uptake data. Distinguishing between more intracellular species would result in more unknown parameters, which could not be estimated reliably from the currently available data.
Previous experimental studies (Table 1) have led to conflicting conclusions regarding the validity of extracellular AUC as a predictor of cytotoxicity. The present results provide a possible explanation. When applied to the data sets of Levasseur et al. [11] and Kurihara et al. [3], the extracellular AUC model yields fairly good fits (Figures 4 and 5), but the Troger et al. [2] data show large systematic deviations from the model predictions (Figure 3). The latter data cover a wider range of exposure times, and the deviations are greatest for the longest exposure time (121 hours), which gives only slightly more cell kill than an 11-hour exposure at the same concentration. For exposures in this range, a decrease in concentration is not fully compensated for by a corresponding increase in exposure time. By definition, the extracellular AUC model cannot show such behavior. The extracellular CnT model incorporates such behavior, but is still unable to provide a good fit to the data for very long exposures.
The model of Levasseur et al. [11] provides a good fit to the experimental data set obtained by the same authors (Figure 4). However, this model is less successful at fitting the Troger et al. [2] data set (Figure 3) because it does not show the correct behavior at low concentrations and long exposure times. When applied to the Kurihara et al. [3] data (Figure 5), this model gives a good fit, but the parameter values then give survival relative to controls substantially greater than 1 at very low concentrations (which would imply that the drug was actually promoting tumor growth).
Similarly, the model of Gardner [16] yields a good fit to the data set of Levasseur et al. [11] but fails to fit the data set of Troger et al. [2] for long exposure times. When applied to the data set of Kurihara et al. [3], it exhibits the “plateau” feature of this model (asymptote to nonzero survival at high concentration). The dependence of this plateau is dictated by the behavior of the curves in the steep descent phase. The inability to adjust these two behaviors independently limits the model's ability to fit cytotoxicity data over a wide range of concentrations.
In the models proposed in the present study, cell kill depends on peak intracellular cisplatin levels. When the extracellular concentration is reduced below a threshold level, the peak intracellular level is correspondingly limited, no matter how long the exposure time (Figure 6). For very long exposure times, the survival relative to controls therefore approaches a limit that depends only on the concentration. This feature of the peak-bound intracellular model accounts for its ability to provide a good fit to the Troger et al. [2] data, including those for 121-hour exposure (Figure 3).
Figure 6.
Survival relative to controls as predicted by the peak-bound intracellular model for a wide range of exposure times, showing a threshold concentration below which no substantial cell kill can be achieved, regardless of exposure time. Parameter values are those for the Levasseur et al. [11] data set.
Under some conditions, the proposed models can yield results similar to the extracellular AUC model, as shown in Figure 4. This may be understood by the following argument. For exposure times that are short enough that the free intracellular concentration remains much less than the extracellular level, the cellular uptake model of Eqs. (2) and (3) can be integrated to give:
| (4) |
Peak intracellular level is then proportional to extracellular AUC, and a close correlation between cell kill and AUC is expected according to the proposed models. The time at which intracellular free concentration approaches the extracellular concentration is on the order of 1/ , and Table 3 shows that this kinetic parameter has different values for different cell lines: 8 hours for the Troger et al. [2] data, 41 hours for the Levasseur et al. [11] data, and 7 hours for the Kurihara et al. [3] data. In the case of Levasseur et al. [11], the exposure times, ranging from 1 to 24 hours, are all less than the intracellular equilibration time. Therefore, the peak intracellular model yields almost identical results to those of the extracellular AUC model (Figure 4).
Of the six models examined, the peak-bound intracellular model consistently yields the best fit to the data considered. The key feature of this model is that cell kill is correlated with the peak intracellular level of DNA-bound platinum. The connection between cytotoxicity and peak intracellular bound concentrations is also supported by results for high-dose cisplatin therapy (Figure 14-4 of Reed et al. [1]) showing that peak platinum-DNA adduct levels correlate with response to treatment. A correlation of cell kill with peak intracellular levels has also been found for doxorubicin [28]: Data on cell kill for various exposure times collapsed onto a single curve when plotted against the intracellular concentration at the time extracellular exposure ended (i.e., the peak intracellular concentration).
The Troger et al. [2] data set provides the most stringent test of the six models, and the validity of this data set is therefore critical for the present study. Ma et al. [12] concluded from their experiments that AUC, corrected for protein binding, predicted cell kill well, and conjectured that the finding of Troger et al. [2]—that cell kill as a function of AUC was substantially different at 121 hours than at shorter times—was an artifact of their not correcting for protein binding in the medium. This reasoning is questionable for several reasons. Firstly, the experiments of Ma et al. [12] covered the range 1 to 20 hours, with no data for exposure times as long as those of Troger et al. [2]. Secondly, Ma et al. [12] used a medium containing albumin to simulate protein binding in plasma. However, albumin may not be the major ligand of platinum in rat serum [29]. Thirdly, Gamelin et al. [30] found that the ratio of ultrafilterable to total platinum in plasma was nearly constant at 6% over time, suggesting that similar correlations should be found whether cell survival is plotted versus total or ultrafilterable platinum. Thus, the findings of Troger et al. [2] of significant time dependence cell survival relative to controls for a given AUC are unlikely to be artifactual as suggested by Ma et al. [12].
It might be thought that the decreased cell kill at the same extracellular AUC, observed by Troger et al. [2] and others at longer exposure times, could be explained simply in terms of the development of a resistant population when cells are exposed to drug for longer times. This possibility was examined under the following assumptions: (i) the cell kill for the sensitive population depends only on AUC; and (ii) both the resistant and sensitive populations grow exponentially at the same rates when untreated, as expected for the conditions of in vitro studies [2]. A simple mathematical analysis shows that, under these assumptions, the increase in survival relative to controls due to the presence of resistant cells is independent of the exposure time.
In summary, of the six models considered, the peakbound intracellular model yields the best fit to three separate sets of data on for cisplatin toxicity involving different types of cultured tumor cells, including a set with long (121 hours) exposure time. Important features of this model are: (i) it is based on the assumption that cell kill depends on the peak level of DNA-bound intracellular platinum; (ii) for relatively short exposure times, it yields predictions similar to those resulting from AUC-type models; (iii) for a given AUC, reduced responses are predicted at very long exposure times; (iv) it predicts that a threshold concentration is needed for any antitumor effect; (v) it involves a small number of fitted parameters, only three if separate cell uptake data are available; and (vi) it is applicable to the case of variable extracellular concentration as a function of time.
Currently, cisplatin is administered intravenously in the clinic either by bolus injection or continuous infusion. Even with bolus injection, much of the AUC comes from a long “tail” of exposure at low concentrations. The present model suggests that this tail of exposure after peak-bound intracellular levels have been achieved provides no therapeutic benefit, although it presumably contributes to toxicity (because mucositis and hematologic toxicity appear to correlate with plasma AUC). The tail could be eliminated by using sodium thiosulfate to deactivate cisplatin systematically. Muldoon et al. [31] gave sodium thiosulfate to guinea pigs 2 hours after cisplatin treatment, and found reduced ototoxicity, suggesting that this toxicity may also be related to the tail of exposure rather than the peak.
The model developed here was based on data for in vitro response of cells to drug. How closely in vivo cellular response correlates with in vitro response is therefore an important question. For some drugs, the in vivo environment, in which each cell is surrounded three-dimensionally by other cells and where cells may not be actively cycling due to limited resources or other reasons, has been found to result in less drug sensitivity. The study of Erlichman et al. [32] found no difference in response to cisplatin between cells grown as spheroids including necrotic cores, cells extracted from xenografts, and cells grown in monolayers. This suggests that for the drug cisplatin, in vivo response can be predicted by in vitro response at the same exposure.
A pharmacodynamic model such as the one proposed here is an essential component of a rational strategy for determining the optimal dose and schedule of cisplatin administration. However, further information about the relationship between plasma exposure and host toxicity, and between plasma exposure and tumor extracellular exposure, is also needed as part of such a strategy. Models similar to the one presented here may also be useful for other drugs that act intracellularly, not only for optimizing the administration of drugs in the clinical setting but also as a framework for analyzing in vitro cytotoxicity data when screening for new drugs.
Abbreviations
- AUC
area under the (concentration-time) curve
Footnotes
This work was supported by National Science Foundation grant DMS-0074985.
References
- 1.Reed E, Dabholkar M, Chabner BA. Cancer Chemotherapy and Biotherapy. Philadelphia: Lippincott-Raven; 1996. Platinum analogues. [Google Scholar]
- 2.Troger V, Fischel JL, Formento P, Gioanni J, Milano G. Effects of prolonged exposure to cisplatin on cytotoxicity and intracellular drug concentration. Eur J Cancer. 1992;28:82–86. doi: 10.1016/0959-8049(92)90391-e. [DOI] [PubMed] [Google Scholar]
- 3.Kurihara N, Kubota T, Hoshiya Y, Otani Y, Kumai K, Kitajima M. Antitumor activity of cis-diaminedichloroplatinum (II) depends on its time x concentration product against human gastric cancer cell lines in vitro. J Surg Oncol. 1995;60:238–241. doi: 10.1002/jso.2930600405. [DOI] [PubMed] [Google Scholar]
- 4.Drewinko B, Gottlieb JA. Action of cis-dichlorodiamineplatinum(II) (NSC-119875) at the cellular level. Cancer Chemother Rep. 1975;59:665–673. [PubMed] [Google Scholar]
- 5.Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, Takiyama W, Ishida K, Isono K, Makuuchi H, Imamura M, Shinoda M, Ikeuchi S, Kabuto T, Yamana H, Fukuda H. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407) Jpn J Clin Oncol. 2001;31:419–423. doi: 10.1093/jjco/hye090. [DOI] [PubMed] [Google Scholar]
- 6.Salem P, Khalyl M, Jabboury K, Hashimi L. cis-Diaminedichloroplatinum (II) by 5-day continuous infusion. A new dose schedule with minimal toxicity. Cancer. 1984;53:837–840. doi: 10.1002/1097-0142(19840215)53:4<837::aid-cncr2820530403>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, Ohno T, Masuhara K, Tanaka Y, Kato K, Nagai H, Yokoyama A, Kurita Y. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39:131–137. doi: 10.1007/s002800050548. [DOI] [PubMed] [Google Scholar]
- 8.Nagai N, Ogata H. Quantitative relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity in rats: importance of area under the concentration-time curve (AUC) as the major toxicodynamic determinant in vivo. Cancer Chemother Pharmacol. 1997;40:11–18. doi: 10.1007/s002800050618. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa S, Sugiyama Y, Mitsuhashi Y, Kobayashi T, Inaba M. Cell killing action of cell cycle phase-non-specific antitumor agents is dependent on concentration-time product. Cancer Chemother Pharmacol. 1988;21:185–190. doi: 10.1007/BF00262767. [DOI] [PubMed] [Google Scholar]
- 10.Nozue M, Nishida M, Todoroki T, Fukao K, Tanaka M. Selection of three out of 24 anti-cancer agents in poorly-differentiated gastric cancer cell lines, evaluated by the AUC/delta IC50 ratio. Anticancer Drugs. 1995;6:291–302. doi: 10.1097/00001813-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Levasseur LM, Slocum HK, Rustum YM, Greco WR. Modeling of the time-dependency of in vitro drug cytotoxicity and resistance. Cancer Res. 1998;58:5749–5761. [PubMed] [Google Scholar]
- 12.Ma J, Verweij J, Kolker HJ, van Ingen HE, Stoter G, Schellens JH. Pharmacokinetic-dynamic relationship of cisplatin in vitro: simulation of an i.v. bolus and 3 h and 20 h infusion. Br J Cancer. 1994;69:858–862. doi: 10.1038/bjc.1994.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erlichman C, Vidgen D, Wu A. Antineoplastic drug cytotoxicity in a human bladder cancer cell line: implications for intravesical chemotherapy. Urol Res. 1987;15:13–16. doi: 10.1007/BF00256328. [DOI] [PubMed] [Google Scholar]
- 14.Skipper HE. The effects of chemotherapy on the kinetics of leukemic cell behavior. Cancer Res. 1965;25:1544–1550. [PubMed] [Google Scholar]
- 15.Adams DJ, Watkins PJ, Knick VC, Tuttle RL, Bair KW. Evaluation of arylmethylaminopropanediols by a novel in vitro pharmacodynamic assay: correlation with antitumor activity in vivo. Cancer Res. 1990;50:3663–3669. [PubMed] [Google Scholar]
- 16.Gardner SN. A mechanistic, predictive model of dose-response curves for cell cycle phase-specific and-nonspecific drugs. Cancer Res. 2000;60:1417–1425. [PubMed] [Google Scholar]
- 17.El-Kareh AW, Secomb TW. Theoretical analyses and simulations of anti-cancer drug delivery. In: Brown DM, editor. Drug Delivery Systems in Cancer Therapy. Totowa, NJ: Humana Press; 2003. in press. [Google Scholar]
- 18.Sadowitz PD, Hubbard BA, Dabrowiak JC, Goodisman J, Tacka KA, Aktas MK, Cunningham MJ, Dubowy RL, Souid AK. Kinetics of cisplatin binding to cellular DNA and modulations by thiol-blocking agents and thiol drugs. Drug Metab Dispos. 2002;30:183–190. doi: 10.1124/dmd.30.2.183. [DOI] [PubMed] [Google Scholar]
- 19.Pascoe JM, Roberts JJ. Interactions between mammalian cell DNA and inorganic platinum compounds: I. DNA interstrand cross-linking and cytotoxic properties of platinum(II) compounds. Biochem Pharmacol. 1974;23:1359–1365. doi: 10.1016/0006-2952(74)90355-4. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JJ, Knox RJ, Friedlos F, Lydall DA. DNA as the target for the cytotoxic and antitumour action of platinum co-ordination complexes: comparative in vitro and in vivo studies of cisplatin and carboplatin. In: McBrien DCH, Slater TF, editors. Biochemical Mechanisms of Platinum Antitumour Drugs. Washington, DC: IRL Press; 1986. pp. 29–64. [Google Scholar]
- 21.Eichholtz-Wirth H, Hietel B. The relationship between cisplatin sensitivity and drug uptake into mammalian cells in vitro. Br J Cancer. 1986;54:239–243. doi: 10.1038/bjc.1986.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecquet B, Leroy A, Lefebvre JL, Peyrat JP, Adenis L. Uptake of platinum compounds in human tumors. In vitro study. Bull Cancer. 1986;73:535–541. [PubMed] [Google Scholar]
- 23.Hromas RA, North JA, Burns CP. Decreased cisplatin uptake by resistant L1210 leukemia cells. Cancer Lett. 1987;36:197–201. doi: 10.1016/0304-3835(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SW, Shen D, Pastan I, Gottesman MM, Hamilton TC. Cross-resistance, cisplatin accumulation, and platinum-DNA adduct formation and removal in cisplatin-sensitive and-resistant human hepatoma cell lines. Exp Cell Res. 1996;226:133–139. doi: 10.1006/excr.1996.0211. [DOI] [PubMed] [Google Scholar]
- 25.Mistry P, Kelland LR, Loh SY, Abel G, Murrer BA, Harrap KR. Comparison of cellular accumulation and cytotoxicity of cisplatin with that of tetraplatin and aminedibutyratodichloro(cyclohexylamine)-platinum(IV) (JM221) in human ovarian carcinoma cell lines. Cancer Res. 1992;52:6188–6193. [PubMed] [Google Scholar]
- 26.Andrews PA, Velury S, Mann SC, Howell SB. cis-Diaminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1988;48:68–73. [PubMed] [Google Scholar]
- 27.Veal GJ, Dias C, Price L, Parry A, Errington J, Hale J, Pearson AD, Boddy AV, Newell DR, Tilby MJ. Influence of cellular factors and pharmacokinetics on the formation of platinum-DNA adducts in leukocytes of children receiving cisplatin therapy. Clin Cancer Res. 2001;7:2205–2212. [PubMed] [Google Scholar]
- 28.Durand RE, Olive PL. Flow cytometry studies of intracellular adriamycin in single cells in vitro. Cancer Res. 1981;41:3489–3494. [PubMed] [Google Scholar]
- 29.Takada K, Kawamura T, Inai M, Masuda S, Oka T, Yoshikawa Y, Shibata N, Yoshikawa H, Ike O, Wada H, Hitomi S. Pharmacokinetics of cisplatin in analbuminemic rats. Biopharm Drug Dispos. 1999;20:421–428. doi: 10.1002/1099-081x(199912)20:9<421::aid-bdd206>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Gamelin E, Allain P, Maillart P, Turcant A, Delva R, Lortholary A, Larra F. Long-term pharmacokinetic behavior of platinum after cisplatin administration. Cancer Chemother Pharmacol. 1995;37:97–102. doi: 10.1007/BF00685635. [DOI] [PubMed] [Google Scholar]
- 31.Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 32.Erlichman C, Vidgen D, Wu A. Cytotoxicity of cisplatin and cisdiamine-1,1-cyclobutane dicarboxylate in MGH-U1 cells grown as monolayers, spheroids, and xenografts. J Natl Cancer Inst. 1985;75:499–505. [PubMed] [Google Scholar]
- 33.Matsushima Y, Kanzawa F, Hoshi A, Shimizu E, Nomori H, Sasaki Y, Saijo N. Time-schedule dependency of the inhibiting activity of various anticancer drugs in the clonogenic assay. Cancer Chemother Pharmacol. 1985;14:104–107. doi: 10.1007/BF00434345. [DOI] [PubMed] [Google Scholar]
- 34.Rupniak HT, Whelan RD, Hill BT. Concentration and time-dependent inter-relationships for antitumour drug cytotoxicities against tumour cells in vitro. Int J Cancer. 1983;32:7–12. doi: 10.1002/ijc.2910320103. [DOI] [PubMed] [Google Scholar]
- 35.Los G, Verdegaal E, Noteborn HP, Ruevekamp M, de Graeff A, Meesters EW, ten Bokkel HD, McVie JG. Cellular pharmacokinetics of carboplatin and cisplatin in relation to their cytotoxic action. Biochem Pharmacol. 1991;42:357–363. doi: 10.1016/0006-2952(91)90723-i. [DOI] [PubMed] [Google Scholar]